
·Basic Research·
Inhibition
of β-elemene
on the expressions of HIF-lα, VEGF and iNOS in diabetic rats model
Yun Zhou, Yan Liu, Jun Chen, Yi-Zhou Sun, Li-Hua Li, Lei
Chen
Department of Ophthalmology, the
First Affiliated Hospital of China Medical University, Shenyang 110001,
Liaoning Province, China
Correspondence to: Lei Chen. Department of
Ophthalmology, the First Affiliated Hospital of China Medical University,
No.155 Nanjing Bei Street, Heping, Shenyang 110001, Liaoning Province, China.
leichen51@hotmail.com
Received:
Abstract
AIM: To evaluate the effect of β-elemene on the expressions of hypoxia-inducible
factor (HIF)-lα,
vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase (iNOS) in a streptozotocin (STZ) induced
diabetic Sprague-Dawley (SD) rat model.
METHODS: SD rats were administered an abdominal injection of
STZ and induced to a diabetic model. After 6wk course of diabetes, the
treatment groups were given β-elemene through periocular and intravitreous injection separately and
the control groups were given blank emulsion injection. HE staining was used to
observe the morphology of retina. The mRNA expressions of HIF-1α, VEGF and iNOS was assayed by
real-time polymerase chain reaction (PCR) and the protein expression was
measured by Western blot and immunocytochemistry methods.
RESULTS: The results indicated that the protein and mRNA
expressions of HIF-1α, VEGF and iNOS after treated by β-elemene periocularly and intravitreally injections
were all found to be reduced compared with the levels in the diabetic rats
group (P<0.05). The inhibitory effect of intravitreal injection was
more remarkable.
CONCLUSION: The results show β-elemene protect the retina of
diabetic rats from high glucose
damage by downregulating the
expression of HIF-1α, VEGF and iNOS.
KEYWORDS: β-elemene; hypoxia-inducible
factor-1α; vascular endothelial growth factor; inducible nitric oxide synthase;
diabetic rat
DOI:10.18240/ijo.2019.11.05
Citation: Zhou
Y, Liu Y, Chen J, Sun YZ, Li LH, Chen L. Inhibition of β-elemene on the expressions of HIF-lα, VEGF and iNOS in diabetic rats model. Int J
Ophthalmol 2019;12(11):1693-1698
INTRODUCTION
Diabetic retinopathy (DR) is one of the most common and
severe microvascular complications of diabetes. Its main causes of blindness
are repeated vetreous hemorrhage and tractive retinal detachment. Recently,
researches of DR become a hot spot of global public healthy research[1]. The most important procedure of DR is hyperglycemia
state, resulting in hypoxia and a series of pathological changes. A number of
studies have shown that hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and inducible nitric oxide synthase (iNOS)
play an important part in the pathogenic process of DR[2-5].
Elemene
(1-methyl-1-vinyl-2,4-diisopropenyl-cyclohex-ane) is extracted from the traditional
Chinese medicinal herb Rhizoma zedoariae. It is studied in vivo and in
vitro as an anticancer agent with gratifying results[6].
Elemene agent extract is a mixture of three isoforms: α, β, and δ[7]. Among the three isoforms, β-elemene is the active
component of elemene and accounting for 60%-72% of the total extract, has been
reported to be useful against amount types of cancers for instance in lung,
gastric, and glioblastoma cancers[6-7].
The possible mechanisms of β-elemene have been reported including the following
aspects: inhibition of tumor cell proliferation, induction of tumor cell
apoptosis, cell cycle arrest, and antiangiogenesis[8-10]. However, new research revealed inhibiting the
expression of VEGF becomes an important procedure. But, the direct targets and
signal transduction pathways and the specificity of β-elemene were all unknown,
so we need further detailed studies. Our research group had already proved that
β-elemene can hold back the occurrence and development of retinal
neovascularization, meanwhile, the effect of intravitreous injection was better
than periocular injection[11]. As far as we know,
no previous researches have studied whether β-elemene inhibits the expression
of HIF-1α, VEGF and iNOS in vivo. So, our study focused on finding the
role of β-elemene in a diabetic Sprague-Dawley (SD) rat model, and detecting
its effect on the expression of HIF-1α, VEGF and iNOS of the retina. This
effect may be a potential therapeutic target for patients with DR.
