
Citation: Mastropasqua L, Di Staso S, D’Aloisio R, Mastropasqua A, Di
Antonio L, Senatore A, Ciancaglini M, Di
Nicola M, Di Martino G, Tognetto D, Toto L. Anatomical and functional
changes after dexamethasone implant and ranibizumab in diabetic macular edema:
a retrospective cohort study. Int J Ophthalmol 2019;12(10): 1589-1597. DOI:10.18240/ijo.2019.10.11
·Clinical
Research·
Anatomical
and functional changes after dexamethasone implant and ranibizumab in diabetic
macular edema: a retrospective cohort study
Leonardo Mastropasqua1,
Silvio Di Staso2, Rossella D’Aloisio3, Alessandra
Mastropasqua1, Luca Di Antonio1, Alfonso Senatore1,
Marco Ciancaglini2, Marta Di Nicola4, Giuseppe Di Martino5,
Daniele Tognetto3, Lisa Toto1
1Department of
Medicine and Science of Ageing, Ophthalmology Clinic, University “G.
d’Annunzio” Chieti-Pescara, Chieti 66100, Italy
2Department of
Life, Health and Environmental Sciences, Ophthalmology Clinic, University of
L’Aquila, L’Aquila 67100, Italy
3Department of
Medicine, Surgery and Health Sciences, Eye Clinic, University of Trieste,
Trieste 34129, Italy
4Department of
Medical, Oral and Biotechnological Sciences, Laboratory of Biostatistics,
University “G. d’Annunzio” Chieti-Pescara, Chieti 66100, Italy
5Department of
Medicine and Science of Ageing, School of Hygiene and Preventive Medicine,
University “G. d’Annunzio” Chieti-Pescara, Chieti 66100, Italy
Co-first
authors: Leonardo Mastropasqua and Silvio Di Staso
Correspondence
to: Rossella D’Aloisio. Department of Medicine, Surgery and Health Sciences,
Eye Clinic, University of Trieste, Trieste, Piazza Ospedale 1 34129, Italy.
ross.daloisio@gmail.com
Received:
Abstract
AIM: To investigate the efficacy and safety of ranibizumab (RZB group) and
dexamethasone implant (DEX group) intravitreal treatments in patients with treatment-naïve
center involved diabetic macular edema (DME) by means of functional and
morphological assessments.
METHODS: This retrospective cohort study included 50 eyes of
50 patients with DME treated either with RBZ or DEX. Best-corrected visual
acuity (BCVA) and microperimetry were evaluated at baseline and during a
6-month follow-up. In addition, central macular thickness (CMT) by means of
structural optical coherence tomography (OCT) and retinal capillary plexus
density and choriocapillary density by means of OCT angiography were assessed
in all cases.
RESULTS: Functional and morphological parameters
significantly improved during the study period in both groups. BCVA improved
significantly in both groups with a greater increase in the DEX group compared
to the RBZ group (P=0.030). Microperimetry significantly differed during
follow-up between the two treatments (P=0.031). In both groups CMT
significantly decreased (P<0.001) without statistically significant
differences between the two groups. A statistically significant increase of
deep capillary plexus density was detected in both groups at 30d after therapy.
The retreatment rate was 0.70±0.10 and 0.65±
CONCLUSION: Both treatments are very effective for DME
treatment during 6mo of follow-up with a lower retreatment rate in DEX group.
KEYWORDS: optical
coherence tomography angiography; diabetic macular edema; intravitreal
dexamethasone implant; intravitreal ranibizumab injections
DOI:10.18240/ijo.2019.10.11
Citation: Mastropasqua
L, Di Staso S, D’Aloisio R,
Mastropasqua A, Di Antonio L, Senatore A, Ciancaglini M, Di Nicola M, Di Martino G, Tognetto D, Toto L. Anatomical and functional changes after
dexamethasone implant and ranibizumab in diabetic macular edema: a
retrospective cohort study. Int J Ophthalmol 2019;12(10): 1589-1597
INTRODUCTION
Diabetic macular edema (DME) is a
leading cause of visual impairment in diabetic retinopathy (DR) and may occur
at any stage of the disease[1-2].
In the past laser photocoagulation
demonstrated its efficacy in prevention of vision loss but did not always
consistently improve visual acuity[3]. Nowadays,
intravitreal treatment either with anti-vascular endothelial growth factor
(VEGF) or steroids agents has become among the most used and effective therapy
for DME condition due to their effect on the retinal vascular permeability and
anti-inflammatory action[4-8].
Anti-VEGF intravitreal treatment is
considered a first-line therapy for center-involved DME improving visual acuity
in a large percentage of patients with best visual results in monthly fixed
regimen compared to pro re nata (PRN) regimen clinical trials[9-12]. Dexamethasone intravitreal
implant (DEX) has demonstrated its efficacy in DME and has been proposed as a
second line therapy in DME refractory to anti-VEGF treatments. Recently some
reports reported DEX use and efficacy in treatment naïve DME[13-17].
