·Brief
Report·
Management
of cataract in keratoconus: early visual outcomes of different treatment
modalities
Nicolas
Arej1,2, Wassef Chanbour2,3, Karen Zaarour1,2,
Mazen Amro2,3, Hala El-Rami2, Fadi Harb2,
Elias Jarade1,2,3,4
1Department
of Ophthalmology, Faculty of Medicine, Saint-Joseph University of Beirut, Beirut 11-5208, Lebanon
2Beirut Eye & ENT Specialist
Hospital, Beirut
116-5311, Lebanon
3Department
of Ophthalmology, Faculty of Medicine, Lebanese
University, Beirut
14-6573, Lebanon
4Mediclinic
Dubai Mall, Dubai 282890, United Arab Emirates
Correspondence to: Elias Jarade. Beirut
Eye & ENT Specialist
Hospital, Al-Mathaf square, Beirut 116-5311, Lebanon. ejarade@yahoo.com
Received: 2018-10-17
Accepted: 2019-04-08
Abstract
A review of 31 eyes with keratoconus who developed
cataract and underwent phacoemulsification. Visual acuities were measured 1mo
postoperatively. Six eyes with a history of good corrected distance visual
acuity (CDVA) and a similar refractive and topographic astigmatic axis were
implanted with toric intraocular lenses (IOLs). The mean postoperative
uncorrected distance visual acuity (UDVA) was 0.2 logMAR with a spherical
equivalent (SE): 0.75D. Eleven eyes with a history of good CDVA and different
refractive and topographic axis were implanted with monofocal IOL+/-Toric
implantable collamer lenses to treat anisometropia and ametropia; mean UDVA was
0.25 logMAR with a mean SE: -0.51 D postoperatively. Six eyes with poor CDVA
were first treated with intra-corneal ring segments, followed by
phacoemulsification, the mean postoperative UDVA was 0.82 logMAR with an SE:
0.22 D. Eight eyes had advanced ectesia and received combined
phacoemulsification and penetrating keratoplasty. Our approach is efficient in
addressing ametropia after cataract surgery in keratoconic eyes.
KEYWORDS: keratoconus; cataract surgery; residual ametropia;
algorithm
DOI:10.18240/ijo.2019.10.21
Citation:
Arej N, Chanbour W, Zaarour K, Amro M, El-Rami H, Harb F,
Jarade E. Management of cataract in keratoconus: early visual outcomes of
different treatment modalities. Int J Ophthalmol 2019;12(10):1654-1658
INTRODUCTION
Keratoconus is a bilateral asymmetric noninflammatory
disorder characterized by progressive thinning and cone-shaped protrusion of
the cornea leading to a decreased visual acuity and irregular astigmatism[1]. Advances have been made in stopping
progression of the disease with the advent of corneal crosslinking[2]. Visual impairment still needs to be managed with
spectacles or rigid gas-permeable (RGP) contact lenses in the early stages of
the disease or may require surgical correction such as with intrastromal
corneal ring segments (ICRSs)[3], phakic toric
implantable collamer lenses (TICL)[4-5],
corneal transplants[6], sometimes combined with a
cataract surgery. The latter becomes of major interest when patients with
keratoconus present with a cataract which contributes to a further visual
decline. Furthermore, it has been shown that keratoconic eyes are more likely
to develop cataracts and at a younger age than the general cataract population[7]. Not to mention the increase of life expectancy
worldwide[8], which by itself increases the
incidence of cataracts in the keratoconus population.
Patients with both cataract and keratoconus present a
unique challenge for ophthalmologists who will need to tailor a particular
treatment for each and every case in a customized approach that usually
encompasses different steps, among which cataract extraction might not always
occur in the first place. In all cases, particular attention should be accorded
to the intraocular lens (IOL) calculation since accurate or reproducible
biometric measurements are hard to attain in keratoconus. First, the relation
between the radius of curvature of the anterior and posterior corneal surfaces
has changed. Second, the corneal apex may be decentered with an anterior
bulging which may lead to a variability in axial length measurements[9] and third, because of the corneal optical multifocality
different measurements of the optical parameters may be obtained in the same
eye[10].
In this study, we retrospectively report the results
of our case series of cataract surgeries in keratoconus and, in the absence of
a standardized protocol for the management of cataract in keratoconic eyes, we
suggest in what follows a treatment algorithm that would help patients achieve
the best visual outcome.
SUBJECTS AND METHODS
Ethical Approval
This is a retrospective
review of patients with keratoconus who underwent cataract surgery at Beirut Eye & ENT
Specialist Hospital
(Beirut, Lebanon) between January 2010 and
October 2017. All patients were older than 18 years of age and had a stable
keratoconus with a cataract in one or both eyes that required surgery at the
time of enrolment. Institutional Review Board (IRB)/Ethics Committee ruled that
approval was not required for this study.
