
·Basic Research·
Rescue
of human corneal epithelial cells after alkaline insult using renalase derived
peptide, RP-220
Luke Potts1, Casie Phillips2, Munok
Hwang3, Samuel Fulcher4,5, Hosoon Choi2,5
1Department of Ophthalmology and
Surgery, Scott and White Eye Institute, Temple, Texas 76508, USA
2Central Texas Veterans Research
Foundation, Temple, Texas 76504, USA
3Department of Biomedical
Informatics, University of Texas Health Science Center at Houston, Houston,
Texas 77030, USA
4Department of Surgery, Central Texas
Veterans Health Care System, Temple, Texas 76054, USA
5Department of Medicine, College of
Medicine, Texas A&M Health Science Center, Bryan, TX 77807, USA.
Correspondence to: Hosoon Choi. 1901 S. 1st St. Room
3R25, Temple, TX 76504-7451, USA. Hosoon.Choi@va.gov
Received:
Abstract
AIM: To study the effect of renalase peptide, RP-220, on cell viability of
human corneal epithelial cells after alkali insult.
METHODS: A dose-response relationship between cell viability
and exposure to NaOH solution were characterized using cultured human corneal
epithelial cells. Viability of corneal epithelial cells was determined using
commercially available MTT and CyQUANT® assays.
RESULTS: At a concentration of 6 mmol/L, insult with NaOH
leads to reduced corneal epithelial cell viability by approximately 30%. This
reduced viability was prevented by treating the cells after initial insult with
the 20-amino acid renalase derived peptide (RP-220).
CONCLUSION: RP-220 has a pro-survival role for RP-220 following
alkaline insult to corneal epithelial cells.
KEYWORDS: corneal alkali injury; renalase;
RP-220; human corneal epithelial cells
DOI:10.18240/ijo.2019.11.01
Citation: Potts
L, Phillips C, Hwang M, Fulcher S, Choi H. Rescue of human corneal epithelial
cells after alkaline insult using renalase derived peptide, RP-220. Int
J Ophthalmol 2019;12(11):1667-1673
INTRODUCTION
Chemical exposure is a significant
cause for ophthalmic morbidity[1]. Injury can
occur by certain household or occupational agents in an accidental or
intentional manner[1]. Military personnel are also
at risk, and the use of chemical agents by terrorist groups presents a unique
challenge in the modern era[2-4].
Of the possible exposures, alkaline agents are known to be particularly
deleterious[1,5]. Notably,
epidemiologic studies conducted using data from US emergency room visits have
shown children from 1-2 years of age are at highest risk for ocular injury from
an alkaline agent, even higher than men ages 18-64, which is another population
at particularly high risk[1]. In contrast to
acidic agents, which cause protein denaturation leading to slowing of further
tissue penetration, alkaline agents cause tissue melting, further enhancing
tissue penetration[6]. Notably, the most
significant injury to the eye has been shown for substances with pH 11-11.5[6].
Corneal injury represents one of the
most significant sources for immediate and delayed ocular morbidity from
alkaline agent exposure. There is a wide variability in the literature on
prognosis after alkaline injury, also revealing a direct correlation between
the severity of initial exposure and prognosis[7-9]. Despite current treatment efforts, individuals
presenting with more severe injury typically have a protracted treatment course
that may or may not result in useful vision[7].
The downstream events triggered by the initial injury may be angiogenic,
fibrotic, or inflammatory in nature, each with potentially devastating
consequences for vision and quality of life[10-12].
Our aim in the current study was to
investigate the potential for corneal epithelial cell rescue after alkaline
insult, using the renalase peptide (RP-220)[13].
Renalase is an amine oxidase secreted from the kidney into the blood[14-15]. There are two prominent
isoforms of the flavoprotein oxidase renalase expressed in humans[16]. Despite early evidence suggesting a physiological
role in catecholamine metabolism, subsequent studies have refuted this claim[17-19]. Rather, renalase has been
found capable of acting as a signaling molecule activating mitogen-activated
protein kinase (MAPK) and extracellular signal-regulated kinase 1/2 (ERK1/2),
thereby exerting cytoprotective effects[13].
