
Citation: Wang SY, Li B, Li DH, Tian Y. Adenovirus-mediated
corneal endotheliitis: a case report. Int J Ophthalmol 2019;12(10):1659-1661
DOI:10.18240/ijo.2019.10.22
·Letter
to the Editor·
Adenovirus-mediated
corneal endotheliitis: a case report
Shuang-Yong Wang, Bei Li, Dong-Hao Li, Ying Tian
The Third Affiliated Hospital,
Guangzhou Medical University, Guangzhou 510150, Guangdong Province, China
Correspondence to: Ying Tian. The Third
Affiliated Hospital, Guangzhou Medical University, Guangzhou 510150, Guangdong
Province, China. tianyi7879@126.com;
ldh71@163.com
Received:
DOI:10.18240/ijo.2019.10.22
Citation:
Wang SY, Li B, Li DH, Tian Y. Adenovirus-mediated corneal endotheliitis: a case
report. Int J Ophthalmol
2019;12(10):1659-1661
Dear Editor,
Corneal endotheliitis is a common
and intriguing clinical entity characterized by corneal edema, keratic
precipitates, and mild to moderate anterior chamber reaction, which occupies
the important pathogenic factor of corneal blindness[1].
Robin et al[2] first described a patient
who suffered from intraocular inflammation and progressive corneal
endotheliitis associated with herpes simplex infection. Since then,
accumulating clinical evidence confirmed that its etiology was mainly
attributed to the family of herpesviridae, including herpes simplex virus, varicella
zoster virus, and cytomegalovirus, which initiated the direct cell damage and
immune- and inflammatory-mediated lesion on endothelial cells[1,3]. However, some other pathogenic microbes were involved
in corneal endotheliitis. We previously reported several cases of uncommon
fungal corneal endotheliitis[4]. Here, we
presented a case diagnosed as adenovirus-mediated endotheliitis. This study was
approved by the Ethical Committee of the Third Affiliated Hospital of Guangzhou
Medical University. The informed consent was obtained from the patient and his
guardian. The treatments of this study followed the Declaration of Helsinki.
Case Presentation A 14-year-old male complained eye
redness, watery discharge, and photophobia in both eyes 10d before, accompanied
by a blurred vision for 1d in the left. His medical history did not show any
systemic disease, ocular trauma, surgery, and infection in both eyes. The
clinical symptoms still progressed even if a levofloxacin eye drop was
administrated by the local medical clinic for 7d. The visual acuity was
counting fingers/
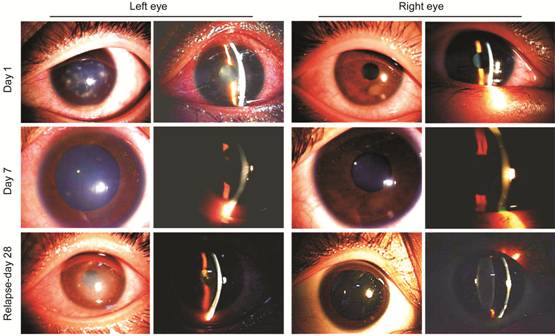
Figure 1 The slit-lamp examination
revealed conjunctival congestion, subepithelial infiltrates, stromal edema,
Descemet’s membrane fold, anterior chamber flare, and keratic precipitates in
the left eye on day 1. The endothelial layer looked blurred. In the right one,
a subepithelial infiltrate was found at 4 o’clock position. The signs and
subjective symptoms improved on day 7. On day 28, corneal endotheliitis
relapsed in the left eye, accompanied by serious iritis, characterized by
stromal edema, endothelial fold, anterior chamber flare, keratic precipitates,
fibrous membranous exudation, and partial posterior synechia of the iris.
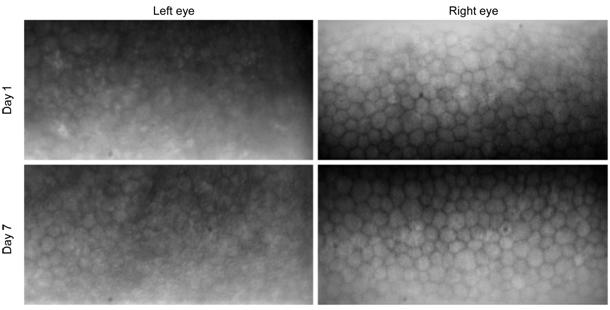
Figure 2 Specular microscope found
that the endothelial layer looked blurred and the outlines of endothelial cells
were obscure in the left eye on day 1. By day 7 after treatment, clear outline
of cells occurred.
Discussion
Human adenovirus is mainly
associated with epidemic keratoconjunctivitis, which characterized by eye
redness, pseudomembrane formation, subepithelial infiltrates, preauricular
lymphadenectasis, and affected people of all ages and regions[5].
There are few published reports on human adenovirus-mediated endotheliitis.
Pflugfelder and Roussel[6] had previously
presented a case of endothelial dysfunction associated with adenoviral epidemic
keratoconjunctivitis. Bilateral disciform keratitis or stromal edema were also
found in the patients who suffered from adenoviral conjunctivitis 3wk before[7-8]. For this case, the reasons for the
initial diagnosis of adenovirus-mediated endotheliitis were as follows: first,
the clinical signs showed initial epidemic keratoconjunctivitis and subsequent
corneal endotheliitis, characterized by eye redness, subepithelial infiltrates,
preauricular lymphadenectasis, stromal edema, Descemet’s membrane folds,
anterior chamber flare, and inflammatory keratic precipitates. Second, corneal
endothelial lesions were near or around the subepithelial infiltrates and their
onsets were consistent with the development of an adaptive immune response,
which suggested that they were associated with direct adenoviral damage and/or
adenovirus-mediated immune response. Third, a remarkable response to antiviral
agents and corticosteroid was a shred of further supportive evidence for the
diagnosis of adenovirus-mediated endotheliitis. Adenoviral etiology was found
in the aqueous humor by RT-PCR during the relapse period, which further
confirmed the initial presumed adenovirus-mediated endotheliitis.
The direct damage and immune
response induced by the pathogens are the basic pathological mechanism of
corneal endotheliitis. It appears that adenovirus is capable of damaging the
affected cells and activating an immune response, which can cause corneal
endotheliitis similar to that caused by herpesviridae or other viruses.
Adaptive immunity to adenovirus hexon mediates complement-mediated lysis of
adenovirus-infected cells and antibody-dependent cell-mediated cytotoxicity.
However, another puzzle that needs to be further clarified is whether any
variations happened to adenoviral etiology, which caused some change of its
biological characteristics, prompted it to invade into the endothelial layer.
Furthermore, it is worth notifying that it is possible to dig into any other
novel or variant pathogens involved in the corneal endotheliitis, not only
herpesviridae.
ACKNOWLEDGEMENTS
Authors’ contributions: Wang SY is the main clinician
managing the patient. Li B is a member of the ophthalmologic team and drafted
the manuscript. Tian Y is responsible for the pathogenic test. Li DH is the
lead consultant in the care of the patient. All authors read and approved the
final manuscript.
Foundation: Supported by the National Natural
Science Foundation of China (No.81870631).
Conflicts of Interest: Wang SY, None; Li B, None; Li DH, None; Tian Y,
None.
REFERENCES