MATERIALS AND METHODS
Ethical Approval All animals were treated according
to the statement of the Society for Vision and Ophthalmology on the use of
animals for ophthalmic and visual research. The experiment was approved by the
Animal Ethics Committee of China Medical University and strictly abide by the
National Institutes of Health guidelines for the care and use of laboratory
animals.
Chemicals and Reagents β-elemene (10 mg/mL) and blank
emulsion were provided by Yuan da Pharmaceuticals (Dalian, China). Antibodies
against HIF-1α and VEGF were purchased from Abcam Biotechnology (Cambridge,
London, UK). β-actin, antibody against iNOS, horse radish peroxidase
(HRP)-conjugated goat anti-rabbit IgG were
purchased from SantaCruz Biotechnology (Dallas, Texas, USA).
Animals Male SD rats (10 weeks old, n=100)
were purchased from the Beijing Vital River Animal Resources, each weighing
198±
Animal Model The rats were randomly divided into
6 groups: A) nomal control group; B) diabetic rats group; C) periocular compare
group; D) periocular treat group; E) intraocular compare group; F) intraocular
treat group, fifteen rats per group. The rats were fasted overnight (12h) and
not limited to drinking water. Of 1% streptozotocin in freshly prepared sodium
citrate buffer (pH 4.5) was injected into the peritoneal cavity once in a dose
of 60 mg/kg to induce diabetes (groups B-F). Rats in group A were injected with
the same volume of sodium citrate buffer. Diabetes was confirmed after 72h of
streptozotocin injection and again on weekly basis during the experiment. Only
the rats with glucose levels higher than 16.67 mmol/L were confirmed diabetic.
Therapeutic Agents and Treatment
Schedule At the time point of six weeks
duration of diabetes, periocular injections were performed with a 5 µL syringe
(Hamilton, Reno, NV, USA) and a 33-gauge needle. Both eyes of the rats were
injected periocularly with 5 µL of blank emulsion and 5 µL of β-elemene in
group C and group D, respectively. Intravitreal injections were performed just
posterior to the pars plana with a 5 µL syringe and a 33-gauge needle. Both
eyes of the rats were injected into the vitreous at the pars plana with 5 µL of
blank emulsion and 5 µL of β-elemene in group E and group F, respectively. The
injections were repeated once every another day, three times a course of
treatment. Rats with any kind of postoperative complication (e.g.
cataract) were excluded from analysis. The rats were executed and the eyeballs
were then fixed, embedded, cut into sections and stained with hematoxylin and
eosin (HE), and the pathological morphology of retina was observed. The
expression of HIF-1α, VEGF and iNOS protein and mRNA was determined using
immunohistochemistry, Western blot technique and real-time polymerase chain
reaction (PCR) technique.
Real-time Polymerase Chain Reaction The retina was removed from the rats and
total RNA was extracted with Trizol (Invitrogen Inc. AQ4) as described by the
manufacturer. Total RNA (1 μg) was reverse transcribed using reverse
transcriptase (Superscript II; Invitrogen-Gibco, USA) and oligo-dT primers
according to the manufacturer’s instructions. Rat HIF-1α, VEGF and iNOS primers
are listed in Table 1. PCR reactions of HIF-1α, VEGF, iNOS and β-actin genes.
AQ5 was performed in a total volume of 20 μL under the same conditions using a
SYBR Green PCR Core Kit (Applied Biosystems, Foster City, California, USA)
according to the supplier’s instructions and an ABI 7900HT (Applied Biosystems)
real-time PCR instrument. The expression levels of HIF-1α, VEGF and iNOS were
corrected by the expression level of β-actin as an endogenous control. The
cycling conditions were
Immunohistochemistry Immunohistochemical staining was
performed as described previously. Briefly, the retinas were sectioned using a
microtome, each having a thickness of 3 μm; dewaxed with xylene, rehydrated;
and subjected to immunohistochemical staining. The activity of endogenous
peroxidase is quenched by the application of hydrogen peroxide and then
subjected to antigen retrieval. Slides were incubated with the appropriate primary
antibody (i.e., antibodies specific for HIF-1α, VEGF and iNOS) overnight
at
Western Blot Western blot assays were performed
using conventional methods. Briefly, the procedure was as follows. The retina
was taken out from the rat and protein was extracted using radioactive
immunoprecipitation buffer (China University of Biotechnology). Protein concentration
was measured using the BCA assay. Total protein (70 μg) was separated on a 12%
SDS-PAGE gel, transferred to a polyvinylidene fluoride membrane (0.45 μm;
Amersham, AQ6, USA), and the primary antibody against rabbit HIF-1α was probed
with a probe. Probing (1:2000 dilution), VEGF (1:2000 dilution) and iNOS
(1:1000 dilution) were carried out overnight at
Statistical Analysis All statistical analyses were
performed using SPSS 17.0 software. First, the software is used to test the
homogeneity of the variance test. Then, each group of values was evaluated by
one-way analysis of variance. Differences in P values less than 0.05
were considered statistically significant.
