
Citation: Hu BJ, Du XL, Li WB, Chang YW, Shi XD, Ma T, Wang Y, He
YH, Niu R, Cui WN. Incomplete fluid-air exchange technique for idiopathic
macular hole surgery. Int J Ophthalmol 2019;12(10):1582-1588. DOI:10.18240/ijo.2019.10.10
·Clinical
Research·
Incomplete
fluid-air exchange technique for idiopathic macular hole surgery
Bo-Jie Hu1, Xue-Li Du1, Wen-Bo Li1,
Yu-Wen Chang2, Xing-Dong Shi3, Teng Ma1, Yong
Wang1, Yan-Hua He1, Rui Niu1, Wei-Na Cui1
1Department of Retina, Tianjin Medical
University Eye Hospital, 251 Fukang Road, Tianjin 300384, China
2People’s Hospital of Hetian District, Hetian
848000, Xinjiang Uygur Autonomous Region, China
3Department of Ophthalmology, Tianjin First
Central Hospital, Tianjin 300192, China
Co-first authors: Bo-Jie Hu, Xue-Li Du and Wen-Bo Li
Correspondence to: Bo-Jie Hu. Department of Retina,
Tianjin Medical University Eye Hospital, 251 Fukang Road, Tianjin 300384,
China. bhu07@tmu.edu.cn
Received:
Abstract
AIM: To explore an improved procedure involving incomplete fluid-air exchange
for idiopathic macular hole (IMH), and the closure rate, visual function, and
the visual field of macular holes (MHs) were evaluated.
METHODS: This prospective randomized controlled study,
included 40 eyes of 40 patients with IMH who were treated with pars plana
vitrectomy and peeling of the internal limiting membrane. They were grouped by
random digital table. Twenty-one eyes underwent incomplete fluid-air exchange
(IFA) and 19 eyes underwent traditional complete fluid-air exchange (CFA) as
the control group. Outcomes included best-corrected visual acuity (BCVA),
intraocular pressure, and optical coherence tomography, light adaptive
electroretinography, and visual field evaluations.
RESULTS: All MHs <400 μm were successfully closed. BCVAs before and 6mo
after surgery were 0.82±0.41 logMAR and 0.28±0.17 logMAR in IFA group and
0.86±0.34 logMAR and 0.34±0.23 logMAR in CFA group, respectively. The electroretinogram
analysis of patients in IFA group revealed increases in b-wave amplitudes at 1,
3, and 6mo after surgery. Additionally, patients in IFA group showed an
amplitude increase of 28.6% from baseline at 6mo (P<0.05), while no
obvious improvements were noted in CFA group. Although there were no
statistically significant improvements in either group, the IFA group showed a
slight increase in mean sensitivity (P>0.05).
CONCLUSION: IFA is a reliable method that offers comparable
closure rate to CFA and facilitates improvements in visual function.
KEYWORDS: best-corrected visual acuity;
electroretinography; internal limiting membrane; macular hole; fluid-air
exchange; visual field defect
DOI:10.18240/ijo.2019.10.10
Citation:
Hu BJ, Du XL, Li WB, Chang YW, Shi XD, Ma T, Wang Y, He YH, Niu R, Cui WN.
Incomplete fluid-air exchange technique for idiopathic macular hole surgery. Int
J Ophthalmol
2019;12(10):1582-1588
INTRODUCTION
Idiopathic macular hole (IMH) refers
the disorganization of retinal tissue in the macular region without related
primary disease[1]. Usually, the symptoms of the
deterioration of central visual acuity and metamorphosis draw patients’ attention
first. IMH predominantly affects women over the age of 50 years[2].
Since Kelly and Wendel[3] first described vitrectomy to treat macular holes (MHs)
in 1991, several vitrectomy-based surgical methods have been implemented for
MH. At present, the standard therapeutic regimen for IMH includes pars plana
vitrectomy combined with internal limiting membrane (ILM) peeling, air or gas
tamponade. Combined with vitrectomy, ILM peeling and fluid-air exchange
achieves a closure rate of >90% for holes smaller than 400 μm[4-5] and an acceptable closure rate of
50%-88% for larger holes[6].