The aim of the study was to
investigate the efficacy and safety of the intravitreal ranibizumab (RZB)
treatment and the DEX in treatment-naïve DME patients by means of a functional
and morphological retrospective study. A comparison between both groups of
treatments, in terms of qualitative and quantitative parameters was performed.
SUBJECTS AND METHODS
Ethical Approval This retrospective cohort study included
fifty eyes of 50 patients with center involved DME treated at the
Ophthalmologic Clinic of University “G. d’Annunzio”, Chieti-Pescara, Italy
between December 2016 and October 2017. This retrospective observational study
adhered to the tenets of the Declaration of Helsinki and our Institutional
Review Board approved the retrospective consecutive chart review. Written
informed consent was obtained from all subjects enrolled.
The inclusion criteria were: 1)
treatment naïve patients with no proliferative moderate DR stage (simplified
version of the ETDRS classification)[18] and
center-involved DME type without subretinal fluid component; 2) central macular
thickness (CMT) >300 µm as measured using the spectral-domain optical
coherence tomography (SD-OCT) at the baseline examination; 3) age >18y; 4)
best corrected visual acuity (BCVA) greater than 0.5 logMAR in the study eye at
baseline examination; 5) treatment with RBZ or DEX implant. If both eyes of a
patient met the inclusion/exclusion criteria, the eye with higher CMT was selected
as the study eye.
The patients treated with RZB
(Lucentis, Genentech, Inc., South San Francisco, California, and Novartis
Pharma AG, Basel, Switzerland), were included if three consecutive monthly
intravitreal injections of 0.5 mg ranibizumab followed by PRN regimen had been
administered.
The patients treated with DEX were
included if an intravitreal implant of 0.7 mg sustained-release dexamethasone
(DEX implant; Ozurdex, Allergan, Irvine, CA, USA) followed by PRN treatment,
administered not before 4mo from the first implant had been administered during
a 6-month follow-up. PRN regimen consisted of a new injection starting from
month
The exclusion criteria were: 1) any
previous ocular surgery in the last 6mo; 2) laser treatments; 3) retinal
vascular diseases; 4) medium lens opacities according to Lens Opacities
Classification System (LOCS)[19].
All patients were diagnosed with DR
and DME using fundoscopy examination, fluorescein angiography (FA), SD-OCT and
were evaluated with a comprehensive ophthalmologic examination.
CMT using SD-OCT (XR Avanti®;
Optovue, Inc., Fremont, CA, USA), foveal and parafoveal vessel density using optical
coherence tomography angiography (OCTA; XR Avanti® AngioVue, Optovue Inc., Fremont, CA, USA,
SSADA software version 2017.1.0.144)[20-22],
BCVA and microperimetry (MP; MP-1 Microperimeter, Nidek Technologies, Padova,
Italy) were assessed at baseline, 30, 60, 90, 120, 150 and 180d after the first
intravitreal injection of ranibizumab and DEX implant.
SD-OCT Angiography with XR
Avanti The XR Avanti AngioVue OCTA is a
device with a high speed of 70 000 axial scans per second that uses a light
source of 840 nm and an axial resolution of 5 μm. This system is based on the
SSADA algorithm (version 2017.1.0.144), which uses blood flow as intrinsic contrast.
Flow is detected as a variation over time in a speckle pattern formed by the
interference of light scattered by red blood cells and adjacent tissue
structures.
OCTA scans were acquired following a
standardized protocol as previously described[23].
Vascular Layer Segmentation Vascular retinal layers were
visualized and segmented as previously described in the superficial capillary
plexus (SCP), the deep capillary plexus (DCP) and the choriocapillaris (CC)[24].
The projection-resolved algorithm
was used to remove projection artifacts from the inner vascular plexus in the
deep vascular plexus. This algorithm retains flow signals from blood vessels
while suppressing projected flow signals in deeper layers. Images were reviewed
by two investigators (Toto L and D’Aloisio R) for segmentation accuracy; if
segmentation errors were observed, then they were corrected using the
segmentation and propagation tool from AngioVue. (Angiovue, Optovue, Freemont
CA, USA). Final images were reviewed again to confirm segmentation placement in
all B-Scans.
Quantitative Vessel Analysis Objective quantification of vessel
density was carried out for each eye using SSADA software. A quantitative
analysis was performed on the OCTA en-face images for each eye using AngioVue
software as previously described[23].
