
Citation: Wang LF, Yan ZY, Li YL, Wang YH, Zhang SJ, Jia X, Lu L,
Shang YX, Wang X, Li YH, Li SY. Inhibition of Obtusifolin on retinal pigment
epithelial cell growth under hypoxia. Int J Ophthalmol 2019;12(10):1539-1547. DOI:10.18240/ijo.2019.10.04
·Basic Research·
Inhibition
of Obtusifolin on retinal pigment epithelial cell growth under hypoxia
Li-Fei Wang1, Zhong-Yang Yan1,
Ya-Lin Li1, Yan-Hui Wang1, Sheng-Juan Zhang1,
Xin Jia1, Lu Lu2, Yan-Xia Shang2, Xin Wang3,
Yun-Huan Li1, Shan-Yu Li1
1Fundus Surgery Ward, Hebei
Provincial Eye Hospital, Xingtai 054001, Hebei Province, China
2Diabetic Eye Disease Ward, Hebei
Provincial Eye Hospital, Xingtai 054001, Hebei Province, China
3Corneal Disease Ward, Hebei
Provincial Eye Hospital, Xingtai 054001, Hebei Province, China
Co-first authors: Li-Fei Wang and Zhong-Yang Yan
Correspondence to: Li-Fei Wang. Fundus Surgery Ward,
Hebei Provincial Eye Hospital, No.399 East Quanbei Street, Qiaodong District,
Xingtai 054001, Hebei Province, China. lifeiw_wanglf@163.com
Received:
Abstract
AIM: To explore the effect of Obtusifolin on retinal pigment epithelial cell
growth under hypoxia.
METHODS: In vitro chemical hypoxia model of
ARPE-19 cells was established using cobalt chloride (CoCl2). Cell
viability was tested by cell counting kit-8 (CCK-8) assay. Western blot and
real-time quantitative polymerase chain reaction were applied to detect
proteins and mRNAs respectively. Flow cytometry was used to examine the cell
cycle. Secretion of vascular endothelial growth factor (VEGF) was tested by
using enzyme linked immunosorbent assay (ELISA).
RESULTS: Under the chemical hypoxia model established by
CoCl2, hypoxia inducible factor-1α (HIF-1α) mRNA and protein levels was up-regulated. Cell
viability was increased and the proportion of S phase was higher. Obtusifolin
could reduce cell viability under hypoxic conditions and arrest cells in G1
phase. Obtusifolin reduced the expression of Cyclin D1 and proliferating cell
nuclear antigen (PCNA) in the hypoxic environment and increased the expression
of p53 and p21. The levels of VEGF, VEGFR2 and eNOS proteins and mRNA were
significantly increased under hypoxia while Obtusifolin inhibited the
increasing.
CONCLUSION: Obtusifolin can inhibit cell growth under hypoxic
conditions and down-regulate HIF-1/VEGF/eNOS secretions in ARPE-19 cells.
KEYWORDS: retinal pigment epithelial cells;
Obtusifolin; vascular endothelial growth factor; hypoxia
DOI:10.18240/ijo.2019.10.04
Citation:
Wang LF, Yan ZY, Li YL, Wang YH, Zhang SJ, Jia X, Lu L, Shang YX, Wang X, Li
YH, Li SY. Inhibition of Obtusifolin on retinal pigment epithelial cell growth
under hypoxia. Int J Ophthalmol
2019;12(10):1539-1547
INTRODUCTION
As a degenerative cause, choroidal
neovascularization (CNV) is the pathological basis of various eye diseases such
as age-related macular degeneration (AMD), myopic macular degeneration (PM) and
central exudative chorioretinopathy (CEC)[1-5].
The mechanism of occurrence and development of CNV is complex, the principle is
not yet clear, and treatment is difficult.
Current treatments for CNV include
surgery to remove or block CNV, intravitreal injections of anti-angiogenic
drugs such as anti-vascular endothelial growth factor (VEGF), and using of
glucocorticoids and inflammatory reactions[6-8]. However, the efficacies of the above methods are not
satisfactory, because of poor long-term efficacy, high recurrence rate, high
price, and many adverse reactions[6-8].
Although modern medicine has developed rapidly in CNV studies, the clinical
efficacy of current CNV is not effective. Therefore, it is of great
significance to explore new treatments.