RESULTS
Inhibitory Effects of β-elemene on
the mRNA Expressions of HIF-1α, VEGF and iNOS HIF-1α, DR, VEGF and iNOS are known
to play an important role in diabetes retinopathy. To investigate the effect of
β-elemene on the expression of HIF-1α, VEGF and iNOS mRNA, real-time PCR
analysis was performed. The primers used are shown in Table 1. The results
indicate that the mRNA levels of HIF-1α, VEGF and iNOS in diabetic rats (group
B) were obviously elevated compared with the control group (group A; Figure 1, P<0.05
vs control group), meanwhile, β-elemene significantly inhibited the mRNA
levels of HIF-1α, VEGF and iNOS compared with the group B (Figure 1, P<0.05 vs
diabetic rats group). These results indicate that β-elemene can protect the
retina from damage under high glucose by down-regulating HIF-1α, VEGF and iNOS
at the mRNA level.
Table1 The sequences of HIF-1α, VEGF
and iNOS primers
|
Gene name |
Sequence ( |
Size |
|
VEGF F |
CCCGACAGGGAAGACAAT |
131 |
|
VEGF R |
TCTGGAAGTGAGCCAACG |
|
|
HIF-1α F |
CCTACTATGTCGCTTTCTTGG |
198 |
|
HIF-1α R |
GTTTCTGCTGCCTTGTATGG |
|
|
iNOS F |
CACCTTGGAGTTCACCCAGT |
135 |
|
iNOS R |
ACCACTCGTACTTGGGATGC |
|
|
β-actin F |
GGAGATTACTGCCCTGGCTCCTAGC |
155 |
|
β-actin R |
GGCCGGACTCATCGTACTCCTGCTT |
|
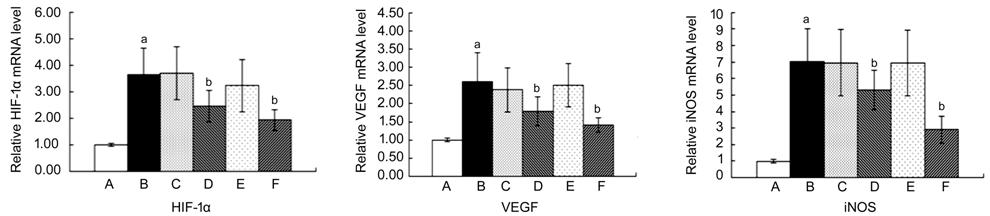
Figure 1 Inhibitory effect of
β-elemene on the mRNA levels of HIF-1α, VEGF and iNOS in diabetic rats A: Normal control group; B: Diabetic
rats group; C: Periocular compare group; D: Periocular treat group; E:
Intraocular compare group; F: Intraocular treat group. aP<0.05 vs control
group; bP<0.05 vs diabetic rats group.
Inhibitory Effects of β-elemene on
the Protein Expression of HIF-1α, VEGF and iNOS To
further investigate the relationship between β-elemene and HIF-1α, VEGF and
iNOS, immunocytochemistry and Western blot analyses were used to detect the
protein expression levels of HIF-1α, VEGF and iNOS. Using immunocytochemistry,
observe the locations of the expressions of HIF-1α, VEGF and iNOS and the
differences. The protein expressions of HIF-1α, VEGF and iNOS were all found to
be reduced compared with the levels in the diabetic rats group (Figure 2; P<0.05).
The results from Western blot analysis were consistent with the immunocytochemistry
results.