Plasma membrane calcium ATPase isoform 4b (PMCA4b, encoded by ATP2B4 gene), has
been identified as a renalase receptor upstream of MAPK activation[17,19].
RP-220, the 20-amino acid peptide
derived from full length renalase sequence, is equally as effective as
full-length recombinant renalase in its cytoprotective properties. RP-220,
specifically, has been demonstrated to rapidly activate ERK1/2 and p38 MAPK in
a human kidney epithelial cell line, suggesting that RP-220 is the critical
region of the renalase protein for interaction with PMCA4b receptors[17,19]. A study involving human
corneal epithelium showed that PMCA4 is the most prominent PMCA isoform and
that splice variant PMCA4b is present in the human cornea[20],
confirming the existence of an RP-220-specific receptor in the human cornea.
The ability of RP-220 to stimulate an increase in ERK1/2 and p38 MAPK activity
would likely protect cells against apoptosis[21]
and ultimately increase cell survival, therefore improving clinical outcomes.
Furthermore, activation of MAPK and ERK1/2 is expected to encourage epithelial
cell migration and proliferation[22], aiding in
the wound healing process.
In vivo, renalase peptide reduced ischemic
or cisplatin-induced kidney injury and in vitro, protected human
proximal tubular (HK-2) cells from H2O2 or
cisplatin-induced apoptosis[13]. The HK-2 cell
line has also demonstrated its ability to upregulate renalase expression upon
hypoxic insult, via hypoxia-inducible factor-1α (HIF-1α)[23]. Renalase expression has also been shown to be
controlled by HIF-1α in human cardiomyocytes and implicated in that context as
protective against cardiac ischemia[24]. In rats,
contrast-induced histological damage to the kidney, as well as apoptosis and
inflammation, were all reduced by treatment with intraperitoneal recombinant
renalase[25]. Renalase has also been shown to
reduce tubulointerstitial fibrosis in a rat model, and it has been suggested
that this is due to its ability to interfere with the TGF-β-induced
epithelial-mesenchymal transition (EMT)[26]. We
have previously shown a marked increase in TGF-β1 expression following alkaline
injury in the rat[27], and TGF-β expression is
closely linked to corneal wound healing[28].
Taken together, the effects of renalase in various injury models suggest that
it may be beneficial in the context of alkaline injury to the cornea.
MATERIALS AND METHODS
Cell Culture Normal primary human corneal
epithelial cells were purchased from American Type Culture Collection (ATCC,
Manassas VA). Cells were thawed and plated on
pH of NaOH Dilutions An Accumet AE150 pH meter (Thermo
Fisher Scientific, Waltham MA) was used to measure the pH of serial dilutions
of a 10 mol/L NaOH stock solution (Sigma Aldrich, St. Louis MO) with sterile
water (Life Technologies Corporation) or 0.9% NaCl solution (Saline solution;
Braun, Irvine CA, USA).
Alkaline Insult to Corneal
Epithelial Cells A stock 50 mmol/L solution of NaOH
was prepared from 10 mol/L NaOH stock (Sigma) solution by dilution with saline
solution (Braun), then filtered through a 0.22 µm filter (Merck Millipore, Cork
Ireland). To each well of a 96-well
plate (CellTreat Scientific) containing cultured cells (passage 3) and 100 µL
of corneal epithelial cell media (ATCC), 100 µL of the 50 mmol/L NaOH solution
were added and mixed gently using a multichannel pipette. Then, 100 µL were
withdrawn and placed in the next column of wells containing cells and media.