Vessel densities of the SCP, DCP and
CC were automatically calculated by software on OCTA 3×3-mm volume scans in the
whole foveal and parafoveal area, foveal area, parafoveal area and in the
superior and inferior hemi-macular areas. Vessel density was defined as the
percentage of the area occupied by vessels in a circular region of interest
(ROI) of
Foveal and Parafoveal Retinal
Thickness Analysis Foveal and parafoveal macular
thickness from the internal
limiting membrane (ILM) to the retinal pigment epithelium (ILM-RPE) were
automatically calculated by software on OCTA 3×3-mm volume scans (XR Avanti1;
Optovue, Inc., Fremont, CA, USA). A circular ROI centred on the foveal
avascular zone with a diameter of
Sample Size Determination and
Statistical Analysis The estimation of the number of eyes
was based on the main endpoint criteria.
A planned sample size of 40 patients was expected to provide 80% power
for a two-sided test with significance level of 0.05, assuming an effect size
of 17% in difference of BCVA after seven days of implantation with between
subjects’ pooled standard deviation of 0.3 logMAR.
A Shapiro-Wilk’s test was performed
to evaluate the departures from normality distribution for each variable.
Student’s test was performed to compare quantitative parameters between DEX and
RZB group. Analysis of variance (ANOVA) for repeated-measures with linear trend
analysis was performed to evaluate the effect of time (within factor), type of therapy
(between factor) and interaction separately for each quantitative parameter.
The Kaplan-Meier method was applied
to estimate the re-treatment rates stratified respect to treatment group (DEX vs
RBZ). The false discovery rate (FDR) correction was used to control the
family-wise type I error rate and an FDR adjusted P value less than 0.05
was determined to be statistically significant. Statistical analysis was
performed using IBM® SPSS Statistics version 20.0 software (SPSS
Inc., Chicago, Illinois, USA).
RESULTS
Demographic Data A total of 50 patients were enrolled
in this study from December 2016 throughout October 2017.
Totally 25 eyes of 25 type 2
diabetic patients (RZB group, 13 males; 12 females; mean age of 61.4±7.3y) with
DME treated with 3 monthly ranibizumab injections followed by a PRN regimen and
25 eyes of 25 type 2 diabetic patients (DEX group, 10 males; 15 females; mean
age of 62.1±6.8y) with DME treated with one DEX followed by a PRN treatment,
were evaluated for the analysis (P=0.752 and P=0.755 for gender
and age, respectively).
No treatment-related complications
were observed during the follow-up, except for two patients of DEX group that
showed intraocular pressure increase requiring hypotonic eye drops.
Thirteen out of 25 eyes in the RZB
group and fourteen out of 25 eyes, in the DEX group were pseudophakic.
Functional Parameters at
Baseline The mean BCVA and 4° MP values of
the two groups of patients at the baseline are reported in Table 1.
Table 1 Baseline parameters of patients
|
Variable |
RZB group (n=25) |
DEX group (n=25) |
Pa |
|
CMT (µm) |
|
|
|
|
Fovea |
460.3±125.2 |
479.1±100.6 |
0.561 |
|
Parafovea |
412.8±73.1 |
447.7±76.0 |
0.104 |
|
SCPD (µm) |
|
|
|
|
Whole |
39.8±4.4 |
40.1±4.2 |
0.806 |
|
Fovea |
26.8±5.8 |
29.3±5.2 |
0.115 |
|
Parafovea |
41.5±4.9 |
41.3±4.3 |
0.878 |
|
Parasuperir |
41.3±5.1 |
40.0±5.8 |
0.404 |
|
Parainferior |
40.8±5.0 |
41.0±6.1 |
0.899 |
|
DCPD (µm) |
|
|
|
|
Whole |
45.9±5.1 |
45.4±5.1 |
0.730 |
|
Fovea |
20.7±7.7 |
19.8±7.4 |
0.675 |
|
Parafovea |
47.7±4.9 |
48.3±3.9 |
0.634 |
|
Parasuperir |
48.8±4.9 |
46.5±6.0 |
0.144 |
|
Parainferior |
47.7±3.9 |
47.5±5.0 |
0.875 |
|
CCD (µm) |
|
|
|
|
Whole |
61.3±7.0 |
63.0±1.8 |
0.254 |
|
Fovea |
61.4±6.2 |
60.8±5.7 |
0.723 |
|
Parafovea |
61.5±6.0 |
63.0±3.3 |
0.279 |
|
Parasuperior |
59.9±8.8 |
62.0±2.1 |
0.252 |
|
Parainferior |
61.5±6.3 |
62.0±3.3 |
0.727 |
|
4° MP (dB) |
5.7±5.5 |
5.8±5.3 |
0.948 |
|
BCVA (logMAR) |
0.4±0.3 |
0.5±0.1 |
0.120 |
aStudent’s t-test DEX group vs
RZB group; CMT: Central macular thickness; SCPD: Superior capillary plexus
density; DCPD: Deep capillary plexus density; CCD: Choriocapillaris density;
MP: Microperimetry; BCVA: Best corrected visual acuity. Data are expressed as
mean and standard deviation.