Melberg and Thomas[7]
first reported visual field defects after MH surgery in 1995. Since then,
numerous studies have confirmed this phenomenon in patients after vitrectomy
for MH, including presentations of scotoma, wedge-shaped defects[8], and arc-shaped defects[9].
Other studies have reported the worsening of visual field loss after the
surgery[10]. Overall, the reported complication
rate for vitrectomy to treat MH ranges from 7%-71%[11-12].
The mechanism by which vitrectomy
produces visual field defects is unclear. Several studies have related this
phenomenon to mechanical damage of the optic nerve by extrusion needle[13-14] and tractional damage to the
peripapillary nerve fiber layer during posterior hyaloid removal[14-15]. Alternatively, some studies
have implicated high intraoperative infusion pressure and optic nerve fiber
dehydration following complete fluid-air exchange in the development of visual
field defects[16-17].
Additionally, the potential influence of impaired retinal or choroidal circulation,
gas contact with the retina, and light toxicity cannot be excluded.
In the present study, we compared
outcomes of an improved incomplete fluid-air exchange (IFA) technique with
those of traditional complete fluid-air exchange (CFA) for IMH surgery. IFA
technology was designed to improve the recovery of visual function and reduce
the occurrence of visual field defects. The IFA technique leaves a small amount
of fluid on the surface of the posterior retina to avoid touching the area
between the superior and inferior vascular arches, especially the ILM sparing
retina and optic disc.
SUBJECTS AND METHODS
Ethical Approval The study protocol followed
Declaration of Helsinki and was approved by the Ethics Committee of Tianjin
Medical University Eye Hospital and registered with Clinicaltrials.gov (study
No.NCT02584062). Patients provided written informed consent for participation
prior to study enrollment.
Study Design and Patients The study, a prospective randomized
controlled study, included a total of 40 patients with diagnosed IMH. And these
patients were divided into IFA group and CFA group based on the random digital
table. The exclusion criteria were high myopia (≥-6.00 diopters), axial length
(AL) >
Ophthalmologic Examination Preoperative and postoperative
ophthalmic examinations were performed at baseline and 1, 3, and 6mo after
surgery and included best-corrected visual acuity (BCVA) using the Snellen
visual chart, intraocular pressure measurement with a non-contact tonometer,
slit-lamp microscopy, indirect ophthalmoscopy, optical coherence tomography
(OCT; TOPCON 3D-OCT-2000; Topcon Corporation, Tokyo, Japan), evaluation of the
30° central field of vision (HAAG-STREIT OCTOPUS900; Haag-Streit, Koenitz,
Switzerland), and light-adapted electroretinography (ERG; ESPION 0-190).
Optometry and LENSTAR examinations were also performed before the surgery. MH
was classified using the Gass classification.
Surgical Procedures All surgeries were performed by the
same experienced surgeon with the same instruments (
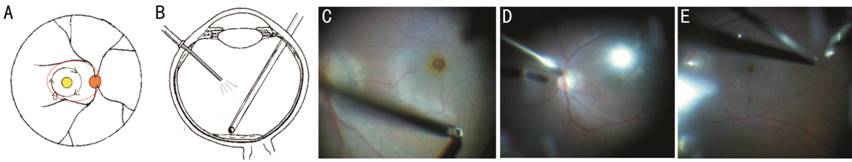
Figure 1 The incomplete fluid-air
exchange procedure A and B are
sketches, and C-E are intraoperative still photographs. For A, the right orange
circle represents the optic disc and the yellow circle indicates the macular
area. The larger black circle represents peeling of the inner limiting membrane
(ILM) of approximately 2-3 papillary diameters. ILM peeling began at the
inferior temporal avascular retina (wide arrow) and continued in the direction
indicated by the black arrows. No additional operations were performed in the
macular area after ILM peeling in the incomplete fluid-air exchange group. For
incomplete fluid-air exchange (B), we left a small amount of fluid on the
posterior retinal surface to avoid mechanical contact with the posterior area
and especially the optic disc and the ILM sparing retina (shown as the oval
region circled with a red line of A). C represents the beginning of the ILM
peeling. D and E represent the operation of incomplete fluid-air exchange.