This process was continued across the plate such that there was treatment of
cells with NaOH concentrations of 25.0, 12.5, 6.2, 3.1, 1.6, 0.78, 0.39, and 0.20
mmol/L. Incubation of cells with each concentration of NaOH was for 1min, with
4 replicates of each and after insult, cells were rinsed twice with 100 µL each
of saline solution (Braun). Cells were then incubated with 100 µL of corneal
epithelial cell media (ATCC) for 24h at
Effects of RP-220 and Scrambled
Peptide on Corneal Epithelial Cells
The RP-220
and scrambled peptide (RP-Scr220) was custom-made according to Wang et al[17] by Selleck Chemicals (Houston TX) and dissolved in
dimethyl sulfoxide (DMSO; Sigma) to yield 10 mg/mL stock solutions. The 100
µg/mL stock solutions of RP-220 and RP-Scr220 were then prepared by diluting
the stock with corneal epithelial media (ATCC). These stock solutions were then
used to treat cultured corneal epithelial cells on a black-walled 96-well plate
(Falcon, Big Flats NY) with serial dilutions of RP-220 and RP-Scr220 at
concentrations of 50, 25, 12.5 and 6.25 µg/mL. Cells treated with corneal
epithelial media only served as control. All treatments were performed with 6
replicates. Cells were incubated with these peptides for 72h, then the plate
was stored at
Rescue of Corneal Epithelial Cells
after Alkaline Insult For rescue assays involving RP-220,
a 6 mmol/L solution of NaOH was prepared from a stock 10 mol/L NaOH (Sigma)
solution, filtered through 0.22 µm filter (Merck Millipore, Cork Ireland).
Cells on a 96-well plate (CellTreat Scientific Products) were treated with the
6 mmol/L NaOH solution for 1min to provide alkaline insult, followed by two
rinses with 100 µL each of saline solution (Braun). Cells were then treated
with 10 or 20 µg/mL RP-220 or RP-Scr220 solution, or with corneal epithelial
media (ATCC) alone. A set of cells treated only with saline solution (Braun)
rather than NaOH, served as control. The cells were incubated with these
solutions for 24h, then MTT assay was performed using the VybrantTM
MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific), measured using a
Varioskan LUX spectrophotometer (Thermo Fisher Scientific) with absorbance at
540 nm. Each treatment was with 4 replicates.
Statistical Analysis The data analyses were performed
with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data were
analyzed using a one-way ANOVA with post-hoc Tukey testing. Differences were
considered significant if P<0.05. Data are presented as mean±standard
error of the mean.
RESULTS
Human corneal epithelial cells were
treated with NaOH solution to assess the impact of alkaline insult. The pH of
the treatment solution was assessed prior to alkali insult. NaOH solution was diluted with either
sterile water or sterile saline solution in various concentrations, and their
pH were measured. As shown in Figure 1, the initial pH of saline and sterile
water was 4.64 and 9.12, respectfully. As the graph shows in Figure 1, though
there was difference in initial pH, no significant pH difference between NaOH
dilutions with either sterile water or sterile saline solution was
observed. Therefore, NaOH solution
was diluted with saline to minimize osmotic pressure. The concentration of NaOH
solution used for corneal epithelial cell insult in these experiments was 6
mmol/L, with pH of around 11.5, as 6 mmol/L was the minimum concentration of
NaOH that reach the maximum pH. There is no significant difference of pH
obtained with 6.2, 12.5 and 25 mmol/L. The pH of the treatment solution
was suitable to assess alkali injury as studies show, the most significant eye
injury was observed with pH range 11 to 11.5[6].
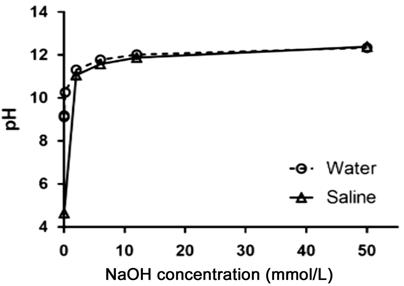
Figure 1 pH change of NaOH solutions
with either sterile water or sterile saline solution (0.9% NaCl) Dashed line shows pH of NaOH dilutions
with sterile water, and solid line shows pH of NaOH dilutions with sterile
saline.