The severity of keratoconus was classified according
to the Amsler-Krumeich grading system[11].
Pentacam Scheimpflug imaging was performed using the WaveLight®
Allegro Oculyzer™ (WaveLight, GmbH, Erlangen,
Germany).
Topographic patterns and K-readings were consequently obtained. Therapeutic
decisions were then made following the algorithm (Figure 1).
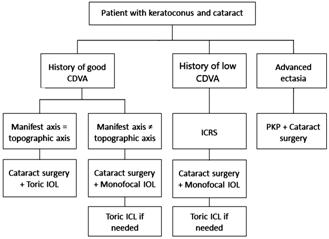
Figure 1 Dr. Jarade’s algorithm for the management of
keratoconic eyes with cataract CDVA: Corrected distance visual acuity; ICRS:
Intrastromal corneal ring segments; PKP: Penetrating keratoplasty; IOL:
Intraocular lens; ICL: Implantable collamer lens.
Eyes with advanced corneal ectasia and/or scarring
were at once scheduled for a combined cataract surgery with a penetrating
keratoplasty (PKP). The remaining cases were addressed in terms of the
corrected distance visual acuity (CDVA) that preceded the installation of
cataract. In eyes with a history of good CDVA, cataract surgery was performed
with IOL implantation. If the astigmatism axis of the manifest refraction
corresponds to that of the corneal topography, a toric IOL was implanted in
order to give the best possible uncorrected distance visual acuity (UDVA)
postoperatively. Whereas, in cases of discrepancy between the astigmatism axes,
a regular monofocal aspheric IOL was initially implanted, followed by the
implantation of a TICL to correct residual ametropia (as indicated by the
patient’s need for emmetropia) or anisometropia. In eyes with a history of low
CDVA with a minimum corneal thickness greater than 400 mm at the optical zone, a single ICRS was
initially inserted according to our protocol[12],
in order to regularize the corneal surface, followed by cataract surgery with
the implantation of a monofocal aspheric IOL and then possibly a TICL as a
separate additional procedure.
IOL calculations were performed by averaging results
from three formulas using standard and corneal topography-derived
keratometries, with the desired refraction aiming for emmetropia. Average
K-reading of central 2.3 optical zone was used for IOL calculation after ICRS
implantation[13]. In patients with post-LASIK
ectasia, K-reading were derived using Jarade’s method for deriving the new
index of refaction[14].
All cataract surgeries were performed by a single
surgeon (Jarade E) using clear-cornea phacoemulsification (or open-sky when
combined with PKP) with implantation of either a monofocal or toric IOL from
Rayner (Rayner Intraocular Lenses Ltd., Worthing, UK) or Alcon AcrySoft®
IQ and SA 60AT (Alcon laboratories, Fort Worth, TX, USA), in the posterior
capsular bag. Patients with severe keratoconus benefited from an RGP contact
lens-assisted cataract surgery in order to enhance optical performance and
decrease optical distortion due to corneal irregularities, which may impede on
the depth of perception and accurate focusing, and could subsequently lead to
an increased risk of posterior capsular rupture[15].
The RGP contact lenses (diameter =11 or 12 mm,
base curve =7.3) were manually customized by trimming their edges to meet the
surgeon’s preferred site of incision. At the end of the cataract procedure, the
incision site was secured with a single 10-0 nylon suture.
UDVA and CDVA were measured with a logMAR scale at
baseline and at 1mo following every therapeutic procedure. Statistical
calculations were performed using MS Excel 2016.
RESULTS
Thirty-one eyes of 23 patients were treated according
to the algorithm in Figure 1. Their characteristics and their treatment
outcomes are displayed in Table 1 and detailed in the following section.
Category 1: Eyes with a History of Good CDVA and a
Manifest Astigmatism Axis Matching with Topography Six eyes had a history of good visual acuity and a
manifest astigmatism axis that matched the topography and, therefore, could
benefit from a cataract surgery with the implantation of a toric IOL. Most of
them (83.33%) had a stage 2 keratoconus; their mean UDVA was 0.5 logMAR (20/60)
at baseline and improved to 0.2 logMAR (20/30) postoperatively. A similar
improvement was noted in terms of CDVA. Spherical equivalent (SE) improved from
-3.35 to 0.75 D. Statistical significance could not be assessed due to the
small number of eyes.