Semen Cassiae is dry, mature seed of
the leguminous plant Cassia obtusifoiia L. or Cassia tora L. It
is an ancient Chinese medicine that can be used as a food and medicine[9]. The main active ingredient of cassia is Obtusifolin,
which has antioxidant and nominal effects[10].
Study has reported that the activity of ciliary lactate dehydrogenase (LDH) in
obtusifolin-fed dogs and rabbits was significantly elevated[8].
Therefore, we speculate that Obtusifolin has effects on the treatment of CNV.
The generation of blood vessels
refers to the process of forming a new capillary network by sprouting or
intussusception after the body or tissue receiving the stimulus[11]. Current research suggests that hypoxia is one of the
most important causes of the occurrence and development of CNV and studies have
confirmed that VEGF plays a key role in the formation of CNV[12-13]. The hypoxia inducible factor-1(HIF-1)/VEGF/eNOS
pathway is mainly induced by hypoxic environment, activates eNOS release of NO
and other factors through signal transduction, regulates cell proliferation,
apoptosis, and migration[14-15].
It is considered that VEGF-related pathways and proteins are overexpressed in
ocular diseases where CNV is the pathological basis[16-17].
This study explored the effects of
Obtusifolin on cell viability and VEGF in human retinal epithelial cells under
hypoxic conditions, and explored its effects on CNV.
MATERIALS AND METHODS
Cells Culture and Observation The human retinal epithelial cells
line (ARPE-19) was purchased from ATCC (USA). The cells were cultured in RPMI
1640 medium containing 10% fetal bovine serum and 100 U/mL of
penicillin-streptomycin mixture in an incubator at
Cell Viability Analysis Cell counting kit-8 (CCK-8) assay
was used to detect cell viability at 12, 24, and 48h after added 0, 50, 100,
150, 200 μmol/L CoCl2. The kit was purchased from Tongren (Japan).
Diluted CCK-8 reagent were added and cultured at
Real-time Quantitative Polymerase
Chain Reaction Analysis Real-time quantitative polymerase
chain reaction analysis (RT-qPCR) was used to detect the mRNA expression
levels of HIF-1α, Cyclin D1, proliferating cell nuclear antigen (PCNA), p53,
p21, VEGF, VEGFR2 and eNOS. The cells were triturated and lysed using Trizol
(TaKaRa, Japan) at
Table 1 The
sequences of primers
|
Primer name |
Sequence ( |
Product size (bp) |
|
HIF-1α-forward |
ACCTATGACCTGCTTCCTGC |
98 |
|
HIF-1α-reverse |
TTTAACTCAAGCTGCCTCGC |
|
|
Cyclin D1-forward |
CTGGCCATGAACTACCTGGA |
245 |
|
Cyclin D1-reverse |
GTCACACTTGATCACTCTGG |
|
|
PCNA-forward |
CACCTTAGCACTAGTATTCGAAGCAC |
137 |
|
PCAN-reverse |
CACCCGACGGCATCTTTATTAC |
|
|
p53-forward |
CTGAGGTCGGCTCCGACTATACCACTATCC |
360 |
|
p53-reverse |
CTGATTCAGCTCTCGGAACATCTCGAAGCG |
|
|
P21-forward |
AGTATGCCGTCGTCTGTTCG |
229 |
|
P21-reverse |
CTTGTCCCCCTCCCAGGTCA |
|
|
VEGF-forward |
CTGGAGCGTGTACGTTGGT |
177 |
|
VEGF-reverse |
TTTAACTCAAGCTGCCTCGC |
|
|
VEGFR2-forward |
CCAGGCAACGTAAGTGTTCGAG |
243 |
|
VEGFR2-reverse |
GGGACCCACGTCCTAAACAAAG |
|
|
eNOS-forward |
ACCCTCACCGCTACAACATC |
217 |
|
eNOS-reverse |
GCTCATTCTCCAGGTGCTTC |
|
|
GAPDH-forward |
CCATCTTCCAGGAGCGAGAT |
222 |
|
GAPDH-reverse |
TGCTGATGATCTTGAGGCTG |
|
Western Blot Western blot was applied to detect
protein expression. Cells were lysed with liquid nitrogen and blocked with RIPA
(Abmole, USA), followed by 1% cleavage in PMSF and phosphatase inhibitors
(Abmole, USA) and lysis for 30min at
Evaluation of Cell Cycle Cell cycle was tested by flow
cytometry. The cells were collected and washed with PBS at
Enzyme Linked Immunosorbent
Assay The VEGF concentration of culture
fluid was tested using enzyme linked immunosorbent assay (ELISA). The kits were
purchased from Nanjing Kaiji Biotechnology Co., Ltd. (China). The primary
antibody was added at
Statistical Analysis All the experimental data were presented
as mean±standard deviation (SD). Statistical analysis used SPSS 20 (SPSS, Inc.,
Chicago, IL, USA). The one-way analysis of variance (ANOVA) following Turkey’s
multiple comparison was carried out to evaluate the differences between the
experimental groups. The statistical significant was expressed as P<0.05.