To assess the alkali insult, human
corneal epithelial cells were treated with various NaOH concentrations (0.2
through 25 mmol/L) for 1min. Cell viability after NaOH insult was observed
using MTT assay at 24h after the initial NaOH exposure. As shown in Figure 2,
the average absorbance values at 540 nm after treatment was decreased in a
dose-dependent manner. While no
significant difference in MTT reading from cells treated with 0.2 to 3.1 mmol/L
NaOH was observed, compared to saline control, cell viability was reduced
significantly with 6.2, 12.5, and 25 mmol/L NaOH insult, approximately 85%,
84%, and 33% of saline control, respectively. This result indicates that NaOH
solution can serve as an alkali insult simulation and 6 mmol/L NaOH is
appropriate concentration to examine the effect of renalase peptide.
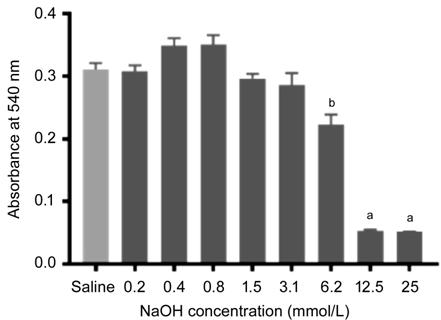
Figure 2 Human corneal epithelial
cell viability after NaOH insult Plot shows
the MTT assay results denoted with average absorbance values at 540 nm after
treatment with various NaOH concentrations. Error bars indicate standard error
of the mean of each group (n=4). aP<0.0001, bP<0.0005
on Tukey’s multiple comparisons test compared with saline control.
CyQUANT® assay was used
to compare the numbers of cells present after 72h incubation with varying
concentrations of either RP-220 or RP-Scr220. These data are presented in
Figure 3. Controls are incubated with corneal epithelial cell media only. Cell proliferation induced by RP-220 or
scrambled peptide was not observed.
The proliferation of cells was significantly reduced in the presence of
50 µg/mL scrambled peptide. Though it is not significant, the cell viability
also reduced on treating cells with 50 µg/mL RP-220. This indicates that higher
concentration of RP-220 or RP-Scr220 can bring adverse effect to cells.
Therefore, based on the CyQUANT® assay, the peptide concentration
for further experiments was limited to 10 µg/mL and 20 µg/mL.
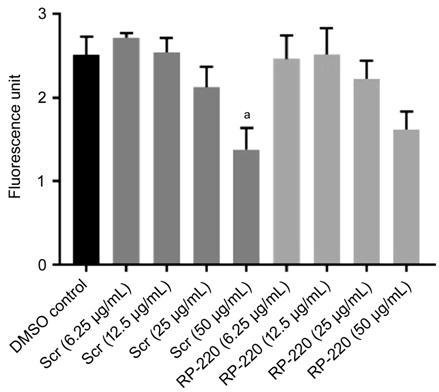
Figure 3 Human corneal epithelial
cell proliferation after treatment with various concentrations of RP-220 or
RP-Scr220 Plot shows CyQuant® assay
results denoted with the average fluorescence values after treatment with
peptide or control, corneal epithelial media. Error bars indicate standard
error of the mean of each group (n=6). aP<0.0005 on
Tukey’s multiple comparisons test compared with control.
To examine whether the renalse
peptide rescue the corneal epithelial cells after the alkali insult as
simulation of eye injury, the cell viability was observed using MTT assay.
Cells were treated with either scrambled or RP-220 following the NaOH insult
for 1min, and cell viability was measured after 24h of treatment. Control cells
treated with saline solution. As shown in Figure 4, NaOH insult reduced cell
viability and following treatment with 10 µg/mL RP-220 significantly rescued
cell viability compared to NaOH insult only cells or scrambled peptide treated
cells. The scrambled peptides showed no effects on the cell viability after the
insult. Higher concentration RP-220
(20 µg/mL) also rescued the cells but it was not significant. This assay
indicates that 10 µg/mL RP-220 is suitable to rescue the cells against alkaline
insult.