Category 2: Eyes with a History of Good CDVA and a
Manifest Astigmatism Axis not Corresponding to Topography Eleven eyes had a history of good visual acuity but their
manifest astigmatism axis did not match the topography. The majority had mild
to moderate keratoconus (stages 1 and 2) and showed an asymmetric bow-tie
pattern (45.45%). Mean UDVA, CDVA and SE were 1.1 logMAR (20/250), 0.18 logMAR
(20/30) and -3.85 D respectively at baseline; 0.25 logMAR (20/35), 0.12 logMAR
(20/25) and -0.51 D postoperatively. Two out of the 11 eyes had an additional
implantation of a TICL in order to correct the remaining astigmatism and they
achieved good UDVA of 0.1 logMAR (20/25) and 0.3 logMAR (20/40) with a residual
cylinder of 0.5 and 0.75 D respectively.
Category 3: Eyes with a History of Low CDVA Six eyes were included in this category, out of which
83.33% had a pellucid-like pattern on the corneal topography; they had
relatively advanced keratoconus (stages 3 and 4). Mean UDVA, CDVA and SE were
1.75 logMAR (20/1100), 0.37 logMAR (20/45) and -6.72 D respectively at
baseline; 0.82 logMAR (20/130), 0.34 logMAR (20/45) and 0.22 D postoperatively.
Noteworthy, one eye which was implanted with a toric IOL after ICRS insertion
achieved a final UDVA of 0 logMAR (20/20) from a baseline UDVA of 0.9 logMAR
(20/160) and a SE of -3.5 D.
Category 4: Eyes with an Advanced Corneal
Ectasia Eight eyes had stage 4 keratoconus with advanced
ectasia and scarring in the pupillary axis and were addressed for combined PKP
and cataract surgery. Mean UDVA, CDVA and SE were 1.34 logMAR (20/440), 0.94
logMAR (20/175) and -15.66 D respectively at baseline; 0.57 logMAR (20/75),
0.18 logMAR (20/30) and 0.85 D postoperatively. In one case, significant
residual ametropia persisted with a UDVA of 2 logMAR (20/2000). A TICL was then
secondarily implanted, UDVA improved to 0.9 logMAR (20/160), CDVA reached 0.2
logMAR (20/30) with an SE of 0 D.
DISCUSSION
Keratoconus is characterized by progressive
steepening of the cornea that leads to a highly irregular astigmatism. While
age hold down the progression of keratoconus, the natural onset of cataract
contributes to further visual impairment in this population. When patients
develop decreased vision, a careful evaluation should be performed to determine
whether the cause is the result of corneal changes, cataract formation, or
other pathology. For the patients with cataract, different options have been
proposed and are largely dependent on the stage of keratoconus and the history
of the patient[16].
Phacoemulsification with the implantation of a toric
IOL has been shown to be a safe and effective procedure in eyes with
topographically stable, fairly regular corneal astigmatism[17-18]. Eyes who benefit the most from this treatment are
those who have mild keratoconus, with an astigmatism that is stable and having
an invariable axis on both manifest refraction and topography. This option can
also be considered in cases of intolerance to RGP[19].
Conversely, toric IOL implantation is not recommended for eyes with markedly
irregular astigmatism or in which RGP are intended to be used postoperatively.
In this study, we included all the cataract surgeries
performed on patients with keratoconus, eyes were categorized into four groups
according to the CDVA, stage of keratoconus, and the similarity between the
refractive and the corneal astigmatism. All the patients were treated according
to Dr. Jarade algorithm (Figure 1) and had favorable outcomes in all the
categories. In category 1, despite the small number of eyes, favorable visual acuity
outcomes were obtained and this was primarily attributable to a careful patient
selection.
Eyes that were unfit for a toric IOL were included in
the category 2 of our series and have also achieved good visual outcomes,
though farther from emmetropia compared to those of the category 1.
Interestingly, final uncorrected visual acuity could be optimized in 2 eyes
that were initially considered as borderline candidates for toric correction,
and this through the implantation of a TICL. Posterior chamber TICLs have been
previously shown to be efficient in the visual correction of phakic keratoconic
eyes[20-21]. However, to our
knowledge, they haven’t been used so far for the purpose of correcting residual
ametropia after cataract surgery in keratoconus.
Alfonso et al[22]
demonstrated that sequential ICRS and IOL implantation provided good visual and
refractive outcomes and was an effective, safe, predictable, and stable
procedure for the treatment of patients with keratoconus and cataract. This
approach was adopted for the eyes in the category 3 of our series, which had a
history of low CDVA (non-RGP correction). Correction of corneal irregularities
prior to cataract surgery is of a particular interest, in terms of enhancing
the intraoperative visibility and improving the predictability of the final
visual outcome after phacoemulsification and IOL implantation. This has also
allowed us to implant a toric IOL in an eye that was found to have a less
irregular astigmatism after ICRS insertion and had an axis that coincide with
the manifest refraction of the patient.