RESULTS
Changes of Cell Viability in
Hypoxia The viable ARPE-19 macrophages were
normal at 100-fold and 200-fold observations (Figure
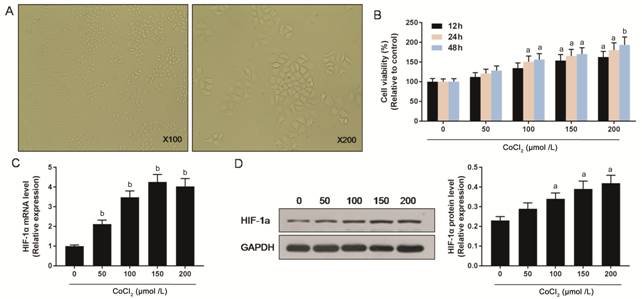
Figure 1 Effects of hypoxia on
ARPE-19 cells A: The morphology of ARPE-19 cells
at 100-fold and 200-fold were observed using microscope; B: The cell viability
of ARPE-19 cells under different CoCl2 concentrations at 12, 24, 48h
were measured using the CCK-8 assay; C, D: Expression levels of HIF-1α mRNA and
protein under different CoCl2 concentrations were tested by RT-qPCR
and Western blot respectively. aP<0.05, bP<0.01
versus 0 μmol /L CoCl2 group.
Effects of Obtusifolin on ARPE-19
Cells under Hypoxia To study the effects of Obtusifolin
on ARPE-19 cells under a hypoxic environment, cells were pretreated with 100,
200, and 400 μg/mL Obtusifolin before adding CoCl2. The cell
viability gradually and cell count were all decreased with the increase of the
concentration of Obtusifolin, and the cell viability in 400 μg/mL
concentrations was similar to that of the control group. This suggests that
Obtusifolin could reduce ARPE-19 cells viability under hypoxic condition
(Figure
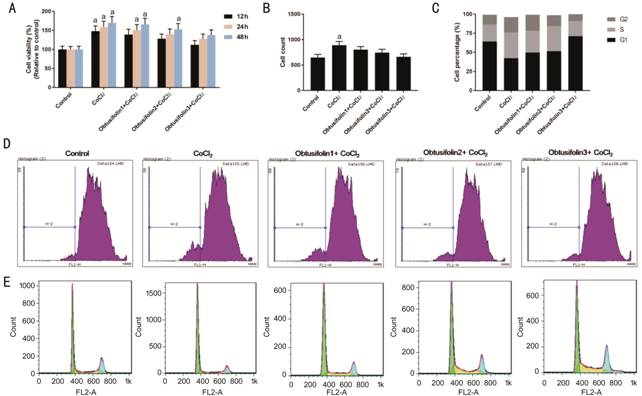
Figure 2 Effects of Obtusifolin on
the cell cycle of ARPE-19 cells under hypoxia A: Cell viability under 100, 200, 400
μg/mL Obtusifolin concentration for 24h in a hypoxic environment; B: Cell count
under 100, 200, 400 μg/mL Obtusifolin for 24h in a hypoxic environment; C-E:
Flow cytometry was applied to detect the cell cycle under 100, 200, 400 μg/mL
Obtusifolin in a hypoxic environment. Obtusifolin1, Obtusifolin2, and
Obtusifolin3 represent 100, 200, and 400 μg/mL concentrations respectively. aP<0.05
versus control group.