Patients with an obviously cloudy
lens underwent combined phacoemulsification and intraocular lens (IOL)
implantation. Whether a patient underwent combined cataract surgery was not
influenced by group and was decided prior to group assignment.
The primary outcome after surgery
was anatomic closure of the MH. Secondary outcomes included the recovery of
visual function measured as BCVA, light ERG findings, and visual field defects
as a complication.
Statistical Analysis A one-way ANOVA was used to assess
the changes between pre- and postoperation for IFA group or CFA group.
Significant effects were further investigated using Bonferroni multiple
comparison tests. Student’s t-tests were used to compare paired data
between the two groups at each time point. Chi-square tests were used to
analyze categorical data. All statistical analyses were performed using
GraphPad Prism6 (GraphPad Software Inc., San Diego, CA, USA). The threshold for
statistical significance was set at P<0.05. Data are expressed as the
mean and standard deviation.
RESULTS
The study included a total of 40
eyes from 40 patients, with 21 eyes in IFA group and 19 eyes in CFA group. All
of the eyes are phakia eyes. There were no significant differences between the
groups in terms of age, disease duration, AL, and MH diameter or stage (Table
1). Four MHs (>400 μm) were not successfully closed at the time of the first
postoperative follow-up; of these, two belonging to IFA group were closed after
CFA, one patient from IFA group underwent an extended ILM peeling and plugging
procedure, and another patient from CFA group refused further treatment. In IFA
group, 12 eyes of them combined cataract and vitrectomy surgery, 2 eyes
underwent cataract surgery in the follow-up period, and the other 4 eyes did
not undergo cataract surgery within 6mo after operation. In CFA group, 11 eyes
combined cataract and vitrectomy surgery, the other 7 eyes did not undergo
cataract surgery within 6mo after operation. No patients were lost to
follow-up. Therefore, we analyzed data for 36 eyes, including 18 eyes in IFA
group and 18 eyes in CFA group.
Table 1 Characteristics of the MH
patients involved in the study
|
Parameters |
IFA group |
CFA group |
t |
P |
|
No. (cases, male/female) |
5/16 |
3/16 |
- |
- |
|
Eye (cases, phakia/pseudophakia) |
21 (21/0) |
19 (19/0) |
- |
- |
|
Age (y, mean±SD) |
67.33±4.52 |
63.56±6.82 |
2.22 |
0.30 |
|
Duration (d, mean±SD) |
73.52±54.76 |
89.33±87.63 |
-0.69 |
0.18 |
|
AL (mm, mean±SD) |
23.39±0.86 |
23.46±0.55 |
-0.31 |
0.31 |
|
MHD (μm, mean±SD) |
427.00±171.86 |
486.53±190.93 |
-1.04 |
0.62 |
|
Stage of MH (Gass stage) |
|
|
|
0.60 |
|
I |
0 |
0 |
|
|
|
II |
12 (57.1%) |
8 (42.1%) |
|
|
|
III |
4 (19.0%) |
4 (21.1%) |
|
|
|
IV |
5 (23.8%) |
7 (36.8%) |
|
|
IFA: Incomplete fluid-air exchange;
CFA: Complete fluid-air exchange; AL: Axial length; MHD: The minimum diameter
of macular hole.
Macular Hole Closure Rate The initial closure rate in IFA
group was 85.71% (18/21) at 1mo after surgery; we successfully closed 100%
(12/12) of holes with a diameter <400 μm and 66.7% (6/9) of holes with a
diameter >400 μm. In CFA group, the initial closure rate was 94.74% (18/19)
including 100% (8/8) of holes with a diameter <400 μm and 90.9% (10/11) of
holes with a diameter >400 μm. There was no significant between-group difference
in the initial closure rate (P=0.673, Chi-square test); also, the
closure rate for large MHs did not have significant differences (P=0.089,
Chi-square test). There were no cases of MH reopening during follow-up. We
present a series of morphological changes in the MH examined by OCT after
surgery with incomplete fluid-air exchange in Figure 2.