Simultaneous PKP with cataract extraction (either by
open-sky extracapsular extraction or phacoemulsification) and IOL implantation,
aka the “TRIPLE” procedure, is the method of choice for combined lens and
corneal opacities[23]. It was applied to all eyes
in category 4 of our series and achieved satisfactory results. Closed-system
phacoemulsification was made possible prior to PKP due to the use of customized
RGP contact lenses which allowed to overcome intraocular image distortions, and
hence, to avoid potential complications such as posterior capsular rupture and
corneal endothelial cell damage.
This was a series of cases of coexisting cataract and
keratoconus that we managed according to the algorithm in Figure 1. Further
studies with a larger number of eyes and longer follow-ups are still needed to
better establish the usefulness of the suggested strategies. The importance of
patient selection has to be highlighted, since the final visual outcome is highly
depended on the preoperative characteristics of each eye; i.e., eyes
with mild keratoconus and relatively regular astigmatism could benefit from a
simple cataract surgery with toric IOL implantation, while eyes with more
advanced disease might require a multi-staged approach in an attempt to better
restore their UDVA. Yet, residual refractive errors can occur, but could often
be anticipated by a careful IOL calculation, or alternatively addressed by a
postoperative correction with spectacles, RGP or even the implantation of TICLs
that could be selectively considered as an option.
In the light of our results, as well as those
published in previous studies and individual case reports, we believe that our
algorithm will be a simple and useful tool for practitioners who encounter
cataract in eyes with keratoconus.
ACKNOWLEDGEMENTS
Conflicts of Interest: Arej N, None; Chanbour W, None; Zaarour
K, None; Amro M, None; El-Rami H, None; Harb F, None; Jarade
E, None.
REFERENCES
|
1
Mas Tur V, MacGregor C, Jayaswal R, O'Brart D, Maycock N. A review of
keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol
2017;62(6):770-783.
https://doi.org/10.1016/j.survophthal.2017.06.009
PMid:28688894
|
|
|
|
2
Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen
crosslinking for the treatment of keratoconus. Am J Ophthalmol
2003;135(5):620-627.
https://doi.org/10.1016/S0002-9394(02)02220-1
|
|
|
|
|
3
Miraftab M, Hashemi H, Hafezi F, Asgari S. Mid-term results of a single
intrastromal corneal ring segment for mild to moderate progressive
keratoconus. Cornea 2017;36(5):530-534.
https://doi.org/10.1097/ICO.0000000000001115
PMid:27984365
|
|
|
|
|
4
Antonios R, Dirani A, Fadlallah A, Chelala E, Hamade A, Cherfane C, Jarade E.
Safety and visual outcome of visian toric ICL implantation after corneal
collagen cross-linking in keratoconus: up to 2 years of follow-up. J
Ophthalmol 2015;2015:514834.
https://doi.org/10.1155/2015/514834
PMid:25874116 PMCid:PMC4383407
|
|
|
|
|
5
Jarade E, Dirani A, Fadlallah A, Khoueir Z, Antoun J, Cherfan G. Visian toric
ICL implantation for residual refractive errors after ICRS implantation and
corneal collagen cross-linking in keratoconus. J Refract Surg 2013;29(7):444.
https://doi.org/10.3928/1081597X-20130617-01
PMid:23820225
|
|
|
|
|
6
Khattak A, Nakhli FR, Al-Arfaj KM, Cheema AA. Comparison of outcomes and
complications of deep anterior lamellar keratoplasty and penetrating
keratoplasty performed in a large group of patients with keratoconus. Int
Ophthalmol 2018;38(3):985-992.
https://doi.org/10.1007/s10792-017-0548-9
PMid:28534231
|
|
|
|
|
7
Thebpatiphat N, Hammersmith KM, Rapuano CJ, Ayres BD, Cohen EJ. Cataract
surgery in keratoconus. Eye Contact Lens: Sci Clin Pract 2007;33(5):244-246.
https://doi.org/10.1097/ICL.0b013e318030c96d
PMid:17873627
|
|
|
|
|
8
Robine JM, Cubaynes S. Worldwide demography of centenarians. Mech Ageing Dev
2017;165(Pt B):59-67.
https://doi.org/10.1016/j.mad.2017.03.004
PMid:28315698
|
|
|
|
|
9
Bozorg S, Pineda R. Cataract and keratoconus: minimizing complications in
intraocular lens calculations. Semin Ophthalmol 2014;29(5-6):376-379.