To explore the factors that
influenced the viability of ARPE-19 cells by Obtusifolin, the cell cycle was
examined by using flow cytometry. Chemical hypoxia caused the ARPE-19 cells to
enter the S phase to accelerate the division. Obtusifolin could restore the
cell cycle under a hypoxic environment similar to the control group. This
indicates that the viability and proliferative capacity of the ARPE-19 cells
were inhibited in the presence of Obtusifolin (Figure
Effects of Obtusifolin on Cell Cycle
Associated Protein To investigate the effects of 100,
200, 400 μg/mL Obtusifolin on the cell cycle under hypoxic environments, the
expression levels of cell cycle-associated proteins and mRNAs were determined
by Western blot and RT-qPCR. When ARPE-19 cells were under hypoxic conditions,
the levels of Cyclin D1 and PCNA protein and mRNA were significantly increased
while the levels of p53 and p21 were decreased (Figure 3). The presence of
Obtusifolin inhibited the expression of Cyclin D1 and PCNA in hypoxic
conditions and up-regulated p53 and p21 levels. With the concentration of
Obtusifolin increased, the effects increased (Figure 3). This suggested that
hypoxia could promote cell proliferation and division by regulating cell
cycle-associated proteins, while Obtusifolin could reduce cell proliferation by
affecting cell cycle-associated proteins and promote cells retention in the G1
phase.
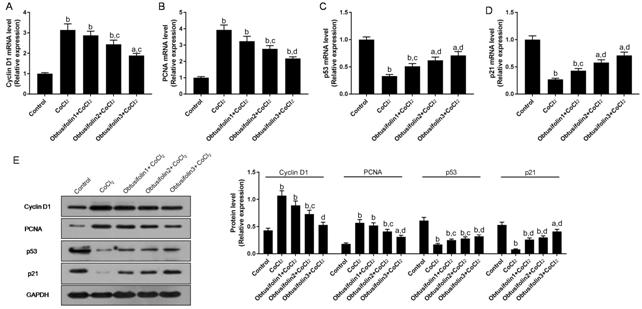
Figure 3 Effecst of Obtusifolin on
cell cycle associated proteins A-D: Cyclin D1, PCNA, p53 and p21
mRNAs were detected by RT-qPCR under 100, 200, 400 μg/mL Obtusifolin; E: Cyclin
D1, PCNA, p53 and p21 proteins were detected using Western blot. Obtusifolin1,
Obtusifolin2, and Obtusifolin3 represent 100, 200, and 400 μg/mL concentrations
respectively. aP<0.05, bP<0.01 versus
control group; cP<0.05, dP<0.01
versus CoCl2 group.
Effects of Obtusifolin on HIF-1,
VEGF, and eNOS To study the effects of Obtusifolin
on the HIF-1, VEGF, and eNOS in the hypoxic cell model, the expression levels
of the relevant mRNA and protein in the pathway were detected by RT-qPCR and
Western blot respectively. When ARPE-19 cells were exposed to hypoxia, the
levels of HIF-1α, VEGF, VEGFR2 and eNOS proteins and mRNA were significantly
increased (Figure 4). Obtusifolin could dose-dependently down-regulate the
expression of the pathway to make it close to the control group (Figure 4). The
level of VEGF secreted by ARPE-19 cells was significantly elevated under the
induction of hypoxia. Obtusifolin dose-dependently down-regulated VEGF
secretion (Figure
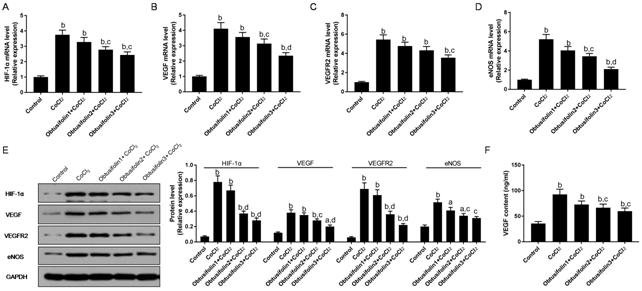
Figure 4 Effecst of Obtusifolin on
HIF-1, VEGF, and eNOS A-D: RT-qPCR was applied to detect
HIF-1α, VEGF, VEGFR2 and eNOS mRNA expressions under 100, 200, 400 μg/mL
Obtusifolin; E: Western blot was used to test HIF-1α, VEGF, VEGFR2 and eNOS
protein expressions under 100, 200, 400 μg/mL Obtusifolin; F: Secretion of VEGF
under 100, 200, 400 μg/mL Obtusifolin were detected by ELISA. Obtusifolin1,
Obtusifolin2, and Obtusifolin3 represent 100, 200, and 400 μg/mL concentrations
respectively. aP<0.05, bP<0.01 versus
control group; cP<0.05, dP<0.01
versus CoCl2 group.