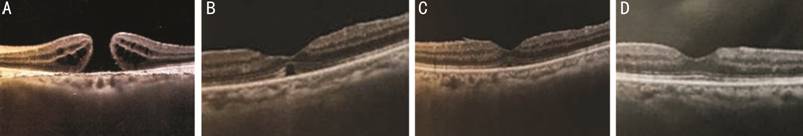
Figure 2 Morphological changes of
the MH examined by OCT after surgery with incomplete fluid-air exchange
technology A: The MH before surgery, showing a
rupture of the whole retinal nerve fiber layer (Snellen visual acuity is finger
counting); B: The MH healing like a bridge 1mo after surgery, showing a focal
loss of photoreceptor cells in subcentral foveal macular (Snellen visual acuity
is 20/67); C: The morphology of MH 3mo after surgery, showing a unbroken IS/OS
layer and a subtle loss of the ellipsoid zone (Snellen visual acuity is 20/100
with a cloudy lens); D: Perfect healing of MH 6mo after surgery (Snellen visual
acuity is 20/25 after phaco and IOL implant surgery).
Visual Function
Changes in best-corrected visual
acuity BCVAs expressed as logMAR values
before and after surgery are shown in Figure 3. Preoperative and postoperative
(1, 3, and 6mo) BCVAs were 0.82±0.41 logMAR (Snellen visual acuity, 20/100),
0.51±0.24 logMAR (Snellen visual acuity, 20/57), 0.38±0.22 logMAR (Snellen
visual acuity, 20/43), and 0.28±0.17 logMAR (Snellen visual acuity, 20/36) in
the IFA group and 0.86±0.34 logMAR (Snellen visual acuity, 20/111), 0.53±0.30
logMAR (Snellen visual acuity, 20/59), 0.40±0.27 logMAR (Snellen visual acuity,
20/45), and 0.34±0.23 logMAR (Snellen visual acuity, 20/41) in CFA group,
respectively. The mean postoperative BCVA was significantly higher at all three
follow-ups in both groups compared to the baseline (P<0.05). There
were no significant between-group differences during follow-up.
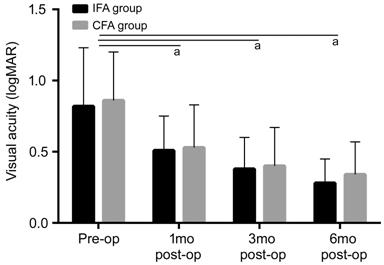
Figure 3 BCVA changes in patients
after MH surgery The mean postoperative BCVA was
significantly higher at all 3 follow-ups in both groups compared to the baseline.
aP<0.05 significantly different from the baseline.
Electrorectinogram amplitudes and
implicit times The b-wave ERG amplitudes and
implicit times are shown in Figure 4. Preoperative and postoperative (1, 3, and
6mo) b-wave amplitudes were 112.16±20.95 mV, 119.13±35.94 mV, 132.52±19.14 mV,
and 144.54±24.58 mV in the IFA group and 124.94±45.21 mV, 132.24±35.51 mV,
130.69±35.25 mV, and 129.31±34.25 mV in the CFA group, respectively. There was
a tendency for higher amplitudes in the IFA group during follow-up; at 6mo,
there was a significant increase in amplitude of 28.6% compared to baseline (P<0.05),
and amplitudes were significantly higher in the IFA group compared to the CFA
group at 3 and 6mo after surgery (P<0.05).