https://doi.org/10.3109/08820538.2014.959193
PMid:25325863
|
|
|
|
|
10
Jarade EF, Abdelmassih Y, El-Khoury S. Reply. Am J Ophthalmol
2017;181:183-184.
https://doi.org/10.1016/j.ajo.2017.07.018
PMid:28784238
|
|
|
|
|
11
Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J
Cataract Refract Surg 1998;24(4):456-463.
https://doi.org/10.1016/S0886-3350(98)80284-8
|
|
|
|
|
12
Jarade EF, Slim E, Cherfan C, El Rami H, Hassan T, Chelala E. Mathematical
analysis of corneal remodelling after intracorneal ring surgery in
keratoconus. Int J Ophthalmol 2017;10(3):348-354.
https://doi.org/10.18240/ijo.2017.03.04
|
|
|
|
|
13
Jarade E, Dirani A, Fadlallah A, Chelala E, Fakhoury H, Cherfan G.
Intraocular lens power calculation after intracorneal ring segment surgery
for the treatment of post-LASIK ectasia. SM Opthalmol J 2015;1(1):1005.
|
|
|
|
|
14
Jarade EF, Abi Nader FC, Tabbara KF. Intraocular lens power calculation
following LASIK: determination of the new effective index of refraction. J
Refract Surg 2006;22(1):75-80.
https://doi.org/10.3928/1081-597X-20060101-15
|
|
|
|
|
15
Oie Y, Kamei M, Matsumura N, Fujimoto H, Soma T, Koh S, Tsujikawa M, Maeda N,
Nishida K. Rigid gas-permeable contact lens-assisted cataract surgery in
patients with severe keratoconus. J Cataract Refract Surg 2014;40(3):345-348.
https://doi.org/10.1016/j.jcrs.2014.01.001
PMid:24491385
|
|
|
|
|
16
Moshirfar M, Walker BD, Birdsong OC. Cataract surgery in eyes with
keratoconus: a review of the current literature. Curr Opin Ophthalmol
2018;29(1):75-80.
https://doi.org/10.1097/ICU.0000000000000440
PMid:28961565
|
|
|
|
|
17
Mol IE, Van Dooren BT. Toric intraocular lenses for correction of astigmatism
in keratoconus and after corneal surgery. Clin Ophthalmol 2016;10:1153-1159.
https://doi.org/10.2147/OPTH.S107305
PMid:27382249 PMCid:PMC4922777
|
|
|
|
|
18
Visser N, Gast ST, Bauer NJ, Nuijts RM. Cataract surgery with toric
intraocular lens implantation in keratoconus: a case report. Cornea
2011;30(6):720-723.
https://doi.org/10.1097/ICO.0b013e31820009d4
PMid:21562460
|
|
|
|
|
19
Kamiya K, Shimizu K, Miyake T. Changes in astigmatism and corneal
higher-order aberrations after phacoemulsification with toric intraocular
lens implantation for mild keratoconus with cataract. Jpn J Ophthalmol
2016;60(4):302-308.
https://doi.org/10.1007/s10384-016-0449-x
PMid:27165708
|
|
|
|
|
20
Esteve-Taboada JJ, Domínguez-Vicent A, Ferrer-Blasco T, Alfonso JF,
Montés-Micó R. Posterior chamber phakic intraocular lenses to improve visual
outcomes in keratoconus patients. J Cataract Refract Surg 2017;43(1):115-130.
https://doi.org/10.1016/j.jcrs.2016.05.010
PMid:28317664
|
|
|
|
|
21
Kummelil MK, Hemamalini MS, Bhagali R, Sargod K, Nagappa S, Shetty R, Shetty
BK. Toric implantable collamer lens for keratoconus. Indian J Ophthalmol
2013;61(8):456-460.
https://doi.org/10.4103/0301-4738.116064
PMid:23925337 PMCid:PMC3775087
|
|
|
|
|
22
Alfonso JF, Lisa C, FernándezVega Cueto L, PooLópez A, MadridCosta D,
Fernández-Vega L. Sequential intrastromal corneal ring segment and monofocal
intraocular lens implantation for keratoconus and cataract: long-term
follow-up. J Cataract Refract Surg 2017;43(2):246-254
https://doi.org/10.1016/j.jcrs.2016.11.044
PMid:28366374
|
|
|
|
|
23
Seitz B, Langenbucher A, Viestenz A, Dietrich T, Küchle M, Naumann GO.
Cataract and keratoplasty: simultaneous or sequential surgery? Klin Monbl
Augenheilkd 2003;220(5):326-329.
https://doi.org/10.1055/s-2003-39429
PMid:12766821
|
|