Effects of Obtusifolin on ARPE-19
Cells The effects of different
concentrations of Obtusifolin on cells was observed under a microscope (Figure
5). The possible mechanism of Obtusifolin was shown in Figure 6.
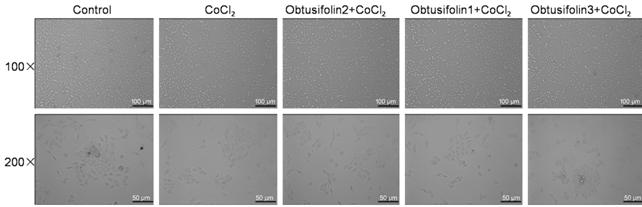
Figure 5 Effecst of Obtusifolin on
cell morphology The effects
of 100, 200, 400 μg/mL Obtusifolin on ARPE-19 cells was observed under a
microscope.
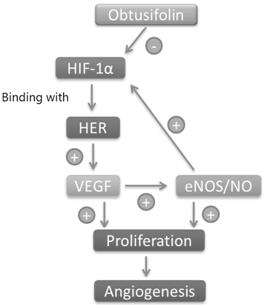
Figure 6 The possible mechanism of
Obtusifolin When retinal
epithelial cells are in anoxic environment, HIF-1α is overexpressed and binds
to HIF-1β to form a dimer. The dimer is gradually transferred to the nucleus in
combination with the hypoxia response element (HER), which promotes
overexpression of the VEGF gene and promotes cell proliferation and
angiogenesis. On the other hand, VEGF can regulate the level of HIF-1α by NO.
Obtusifolin may inhibit cell proliferation and angiogenesis by regulating
oxidative stress levels or inhibiting HIF-1α expression.
DISCUSSION
The main pathological basis of
angiogenesis caused by hypoxia or inflammatory cytokines is overexpression of
VEGF[18]. Angiogenesis is a complex process that
involves the proliferation, migration and tube formation of vascular
endothelial cells[19-20]. CNV
blood vessels mainly come from the retinal pigment epithelial cell[18], so this study uses human retinal pigment epithelial
cells line ARPE-19 cells as the research object. CoCl2 has a low
affinity with O2 and does not have the effect of regulating O2
concentration. However, Co2+ can replace the chelation of Fe2+
and hemoglobin, which destroys the ability of cells to sense hypoxia and thus
mimic the hypoxic microenvironment[21]. The study
also finds that CoCl2 can protect cells through anti-apoptosis
pathways, and the method is simple, stable and easy to control[22]. Therefore, CoCl2 was used to simulate an in
vitro chemical hypoxia microenvironment in this study. The results showed
that the HIF-1α and cell viability were increased in a dose-dependent manner in
the ARPE-19 cells treated with CoCl2, demonstrating the successful
establishment of an in vitro chemical hypoxia model.
Obtusifolin, including Emodin,
Chrysophanol, Rhein, and Aloe-emodin, has a variety of biological activities,
of which the eyesight is one of its most importance[10].
The results of this study showed that Obtusifolin had the effect of reducing
the cell viability of ARPE-19 cells under hypoxic conditions. Further studies
have also found that Obtusifolin could promote the retention in the G1 phase
and inhibit the proliferation of ARPE-19 cells. Hou et al[23] found that Obtusifolin has the effect of promoting
apoptosis of retinal capillary cells in diabetic retinopathy rats. And other
studies suggest that for hyperlipidemic rats, Obtusifolin shows anti-oxidation
and NO regulation[24]. This showed that
Obtusifolin inhibits the proliferation and differentiation of ARPE-19 cells,
suggesting that it has a certain anti-angiogenic ability.