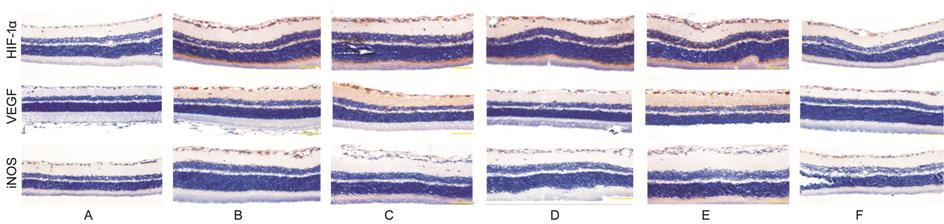
Figure 2 Expression of HIF-1α, VEGF
and iNOS protein of the retina of rats examined by immunocyto-chemistry method
(400×) A: Normal control group; B: Diabetic
rats group; C: Periocular compare group; D: Periocular treat group; E:
Intraocular compare group; F: Intraocular treat group.
Immunocytochemistry results Group A, less HIF-1α positive expression
located in ganglial cell layer, outer plexiform layer; Group B, positive
expression mostly located in ganglial cell layer, inner plexiform layer, inner
nuclear layer, outer plexiform layer and layer of rods and cones. Groups D, F,
positive expression was less than group B, especially in group F. Groups C, E
have no obvious differences between group B.
Group A, weak VEGF positive
expression observed; Group B, positive expression located in inner layers of
retina. Groups D, F, positive expression was less than group B, especially in
group F. Groups C, E have no obvious differences between group B.
Group A, no iNOS positive expression
observed; Group B, positive expression mostly located in ganglial cell layer
and layer of rods and cones. Groups D, F, positive expression was less than
group B, especially in group F. Groups C, E have no obvious differences between
group B.
Western blot results The protein expressions of HIF-1α,
VEGF and iNOS after treated by β-elemene periocularly and intravitreally
injections were all found to be reduced compared with the levels in the
diabetic rats group (Figure 3; P<0.05). The inhibitory effect of
intravitreal injection was more remarkable.
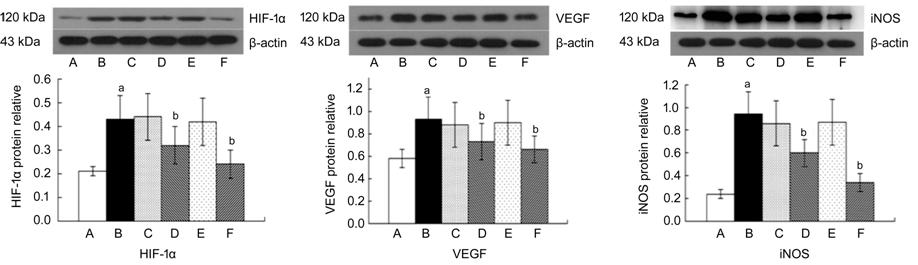
Figure 3 The expression of HIF-1α,
VEGF and iNOS detected by Western blot analysis A: Normal control group; B: Diabetic
rats group; C: Periocular compare group; D: Periocular treat group; E:
Intraocular compare group; F: Intraocular treat group. aP<0.05
vs control group; bP<0.05 vs diabetic rats
group.`
The results showed that the
expression of HIF-1α, VEGF and iNOS in diabetic rats were significantly
elevated comparing with the control group (P<0.05 vs control
group). β-elemene could inhibit the expression of HIF-1α, VEGF and iNOS both by
periocular injection and intraocular injection (P<0.05 vs diabetic
rats group), while the effect was more obvious by intraocular injection.
DISCUSSION
In normal eye tissues, the stability
of angiogenesis is controlled by the balance of the stimulus and inhibitory
substances. Mostly, the inhibitory factors are dominant and it keeps the
vessels quiet in normal tissues. VEGF is found to be the strongest angiogenesis
promoting factor[12]. The expression of VEGF is
increased in the visual cells of diabetic patients, and the concentration of
VEGF is elevated in the aqueous humor and vitreous of the patients with DR.