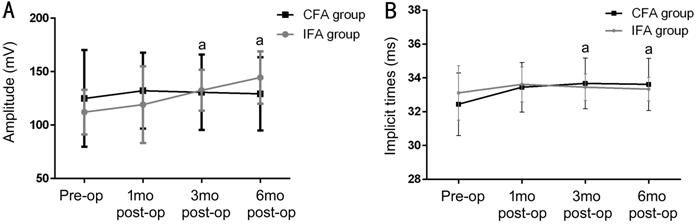
Figure 4 ERG changes over time in
patients after MH surgery A:
b-wave amplitudes of ERG changes. There was a tendency for higher amplitudes in
the IFA group during follow-up; at 6mo, there was a significant increase in
amplitude of 28.6% compared to baseline (P<0.05) and amplitudes were
significantly higher in the IFA group compared to the CFA group at 3 and 6mo
after surgery (P<0.05). B: Implicit times of ERG changes. Implicit
times were prolonged in both groups at 1mo after surgery (P>0.05).
Values gradually returned to baseline over the 6-month follow-up period in the
experiment group but not in the CFA group; between-group differences were
statistically significant at the 3 and 6mo follow-ups (P<0.05). aP<0.05
significant difference between two groups.
We also examined the implicit times
of b-waves (Figure 4B). Preoperative and postoperative (1, 3, and 6mo) implicit
times were 33.11±1.61ms, 33.61±1.04ms, 33.44±0.78ms, and 33.33±0.69ms in the
IFA group and 32.44±1.85ms, 33.44±1.46ms, 33.67±1.50ms, and 33.61±1.54ms in the
CFA group, respectively. Implicit times were prolonged in both groups at 1mo
after surgery (P>0.05). Values gradually returned to baseline over
the 6-month follow-up period in the experiment group but not in the CFA group;
between-group differences were statistically significant at the 3 and 6mo
follow-ups (P<0.05).
Changes in the visual field Changes in the central 30° visual
field represented by mean sensitivity and mean deviation are shown in Figure 5.
Preoperative and postoperative (1, 3, and 6mo) mean sensitivity values were
23.21±4.96, 23.19±5.22, 24.15±4.11, and 25.13±
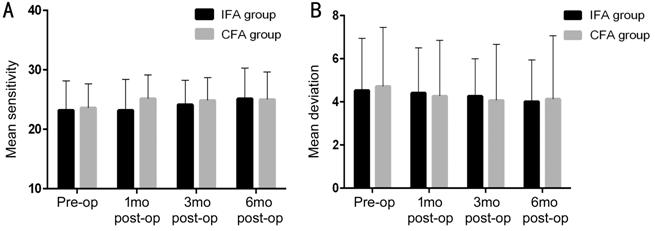
Figure 5 Mean sensitivity changes
over time in patients after MH surgery
A: Mean
sensitivity changes. Although there were no obvious improvements in either
group, there was a non-significant increase in the IFA group; however, there
were no significant between-group differences. B: Mean deviation changes. There
were also no significant changes in either group.
Preoperative and postoperative (1,
3, and 6mo) mean deviations of the central visual field were 4.53±2.41, 4.41±2.10,
4.26±1.74, and 4.02±
Ocular and Systemic
Complications Totally 2 of the patients underwent
cataract surgery during the follow-ups. One case occurred at 3mo after MH
surgery, and the other one occurred at 5mo after vitrectomy. No systemic
complications were noted during the 6mo follow-up.
DISCUSSION
The greatest difference between our
study and other is that here, we improved a procedure of IFA for MH surgeries.
During IFA, we leave a small amount of fluid on the surface of the posterior
retina to avoid touching the ILM sparing retina and optic disc.
Here, we summarize our experience
with IFA technology compared to CFA for the surgical treatment of IMH. The
results indicated that closure rates were similar between the two procedures,
although CFA was associated with a comparable closure rate for MHs >400 μm.
The factors affecting MH closure are mainly linked to residual traction from
the ILM or epiretinal membrane, shorter maintenance tamponade, poor compliance
with a prone position, and large hole diameters[18-21]. In other cases, the exact reasons for poor hole
closure are unclear[22]. Previous studies have
consistently associated lower closure rates with MH >400 μm[23-26]; therefore, we do not believe
that IFA was a direct reason for poorer closure of large MHs in IFA group in
this study. This notion is supported by the successful closure of stage 2/3 MHs
in IFA group.