No statistically significant
difference was found between the two groups of patients in terms of BCVA (P=0.120)
and microperimetry sensitivity (P=0.948). The mean BCVA at the baseline was
0.4±0.3 logMAR in the RZB group and 0.5±0.1 logMAR in the DEX group (Table 1). The mean microperimetry sensitivity
at the baseline was 5.7±5.5 dB in the RZB group and 5.8±5.3 dB in the DEX group
(Table 1).
Morphological Parameters at
Baseline At the baseline no statistically significant
difference was found between the RZB group and DEX group in terms of
morphological parameters (Table 1). The mean CMT in the foveal area was
460.3±125.2 μm (RZB group) and 479.1±100.6 μm (DEX group) (Table 1). The mean
superficial capillary plexus density (SCPD), the mean deep capillary plexus
density (DCPD) and the mean choriocapillaris density (CCD) were not
significantly different (Table 1).
Post Treatment Analysis During the entire follow-up period
BCVA improved significantly in both groups with a greater increase in the DEX
group compared to the RBZ group (P=0.030; Table 2). Variation of retinal
sensitivity at microperimetry significantly differed during follow-up between
the two treatments (P=0.031).
Table 2 Two-way ANOVA for repeated
measures of morphological and functional parameters from baseline to 180d after
therapy
|
Variable |
Group |
Baseline |
30d |
60d |
90d |
120d |
150d |
180d |
Pa |
Pb |
Pc |
|
No. of eyes (DEX/RZB group) |
25/25 |
25/25 |
25/25 |
25/12 |
14/12 |
13/10 |
13/9 |
|
|
|
|
|
CMT (µm) |
|
|
|
|
|
|
|
|
|
|
|
|
Fovea |
DEX group |
479.1±100.6 |
302.7±49.1 |
284.2±49.6 |
306.4±51.7 |
292.7±21.0 |
299.0±31.4 |
300.1±26.7 |
<0.001d |
0.091 |
0.920 |
|
|
RZB group |
460.3±125.2 |
342.1±65.8 |
322.8±55.6 |
319.5±98.4 |
249.0±33.1 |
256.5±41.7 |
250.2±28.7 |
|
|
|
|
Parafovea |
DEX group |
447.7±76.0 |
348.8±24.1 |
345.2±22.4 |
360.5±30.4 |
350.0±14.1 |
355.4±16.7 |
357.3±23.9 |
<0.001d |
0.567 |
0.039 |
|
|
RZB group |
412.8±73.1 |
354.7±33.5 |
350.2±30.3 |
345.8±34.9 |
324.6±24.0 |
327.4±31.0 |
330.5±25.3 |
|
|
|
|
4° MP (dB) |
DEX group |
5.8±5.3 |
7.9±4.4 |
7.9±4.3 |
7.6±5.2 |
6.4±5.5 |
6.5±5.4 |
7.0±5.9 |
0.126 |
0.122 |
0.031d |
|
|
RZB group |
5.7±5.5 |
4.5±3.6 |
6.0±4.4 |
5.4±4.1 |
5.8±3.8 |
5.9±4.0 |
5.8±4.5 |
|
|
|
|
BCVA (logMAR) |
DEX group |
0.5±0.1 |
0.4±0.2 |
0.3±0.2 |
0.3±0.2 |
0.3±0.2 |
0.3±0.2 |
0.3±0.1 |
<0.001d |
0.030 |
<0.001d |
|
|
RZB group |
0.4±0.3 |
0.3±0.2 |
0.3±0.2 |
0.3±0.2 |
0.3±0.4 |
0.3±0.3 |
0.3±0.4 |
|
|
|
Data are expressed as mean and
standard deviation. CMT: Central macular thickness; aP-value relative to effect of period;
bP-value relative to effect of type of
therapy; cP-value relative to interaction term
(time×therapy). dSignificant after FDR correction.
In both groups CMT significantly
decreased (Table 2; P<0.001) in the foveal and parafoveal area
without statistically significant differences between the two groups (Figure
1).
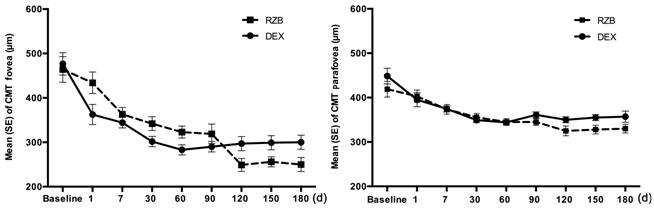
Figure 1 Central macular thickness
modifications during time Central foveal and parafoveal
thickness modifications during time in the DEX group and RBZ group.
In the RZB group CMT in the foveal
area significantly decreased at the postoperative controls from 460.3±125.2 μm
to 342.1± 65.8 μm at 30d and 322.8±55.6 at 60d and in the parafoveal area from
412.8±73.1 μm to 354.7±33.5 μm at 30d and 350.2±30.3 μm at 60d (Table 2 and
Figure 1).