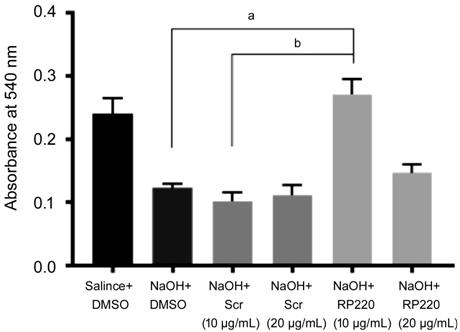
Figure 4 Human corneal epithelial
cell viability after insult with 6 mmol/L NaOH, or 6 mmol/L NaOH followed by
RP-220 or scrambled peptide for rescue Plot shows the MTT assay results denoted
with average absorbance values at 540 nm after treatment. Error bars indicate
standard error of the mean of each group (n=4). aP<0.0001,
bP<0.0005 on Tukey’s multiple comparisons test compared
between groups.
DISCUSSION
In this study, we have demonstrated
a pro-survival role for the renalase derived peptide (RP-220) following insult
of cultured human corneal epithelial cells with NaOH solution. The alkaline
nature of the insult was demonstrated by testing pH of various dilutions of
NaOH with sterile water or saline solution, and a reduction in corneal
epithelial cell viability shown. We have previously characterized an alkaline
injury model in the rat using 1 mol/L NaOH which demonstrated that corneal
epithelial injury is a key early pathologic event after alkaline injury and
finding ways to alleviate this insult is thus important for limiting the
overall damage that results with time after alkaline injury[27].
In the studies outlined herein, we
used commercially available human corneal epithelial cells at passage 3. The
cells were grown to 70% confluence prior to use, which is slightly lower than that
used in some other studies of corneal epithelial cell cultures[29-30]. It has been shown that corneal
epithelial cells express more proliferation-type genes at this lower confluence
compared to higher confluence[31]. This is
relevant in the context of studying alkali injury since cells are in a more
proliferative phase during wound healing than they may be at other times. Of
note, at later passages we did observe significant phenotypic changes in the
cells, taking on a more fibroblast-like appearance. Hence, care was taken to examine culture
plates prior to use for the experiments in this study to ensure that the
predominant morphology of the cultured cells was epithelial. In future studies, it will be important
to further characterize the genotypic expression profile of these cells at
different passages and confluences.
Using the commercially available MTT
assay, we identified concentrations of NaOH that resulted in reduced numbers of
viable corneal epithelial cells after 1min of insult. MTT assay reading showed
slight increase, but not significantly different, in 0.2 and 0.4 mmol/L than in
saline control (Figure 2). This can be partially explained as MTT reading
increases in higher pH according to Plumb et al[32]
1989. The higher pH can decrease the cell number, but the part of the effects
can be counteracted by pH dependent MTT absorption increase. There were no
statistically significant differences among 0 to 3.1 mmol/L NaOH. Although
there was a significant decrease in viability with use of 12.5 and 25 mmol/L
NaOH, versus 6.2 mmol/L NaOH, the pH values do not differ much between these
solutions (Figure 1). The damage to the cells using 12.5 mmol/L and above was
too severe to the cells. During the cornea chemical damage, the cells with high
degree of damage cannot be recovered as many cells are subject to immediate
cell death. This study was designed to evaluate an alkaline insult that led to
reduced viability of cells, yet feasible to potentially prevent or reverse.
Accordingly, we choose 6 mmol/L NaOH as that is the minimum concentration
showed significant difference with no insult control. It will be informative
to repeat the rescue studies using a higher concentration of NaOH to see
whether a rescue effect is still observed.