During surgery, the range of ILM
peeling (2-3 papillary diameters) did not just cover the fovea; therefore, we
performed full-field ERG, which is more valuable for assessing retinal function
than BCVA. Both groups showed postoperative improvements in visual function as
measured by BCVA and b-wave amplitude recovery, the latter of which was better
in IFA group. Some patients underwent combined phacoemulsification and IOL
implantation surgery, which also improves BCVA. Although we did not utilize a
uniform standard to quantify BCVA (e.g., use of cataract surgery
together with posterior capsule incision in all patients), our outcome is still
meaningful in a real-world context. Consistent with our study, previous studies
have reported notable improvements in visual function after IMH closure[27-29].
Improvements in BCVA and on ERG
suggest that better visual function was achieved in IFA group. A previous study
concluded that surgeon experience is an important factor affecting functional
results and suggested that, when possible, surgeons should minimize contact
with the inner retinal fiber[30]. Moreover,
improvements in BCVA were noted earlier than those in ERG findings; we found
that ERG recovery occurred 6mo after vitrectomy. One possible explanation is
that improvements in BCVA were attributed to substitution of the IOL as well as
MH closure. Alternatively, it can be considered that electrophysiological
recovery is delayed by damage to the inner retinal layer, damage during removal
of the posterior vitreous cortex, and ILM. As shown by a previous study, ERG
objectively reflects the electrophysiological responses of cones and the inner
retinal layer, including Müller and bipolar cells.
No significant visual field defects
were noted in our study. We consider that this result may have been partly
related to the use of IFA, which maintained a humid environment in the macular
area as well as a constant intraocular pressure of
The final closure rate in this study
was 97.5% (39/40). Failure close MH in one patient was partly related to
refusal of further treatment. All four MHs that were not closed after the
initial procedure were Gass stage 4 holes with symptom durations varying from
2mo to 2y. Of note, we used sterile air tamponade in the vitreous cavity rather
than an expansive gas. Thus, we speculate that larger hole diameter, shorter
duration of intraocular tamponade, and potential poor compliance with a prone
position were the main reasons for initial failure to close the MH. Also of
note, all four diameters were >400 μm with complete posterior vitreous
detachment and MH edges that were smooth, regular, round, and hydropic. In a
study by Brockmann et al[38], perifoveal
pseudocysts on OCT were beneficial for MH closure. A possible mechanism
underlying this observation is that compensatory glia cell swelling reduced the
minimal diameter of larger holes[39]. In
contrast, OCT in all four cases of unclosed MHs in this study revealed
pseudocysts at the IMH rim. Moreover, some studies have confirmed that the
basal diameter and minimum linear diameter of the MH affect anatomical closure[40-41], and that diameters >400 μm
have lower closure rates than smaller diameters[42-43]. The relationship between the course of disease and
MH closure remains controversial[44-45].
As mentioned above, the present
study had some limitations. First, because of the limitation of our conditions,
it was a great pity that we could not use more accurate multifocal
electrophysiology and microperimetry examinations. We hope moreover, we included
a small number of patients. A future long-term randomized controlled study is
needed to verify the safety and efficacy of IFA for IMH surgery.
In conclusion, there was no
significant effect of IFA technology on the success of anatomical MH closure;
however, use of this technique facilitated functional recovery and reduced the
occurrence of visual field defects. Given the observation of a general lower
closure rate for stage 4 MHs, we recommend an IFA approach for early stage 2-3
MHs. For larger (>400 μm) MHs, IFA may also be chosen after surgeons weigh
the pros and cons of this approach.
ACKNOWLEDGEMENTS
Foundation: Supported by National Natural
Science Foundation of Xinjiang Autonomous Region, China (No.81460089)
Conflicts of Interest: Hu BJ, None; Du XL, None; Li WB,
None; Chang YW, None; Shi XD, None; Ma T, None; Wang Y,
None; He YH, None; Niu R, None; Cui WN, None.
REFERENCES