
·Clinical Research·
Comparison
of anti-inflammatory effects of intense pulsed light with
tobramycin/dexamethasone plus warm compress on dry eye associated meibomian
gland dysfunction
Yu-Fei Gao1, Rong-Jun Liu1, Ya-Xin
Li1,2, Chenmilu Huang1,3, Yi-Yun Liu1, Chen-Xi
Hu1, Hong Qi1
1Department of Ophthalmology; Beijing
Key Laboratory of Restoration of Damaged Ocular Nerve, Peking University Third
Hospital, Beijing 100191, China
2The First Hospital of Fangshan
District, Beijing 102400, China
3Beijing No.6 Hospital, Beijing
100007, China
Co-first authors: Yu-Fei Gao and Rong-Jun Liu
Correspondence to: Hong Qi. Department of
Ophthalmology, Peking University Third Hospital, 49 North Garden Road, Haidian
District, Beijing 100191, China. doctorqihong@163.com
Received:
Abstract
AIM: To compare the anti-inflammatory effects of intense pulsed light (IPL)
with tobramycin/dexamethasone plus warm compress through clinical signs and
cytokines in tears.
METHODS: Eighty-two patients with dry eye disease (DED)
associated meibomian gland dysfunction (MGD) were divided into two groups.
Group A was treated with IPL, and Group B was treated with
tobramycin/dexamethasone plus warm compress. Ocular Surface Disease Index (OSDI),
tear film breakup time (TBUT), corneal fluorescein staining (CFS), meibomian
gland expressibility (MGE), meibum quality, gland dropout and tear cytokine
levels were evaluated before treatment, 1wk and 1mo after treatment.
RESULTS: TBUT in Group A was higher (P=0.035), and
MGE score was lower than Group B at 1mo (P=0.001). The changes of
interleukin (IL)
CONCLUSION: Treatment with IPL can improve TBUT and MGE and
downregulate levels of IL
KEYWORDS: intense pulsed light; meibomian
gland dysfunction; dry eye disease; interleukin
DOI:10.18240/ijo.2019.11.07
Citation: Gao
YF, Liu RJ, Li YX, Huang C, Liu YY, Hu CX, Qi H. Comparison of
anti-inflammatory effects of intense pulsed light with tobramycin/dexamethasone
plus warm compress on dry eye associated meibomian gland dysfunction. Int J
Ophthalmol 2019;12(11):1708-1713
INTRODUCTION
Meibomian gland dysfunction (MGD) is
mainly characterized by terminal duct obstruction and abnormality in meibum
secretion[1],
which alters the tear film and decreases its functional integrity[2]. MGD may occur as
an isolated disorder, but it may also be accompanied by dry eye disease (DED)[3]. DED is a
multifactorial disease of the ocular surface characterized by a loss of
homeostasis of the tear film, and accompanied by ocular symptoms[4]. DED has been
divided into evaporative and aqueous deficiency subtypes, and MGD is the most
common cause of evaporative dry eye[3-5].
According to some studies, the overall prevalence of MGD varies widely from
3.5% to 70% and the prevalence of DED ranges from 5% to 50%, both of which is
related to age, race and district[6-7].
It’s reported by the Dry Eye Workshop II (DEWS II) that 32.9% of dry eye
patients associates with MGD[7].
Tear instability and tear
hyperosmolarity associated with MGD, could activate stress signaling pathways
in the ocular surface epithelium and resident immune cells, therefore trigger
production of inflammatory cytokines. It’s regarded as a self-perpetuating “dry
eye inflammatory vicious cycle”[8]. Several studies have reported the levels of
interleukin (IL)-6, IL
Treatments recommended for DED
associated MGD includes warm compress, lid massage, antibiotic and
anti-inflammatory ointments, and artificial tears[15]. Unfortunately, warm compress is
hard to standardize and its troublesome procedure reduces patients’ compliance[16-17]. Some anti-inflammatory
ointments can’t be used consecutively because of their side effects, which may
decrease the treatment efficiency. To avoid the drawback of conventional
treatment, a new therapy named intense pulsed light (IPL) was proposed by some
researchers[18-23]. IPL devices
could direct light extended from 515 nm to 1200 nm to the skin near the eye
lid. The probable mechanisms of IPL treating DED associated MGD include heat
transfer, antibiotic effect and preventing inflammatory mediators from the
meibomian glands[24].