In the DEX group CMT in the foveal
area significantly decreased at the postoperative controls from 479.1±100.6 μm
to 302.7±49.1 μm at 30d and 284.2±49.6 μm at 60d and in the parafoveal area
from 447.7±76.0 μm to 348.8±24.1 μm at 30d and 345.2±22.4 μm at 60d (Table 2
and Figure 1).
At 90 and 120d after injection,
central foveal and parafoveal thickness continued to decrease (Table 2 and
Figure 1). At 6-month follow-up, foveal CMT was 250.2±28.7 μm and 300.1±26.7 μm
respectively in RZB and DEX groups and the parafoveal CMT was 330.5±25.3 μm and
357.3±23.9 μm respectively in RZB and DEX groups. Overall, SCPD did not modify
significantly during the follow-up in both groups, while a difference was found
between the two groups of treatment regards to parainferior SCDP (P=0.023;
Figures 2, 3, Table 3).
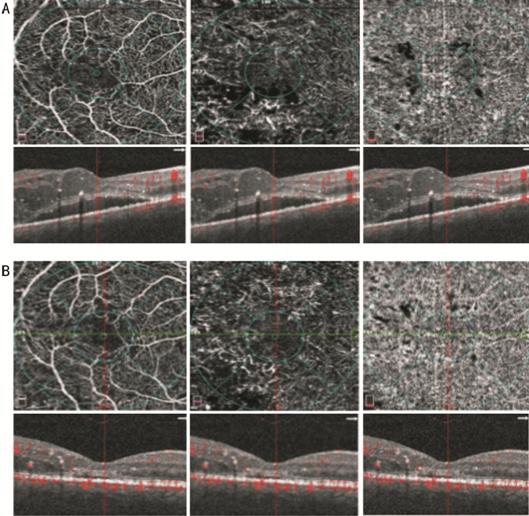
Figure 2 OCTA images of the SCP (A and B,
left panel), DCP (A and B, middle panel) and CC (A and B, right panel) At baseline (A) vessel rarefaction
surrounding the foveal avascular zone in the SCP and DCP, microaneurysms and
diffuse vessel rarefaction in the DCP and focal areas with no apparent flow in
the CC can be observed; corresponding structural SD-OCT images centred on the
fovea (A and B, left, middle and right panel with overlying segmentation bands
at the level of the SCP, DCP and CC, respectively) show increased retinal
thickness due to cystoid macular edema and presence of subretinal fluid. After
a loading dose of ranibizumab injection (B) a restoration of vessel density
mainly in the DCP can be observed with corresponding resolution of macular
edema and partial resolution of subretinal fluid.
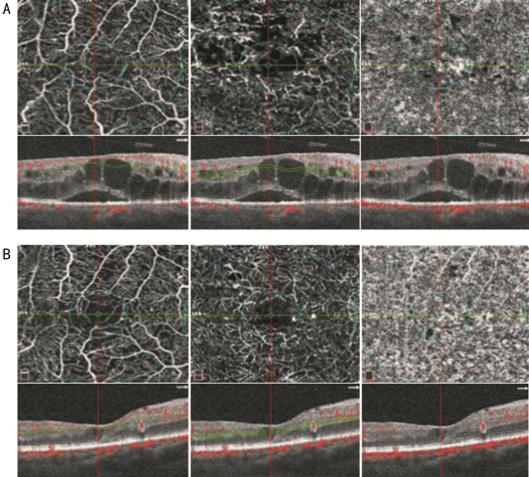
Figure 3 OCTA images of the SCP (A
and B, left panel), DCP (A and B, middle panel) and CC (A and B, right
panel) At baseline (A) vessel rarefaction
surrounding the foveal avascular zone in the SCP and DCP, microaneurysms and
diffuse vessel rarefaction in the DCP and focal areas with no apparent flow in
the CC can be observed; corresponding structural SD-OCT images centred on the
fovea (A and B, left, middle and right with overlying segmentation bands at the
level of the SCP, DCP and CC, respectively) show increased retinal thickness
due to cystoid macular edema and presence of subretinal fluid. After
dexamethasone implant (B) a restoration of vessel density mainly in the DCP can
be observed with corresponding resolution of macular edema and subretinal
fluid.