In our in vivo model of alkaline injury, we have observed severe
loss of epithelial cells after 30s treatment with 1 mol/L NaOH[27]. At this much higher concentration, cell-cell contact
is disrupted and mechanical sloughing of cells from the surface occurs. This
likely occurs in our in vitro studies as well, during rinses and various
solution changes. The use of control wells where cells were exposed to
identical numbers of solution changes was important to account for the loss of
cells simply from loss of adhesion and subsequent removal from pipette
activity.
Notably, the MTT tetrazolium
dye-based assay is dependent upon metabolic activity of cells[33]. Although we have attempted to control for this in
our experiments, it is possible that the assay is affected in some way
independent of the viability of the cells. One example of the way in which
results could be impacted is the variation of metabolic activity (and thus
potentially assay results) with cell density[34].
To fully understand the data, it will be important to look at viability with
the MTT assay with varying corneal epithelial cell densities. This is an
important limitation of the results and it will be useful to further validate
the NaOH insult dose-response results using an assay that is independent of
cellular metabolism. These limitations also apply to the data presented in
Figure 4, which were also obtained using the MTT assay.
Cell proliferation was not affected
by incubation with varying concentrations of RP-220 or control scrambled
peptide, as assayed by CyQUANT®, except at the highest concentration
(50 µg/mL) of the scrambled peptide.
Insignificant trends toward reduced viability were seen with 50 µg/mL
RP-220 and with 25 µg/mL RP-220 and scrambled peptide, as illustrated in Figure
3. These findings may be related to the concentration of DMSO present as a
solvent for these peptides, which would be 0.5% (remainder corneal epithelial
media) at the highest peptide concentration. The difference between the 50
µg/mL viabilities for RP-220 versus scrambled peptide may be related to
potential proliferation-promoting effect of the RP-220 on corneal epithelial
cells. It seems that at the concentration of peptide used for the experiments,
there are no adverse effects on the cells from either solvent or peptide. It
will be important in future studies to ascertain what effect lower
concentrations of peptide, as well as solvent controls, have on corneal
epithelial cell proliferation with time. Notably, renalase has been shown to
increase proliferation of melanoma cells[35], but
whether this also occurs in corneal epithelial cells is unknown.
In this work, we have shown that
RP-220 at a concentration of 10 µg/mL has a pro-survival effect on corneal
epithelial cells after alkaline insult.
Interestingly, this effect is absent at the next highest concentration
of 20 µg/mL. This may be due to
DMSO solvent effect, although the data presented in Figure 3 would suggest that
this effect is negligible. These data would also suggest minimal to no adverse
effect from the peptide itself. Studies in HK-2 cells have previously shown
anti-apoptotic effects of renalase at concentrations as low as 10 µg/mL[13]. Although signaling mechanism was not analyzed in
this work, renalase has been proposed to promote cell survival through
activation of STAT3, ERK, p38 and AKT downstream activation[19].
Epithelial wound healing has been shown to be dependent upon p38 and ERK1/2
activation in rabbit and human corneal epithelial cells[22].
This was due to a combination of increased proliferation and migration[22].
As noted previously, we have shown
that there is significant upregulation of TGF-β
RP-220 signaling is initiated via
PMCA4b as a receptor[17]. Importantly, this
isoform is expressed in human corneal epithelial cells[20],
and ostensibly this is an early event in pro-survival signaling in these cells
leading to the findings in this study. Further work is needed to confirm the
expression of this isoform under the culture conditions used in these
experiments, and to confirm its role in the protective effect. We will also be
examining the expression of PMCA isoforms in the rat given the use of this
animal model for in vivo studies of renalase. The findings in this study
provide a foundation for several new lines of experimental questions relating
to the renalase peptide and its potential as a therapeutic in the context of
alkali injury.
ACKNOWLEDGEMENTS
Foundations: Supported by the resources of the
Central Texas Veterans Health Care System (Temple, TX); the Central Texas
Veterans Health Care System Research Service.
Conflicts of Interest: Potts L, None; Phillips C, None;
Hwang M, None; Fulcher S, None; Choi H, None.
REFERENCES