To further explore the mechanism of
the effect of Obtusifolin on cell viability, we studied cell cycle-related
proteins by Western blot and RT-qPCR. The results showed that Obtusifolin could
dose-dependently down-regulate Cyclin D1 and PCNA in ARPE-19 cells under
hypoxia and up-regulate p53 and p21 levels. Cyclin D1 is one of the most
important proteins that regulate cell cycle, it can bind and activate the
unique cyclin-dependent kinase CDK4 during G1, promoting cell cycle progression
from G1 to S, thereby promoting cell proliferation[25].
PCNA is involved in cellular DNA synthesis. PCNA was not expressed in G0-G1
phase cells, but it was significantly increased in the late G1 phase, and PCNA
was a sensitive indicator of cell cycle response[26].
As a tumor suppressor gene, p53 has the effect of inhibiting cell proliferation
by tissue cycle[27]. The p21 gene is a member of
the Clp family and it is a cyclin-dependent kinase inhibitor downstream of the
p53 gene[28]. P21 can together with p53
constitute the cell cycle G1 checkpoint[29]. The
results of this study suggest that Obtusifolin could inhibit cell proliferation
by up-regulating tumor suppressor genes and down-regulating cyclin proteins.
The proliferation of cells is
affected by a variety of cellular pathways. For the proliferation of retinal
pigment epithelial cells and the formation of blood vessels, the largest
influencing factor is the hypoxic microenvironment, and overexpression of VEGF
is the main cause of vascular proliferation[30-31]. HIF-1 is a key upstream transcription factor in
angiogenesis signaling pathway, HIF-1 can be divided into HIF-1α and HIF-1β[32]. When the body is under hypoxia, it will induce high
expression of HIF-1α, and it will up-regulate the expression of VEGF after
binding with VEGF gene through hypoxia response element (HER)[33]. As a key protein in the pathway, VEGF mainly
promotes the release of eNOS and NO through the activation of PI3K/Akt, MAPK
and JAK/STAT pathway[34-36].
NO is an essential angiogenesis profile factor[37-38]. On the other hand, PI3K/Akt and other pathways also
have the effect of promoting cell proliferation and anti-apoptosis[39-40]. The results of this study
indicated that the hypoxic microenvironment could promote the expression and
secretion of VEGF by increasing the expression of HIF-1α, and promote the
expression of VEGFR2 and eNOS. Obtusifolin could down-regulate the HIF-1α,
decrease the expression and secretion of VEGF. Previous studies have shown that
improving the hypoxic state can play an anti-angiogenic role by inhibiting VEGF[41-42]. Studies have also found that
inhibiting the expression and secretion of VEGF can exert an anti-angiogenic
effect by inhibiting the expression of NO[43].
Tang and Zhong’s study[44] shows that Obtusifolin
can regulate oxidative stress levels associated with obesity and diabetes.
Obtusifolin regulates the levels of SOD and MDA to down-regulate oxidative
stress levels. In addition, the study also finds that Obtusifolin regulates the
level of NO[44]. Study has also shown that
Obtusifolin could reduce the level of inflammatory factors by inhibiting
nuclear factor-kappa B, which might also be related to angiogenesis[45]. This study first discovered that Obtusifolin could
inhibit angiogenesis by inhibiting signal transduction by downregulating HIF-1α
and reducing VEGF expression. In addition, Obtusifolin may also inhibit VEGF
expression and may also inhibit cell proliferation by inhibiting VEGF related
pathways and further studies are needed. Hypoxia could promote angiogenesis
possibly by inducing the expression of VEGF, while Obtusifolin could inhibit
the expression and secretion of VEGF by down-regulating HIF-1α, thereby
reducing the inhibition of angiogenesis by eNOS.
ACKNOWLEDGEMENTS
Authors’ contributions: Substantial contributions to
conception and design: Wang LF, Yan ZY; Data acquisition: Li YL, Wang YH; Data
analysis and interpretation: Zhang SJ, Jia X; Drafting the article or
critically revising it for important intellectual content: Lu L, Shang YX;
Final approval of the version to be published: Wang X, Li YH; Agreement to be accountable
for all aspects of the work in ensuring that questions related to the accuracy
or integrity of the work are appropriately investigated and resolved: Li SY;
All authors read and approved the final manuscript.
Conflicts of Interest: Wang LF, None; Yan ZY,
None; Li YL, None; Wang YH, None; Zhang SJ, None; Jia
X, None; Lu L, None; Shang YX, None; Wang X, None; Li
YH, None; Li SY, None.
REFERENCES