Table 3 Two-way ANOVA for repeated
measures of morphological parameters from baseline to 180d after therapy
|
Variable |
Group |
Baseline |
30d |
60d |
90d |
120d |
150d |
180d |
Pa |
Pb |
Pc |
|
No. of eyes (DEX/RZB group) |
25/25 |
25/25 |
25/25 |
25/12 |
14/12 |
13/10 |
13/9 |
|
|
|
|
|
SCPD (µm) |
|
|
|
|
|
|
|
|
|
|
|
|
Whole |
DEX group |
40.1±4.2 |
40.8±3.0 |
41.4±3.5 |
41.5±2.9 |
41.1±3.2 |
39.7±0.1 |
39.8±1.1 |
0.077 |
0.069 |
0.035 |
|
|
RZB group |
39.8±4.4 |
40.7±6.2 |
42.1±3.4 |
43.2±4.4 |
45.2±3.6 |
45.5±3.1 |
45.7±3.1 |
|
|
|
|
Fovea |
DEX group |
29.3±5.2 |
25.2±7.2 |
23.4±8.9 |
28.0±8.6 |
28.1±2.9 |
28.1±1.8 |
28.0±1.9 |
0.188 |
0.582 |
0.701 |
|
|
RZB group |
26.8±5.8 |
27.2±7.1 |
25.3±5.2 |
28.6±5.0 |
26.3±7.1 |
25.6±6.0 |
25.0±7.5 |
|
|
|
|
Parafovea |
DEX group |
41.3±4.3 |
41.0±5.3 |
41.7±5.0 |
40.8±3.3 |
41.2±3.4 |
40.7±1.3 |
41.3±1.1 |
0.059 |
0.088 |
0.040 |
|
|
RZB group |
41.5±4.9 |
43.0±5.0 |
44.8±2.9 |
46.0±3.1 |
46.1±2.7 |
46.5±2.4 |
47.1±3.1 |
|
|
|
|
Parasuperior |
DEX group |
40.0±5.8 |
42.2±5.1 |
42.3±5.0 |
41.1±3.3 |
41.9±3.3 |
40.7±.2.1 |
40.5±1.8 |
0.478 |
0.074 |
0.285 |
|
|
RZB group |
41.3±5.1 |
43.4±5.4 |
44.5±3.9 |
46.0±3.0 |
45.1±3.7 |
46.0±2.9 |
46.1±3.9 |
|
|
|
|
Parainferior |
DEX group |
41.0±6.1 |
42.8±4.3 |
40.4±4.1 |
41.2±3.9 |
41.5±3.8 |
40.7±0.2 |
41.1±0.5 |
0.585 |
0.028 |
0.023 |
|
|
RZB group |
40.8±5.0 |
43.1±5.0 |
45.2±2.9 |
46.5±2.7 |
46.9±3.0 |
47.2±3.1 |
46.7±3.1 |
|
|
|
|
DCPD (µm) |
|
|
|
|
|
|
|
|
|
|
|
|
Whole |
DEX group |
45.4±5.1 |
48.3±3.8 |
47.2±4.4 |
43.0±6.2 |
46.5±5.7 |
46.5±3.1 |
46.4±2.7 |
0.482 |
0.788 |
0.344 |
|
|
RZB group |
45.9±5.1 |
47.5±5.1 |
47.7±4.9 |
50.0±4.5 |
49.1±3.5 |
49.3±2.9 |
49.0±3.2 |
|
|
|
|
Fovea |
DEX group |
19.8±7.4 |
26.1±8.0 |
24.8±6.0 |
27.1±6.1 |
27.5±8.2 |
27.6±8.0 |
27.7±7.4 |
0.001d |
0.821 |
0.199 |
|
|
RZB group |
20.7±7.7 |
26.2±8.0 |
24.4±7.2 |
28.8±7.0 |
27.8±10.4 |
27.2±6.4 |
27.4±7.0 |
|
|
|
|
Parafovea |
DEX group |
48.3±3.9 |
50.8±4.0 |
48.4±4.0 |
47.4±5.1 |
48.4±6.0 |
47.8±3.9 |
48.1±3.8 |
0.622 |
0.654 |
0.199 |
|
|
RZB group |
47.7±4.9 |
49.1±6.4 |
49.4±4.8 |
51.3±4.5 |
50.2±3.1 |
50.3±4.0 |
50.7±4.0 |
|
|
|
|
Parasuperior |
DEX group |
46.5±6.0 |
50.8±4.6 |
49.4±4.0 |
47.3±6.3 |
50.1±4.7 |
47.8±3.1 |
47.4±2.2 |
0.301 |
0.901 |
0.284 |
|
|
RZB group |
48.8±4.9 |
50.4±6.8 |
49.7±6.0 |
51.0±4.4 |
51.4±4.4 |
50.8±4.0 |
49.9±4.3 |
|
|
|
|
Parainferior |
DEX group |
47.5±5.0 |
50.3±4.9 |
47.7±5.0 |
45.4±5.4 |
47.1±7.2 |
47.7±4.4 |
47.3±5.0 |
0.411 |
0.561 |
0.310 |
|
|
RZB group |
47.7±3.9 |
48.7±5.0 |
49.1±4.4 |
51.3±4.2 |
50.1±3.8 |
51.2±3.0 |
51.2±4.0 |
|
|
|
|
CCD (µm) |
|
|
|
|
|
|
|
|
|
|
|
|
Whole |
DEX group |
63.0±1.8 |
65.0±1.8 |
65.5±1.5 |
64.4±1.8 |
60.8±6.8 |
57.7±8.4 |
58.0±9.0 |
0.511 |
0.399 |
0.301 |
|
|
RZB group |
61.3±7.0 |
64.5±2.2 |
64.0±2.0 |
65.3±2.2 |
65.5±1.7 |
65.5±1.4 |
65.5±1.3 |
|
|
|
|
Fovea |
DEX group |
60.8±5.7 |
65.9±2.8 |
65.4±5.4 |
64.5±1.8 |
59.3±8.0 |
54.8±6.0 |
55.4±7.1 |
0.522 |
0.822 |
0.598 |
|
|
RZB group |
61.4±6.2 |
63.7±2.5 |
62.7±5.0 |
64.7±4.0 |
63.3±3.4 |
64.9±1.9 |
65.2±2.8 |
|
|
|
|
Parafovea |
DEX group |
63.0±3.3 |
64.8±1.9 |