Recent years, studies found that in the VEGF signal
regulating pathway of hypoxia, HIF-l plays a role of central link. And its
function concludes not only increasing VEGF mRNA stability and increasing the
transcription activity of VEGF. A large number of animal experiments confirmed
that HIF-lα played an important
role in the occurrence of DR. HIF-lα is the functional subunit of HIF-1. Under
the condition of hypoxia, HIF-lα is activated, combined with a common base
sequence (
In our experiment, we established a
diabetic rats model, took a β-elemene injection in two ways: periocular
injection and intravitreous injection. According to the results, we got fine
effects in both two ways of injection by paying precise attention to the
microscope operations. β-elemene is a kind of emulsion. In order to observe the
effect of the emulsion on the retina, we set a blank emulsion control in our
experiment. We proved that emulsion had no toxicity response on the retina, and
it was safe to be used in diabetic rats. And we also compared the differences
between two ways of injection. Studies have revealed that at the time point of
one month of diabetic course, the HE staining of retina had no obvious changes,
but at the time point of three months of diabetic course, the ganglial cell
layer had obvious changes: the number of cells decreased with loosely arranged
positions and fuzzy boundaries. At the time point of 2wk of diabetic rats
course, HIF-1α, VEGF mRNA and protein expression was elevated, and at 8
to 10wk, HIF-1α expression reached peak, while at 12wk the expression
began to decline. The VEGF expression was continued rising[29].
There is no research to observe the retinal form of diabetic rats for the
duration of 6wk. Due to diabetic rats course of late mortality rate is high,
the cost is too high, and all the comprehensive factors, this study adopted a
diabetic rats for six weeks of disease course, and then we conducted local
injections of β-elemene and blank emulsion.
In this study, HE staining in
diabetic rats for the duration of 6wk revealed that in diabetic rats group,
retinal cells appeared arranging disorder, fuzzy boundaries, and sparse nucleus
could be observed sometimes, nucleus dyeing quality appeared fuzzy, and in
periocular and intraocular treat groups, retinal cells were arrayed better than
diabetic rats group, while in periocular and intraocular compare groups, we did
not see any improvement. Therefore, we deduced β-elemene had non-toxic
reactions to the retina of diabetic rats at 6wk of diabetic course, and the
morphology of the retina could be improved to a certain extent.
Immunocytochemistry results showed in diabetic rats without β-elemene
injection, positive expression of HIF-1α mostly located in ganglial cell layer,
inner plexiform layer, inner nuclear layer, outer plexiform layer and layer of
rods and cones while positive expression of VEGF located in inner layers of
retina and also positive expression of iNOS mostly located in ganglial cell
layer and layer of rods and cones. After injection, the expression of HIF-1α,
VEGF, iNOS in the retina of diabetic rat had dropped especially in the group of
intraocular injection. So whether the protective effects of β-elemene to the
retina was related to the inhibition of HIF-1α, VEGF, iNOS expression? We
detected the expression of HIF-1α, VEGF, iNOS in the retina of diabetic rats
using real-time PCR method and Western Blot method respectively from the
mRNA and protein levels. The results confirmed that β-elemene can inhibit the
expression of HIF-1α, VEGF and iNOS in early diabetic rats course, and the
effect of intravitreous injection is much better than periocular injection. But
the definite mechanism is unknown, so we need a forward mechanism research.
In summary, this study proves that
injection of β-elemene periocularly and intraocularly can protect the retinal
form and can inhibit the expression of HIF-1α, VEGF and iNOS of diabetic rats
in the course of 6wk. And the effect of intraocular injection is better in
vitro. In the future work, we will focus on whether this downregulation
effect of HIF-1α, VEGF and iNOS expression occurs in vivo, as well as
the probable signaling pathway involved.
ACKNOWLEDGEMENTS
The authors thank Dr. Wei-Ping Teng
(Department of Endocrinology, China Medical University, China) and Dr.
Feng-Ping Shan (Department of Immunization, China Medical University, China),
who helped with the experimental design; Dr. Wei Yang (Department of Laboratory
Animal Science, China Medical University, China), who assisted with the animal
experimental techniques; and Dr. Jing-Pu Shi (Department of Epidemiology, China
Medical University, China), who helped with data analysis.
Conflicts of Interest: Zhou Y, None; Liu Y, None; Chen
J, None; Sun YZ, None; Li LH, None; Chen L, None.
REFERENCES