65.1±1.8 |
64.4±1.4 |
58.7±7.1 |
57.8±12.7 |
57.3±11.0 |
0.488 |
0.374 |
0.362 |
|
|
RZB group |
61.5±6.0 |
63.4±3.1 |
63.9±2.0 |
65.2±2.7 |
65.8±1.9 |
66.8±1.5 |
66.9±1.7 |
|
|
|
|
Parasuperior |
DEX group |
62.0±2.1 |
64.7±1.9 |
65.9±1.4 |
63.8±1.9 |
61.9±6.4 |
56.7±12.4 |
56.8±9.7 |
0.188 |
0.878 |
0.154 |
|
|
RZB group |
59.9±8.8 |
61.8±2.2 |
63.8±2.7 |
65.1±3.8 |
65.3±2.7 |
64.5±4.3 |
64.7±2.3 |
|
|
|
|
Parainferior |
DEX group |
62.0±3.3 |
65.0±1.7 |
65.4±1.9 |
65.0±2.0 |
58.9±7.7 |
57.2±10.0 |
58.0±8.9 |
0.502 |
0.368 |
0.448 |
|
|
RZB group |
61.5±6.3 |
63.2±2.5 |
63.6±2.4 |
65.5±2.9 |
65.6±1.8 |
65.9±1.6 |
66.2±1.7 |
|
|
|
Data are expressed as mean and
standard deviation. CMT: Central macular thickness; SCPD: Superior capillary
plexus density; DCPD: Deep capillary plexus density; CCD: Choriocapillaris
density. aP-value relative to effect of period;
bP-value relative to effect of type of
therapy; cP-value relative to interaction term
(time×therapy). dSignificant after FDR correction.
A statistically significant increase
in foveal DCPD was detected in both groups at 30d after therapy (Table 3; P<0.001).
At 6-month follow-up both groups showed a significant rise in foveal DCPD from
the baseline (from 19.8±7.4 μm to 27.7±7.4 μm in DEX group; from 20.7±7.7 μm to
27.4±7.0 μm in RZB group; Figures 2, 3).
Overall, no statistically
significant difference in CCD was detected during the follow-up and between the
two types of treatment (Table 3; Figures 2, 3).
The percentage of patients requiring
retreatment during follow-up was different between the two groups at different
follow-up controls. The 120- and 180-day re-treatment rates were 0.70±0.10 and
0.65±0.10 respectively, for patients in the RBZ group and 0.65±0.10 and
0.50±0.11, respectively, for patients in DEX group (Figure 4).
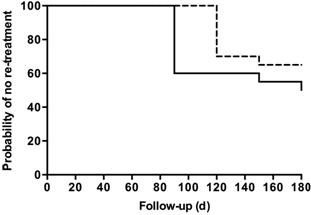
Figure 4 Kaplan-Meier curve of
re-treatment according to group Continuous line is relative to
patients in the RBZ group while dotter line is relative to patients in DEX
group.
DISCUSSION
Management of DR and its most common
complications, such as DME, have improved with the development of different
intravitreal drugs[4-8].
Intravitreal treatment of anti-VEGF,
specifically targeting the VEGF and corticosteroids with their action of
blockage the inflammatory mediators’ production, are largely widespread in the
treatment of the DME condition[25].
Ranibizumab, a monoclonal anti-VEGF
antibody fragment, is a safe treatment for DME with the early effects
detectable as early as 7d after the first injection[9-11,26].
Similarly, dexamethasone, an
anti-inflammatory agent approved by Food and Drug Administration in 2014 for
intravitreal treatment, represents an efficacy DME treatment[27].
In our retrospective 6-month
follow-up study no significant difference in terms of morphological and
functional parameters was found between the two groups that underwent DEX and
RBZ for DME treatment.
CMT showed a significant decrease in
both groups compared to preoperative values. Patients treated with DEX showed a
tendency to a higher decrease in comparison with RZB in the short-term period.
In DEX group, the greatest reduction of foveal CMT was observed at 2mo.
Conversely, in RZB group the greatest reduction of foveal CMT was detected at
120d. The effect peak of the dexamethasone implant has been already previously
reported to be at 30d with a mean duration of the treatment being at 4mo[28]. Several studies have already demonstrated DEX
efficacy in DME improvement[6,29].
It has been described 34% of CMT reduction at 30d after DEX implantation[16]. Similarly, in literature, the ranibizumab efficacy
in CMT decrease of DME patients has been reported[30].
Callanan et al have compared dexamethasone with ranibizumab for the treatment
of DME and demonstrated that the mean decrease in CMT from baseline was greater
with the corticosteroids than the anti-VEGF at 1 and 2mo after injections[30]. In our study the re-treatment rate in patients
treated with anti-VEGF injections was higher than those treated with DEX
implantation. A three-year randomized sham-controlled trial reported a mean of 4-5
injections over 3y in DME patients treated with DEX[6].
Similarly to our findings, the Bevordex study[31]
reported a comparison between anatomic and functional outcomes using DEX and
bevacizumab during over 12-month follow-up. Anatomic findings were
significantly better in patients treated with DEX with fewer injections (mean
of 2.7 injections) compared to patients treated with the anti-VEGF (8.6
injections).
However, the final CMT was
300.1±26.7 µm in DEX group and 250.2±28.7 µm in the RZB group at 180d in our
series thus probably leading to earlier retreatment in the DEX group.
Using OCTA analysis, we also
investigated retinal superficial and deep vessel densities and CC density in
both treatment groups. Nowadays, in clinical practice routinely use of OCTA
allows for a better and precise evaluation of the microvascular retinal changes
in DR and DME patients[16,32].
Some studies have already reported retinal capillary network and CC
modifications in DR patients, such as a decrease of vessel density and a
significant decrease of capillary perfusion density values as retinopathy
progresses[32-33]. It has been
described that the reduction of vessel density was more evident in the DCP
compared to the superficial plexus[32-33].
In our study, deep vessel density
increased significantly after both RZB and DEX injections; on the contrary, we
did not find significant modifications of foveal and parafoveal retinal
superficial vascular density after the two treatments.
In several studies it has been
observed that in DME patients DCP is severely damaged showing reduced density,
ectatic vessels, no flow areas corresponding to cysts[32,34-35]. In particular, sites of
macular edema are mainly localized in the deep plexus in regions of reduced or
absent flow.
It has been speculated that DCP
could be a potential predictor of the effectiveness of the DME treatment. Lee et
al[32] found a significant correlation
between the status of DCP and the therapy response. We hypothesize that the
modification of vessel density after treatment could be related to two factors:
disappearance of macular edema and steroid and anti-VEGF effect on vessel
diameter.
The modification of vessel density
in DR complicated by DME could be in part related to vessel displacement by
intraretinal fluid particularly when retinal cysts are present, thus edema
reduction or resolution could modify the vessel distribution.
In addition, vessel caliber could
change due to the drug effect thus influencing vessel density assessment. The
blockage of VEGF due to intravitreal steroid such as dexamethasone or anti-VEGF
injections can lead to a reduction of arteriolar or venular vessel diameter
with a resolution or improvement of macular edema[36-37].
The CCD after treatment did not show
any significant increase in both groups.
Capillary perfusion density has been
found reduced in patients suffering from DR with greater reduction at
increasing disease severity[33]. As previously
reported in retinal vein occlusion complicated by macular edema it can be
hypothesized that overlying retinal edema could attenuate the OCT signal of the
CC[38]. A role of anti-VEGF and dexamethasone in
influencing directly CCD could be considered and investigated.
Regarding functional parameters,
overall retinal sensitivity detected with microperimetry increased
significantly after therapy. It is probably related to a rearrangement of
foveal architecture and to the status of photoreceptors, also after the
improvement of DME[39].
On the contrary, the BCVA showed a
statistically significant increase in both groups of treatment, with the
highest gain at 60d post implant in the patients treated with DEX.
This study has some limitations such
as the relatively small sample of eyes examined presenting only no
proliferative moderate DR stage, the short follow-up and the retrospective
nature.
In conclusion, RBZ and DEX appeared
both safe and effective therapies for DME. The corticosteroid medication showed
an earlier short-term effect with a lower retreatment rate compared to
ranibizumab. Nevertheless, the two different intravitreal treatments both
allowed a fast improvement of the pathology in terms of anatomical and
functional outcomes.
ACKNOWLEDGEMENTS
Conflicts of Interest: Mastropasqua L, None; Di
Staso S, None; D’Aloisio R, None; Mastropasqua A, None; Di Antonio L, None; Senatore
A, None; Ciancaglini M, None; Di Nicola M, None; Di Martino G, None; Tognetto D, None; Toto L, None.
REFERENCES