Although the clinical effects of IPL on DED associated MGD has already been
proved by several studies[19,21-23], no one has compared the
anti-inflammatory effects of IPL with tobramycin/dexamethasone plus warm
compress. This study aimed at comparing IPL with tobramycin/dexamethasone plus
warm compress on DED associated MGD from perspective of anti-inflammatory
effects.
SUBJECTS AND METHODS
Ethical Approval The study continued for one year from November 2016 to
November 2017. Patients who had been diagnosed as DED associated MGD were
recruited. Informed consents were obtained from all patients before the study.
The study was approved by the biomedical ethics committee of Peking University
Third Hospital and adhered to the tenets of the Declaration of Helsinki. The
study was registered at clinicaltrials.gov (NCT 02958514).
Patients were diagnosed as MGD on the basis of the
criteria provided by the Tear Film and Ocular Surface Society (TFOS)[25-26]: 1) ocular symptoms; 2) abnormal
morphologic lid features; 3) alterations of meibomian gland secretion. Patients
with either 1) + 2) or 1) + 3) could be diagnosed as MGD. Meanwhile, patients
were also diagnosed as DED based on criteria provided by the Dry Eye Workshop
(DEWS)[27]:
1) the Ocular Surface Disease Index (OSDI) >13; 2) tear film breakup time
(TBUT) ≤5s or 5s<TBUT≤10s with positive corneal fluorescein staining (CFS).
Patients were excluded from the
study if they met each criteria as follow: 1) under the age of 18y; 2) ocular
infection and allergy; 3) allergic to hormonal drugs; 4) abnormalities of
anatomy or movements of eyeballs; 5) ocular surgical history or trauma within
3mo; 6) Fitzpatrick Skin Types Ⅳ, Ⅴ and Ⅵ[28]; 7) with tattoos, pigmented lesions or skin cancer in the treatment area;
8) radiotherapy or chemotherapy history within 1y; 9) pregnancy or lactation;
10) autoimmune disease.
Intervention Procedure Patients were divided into two groups randomly according
to a computer-generated randomization program. Patients received bilateral
treatment, but only the severer eye was enrolled in the study. Patients in
Group A were treated with IPL once per month, and sodium hyaluronate eye drops
(Hycosan, EUSAN GmbH, Germany) four times a day. Patients in Group B were given
tobramycin/dexamethasone ointment (Tobradex, Alcon, Belgium) plus warm compress
once every night and sodium hyaluronate eye drops four times a day.
The IPL device (M22, Lumenis, USA)
was used in this study. Pulse intensity ranged from 12 to 14 J/cm². Pulse width
was 6ms. IPL treatment was performed by a same doctor and was given as follow:
1) Clean the treatment area on both upper and lower eyelids with cotton swabs;
2) Apply compound lidocaine cream (Beijing Unisplendour Pharmaceutical Co.,
Ltd.) for anesthesia for 30min; 3) Protective shield was placed over the cornea
and sclera, and the other eye was protected by an eyeshade; 4) IPL was
administered to the periocular area on both upper and lower eyelids (
As for Group B, patients received
tobramycin/dexamethasone ointment and a 10-minute warm compress (
Clinical Evaluation To compare the clinical effects of the two groups, tests
were conducted in the same order that minimized the extent to which one test
influenced the tests that followed. 1) Subjective symptoms of patients were
evaluated by the OSDI questionnaire. 2) Measurement of TBUT was facilitated by
viewing with a blue exciter filter after instilling sodium fluorescein onto the
bulbar conjunctiva with a fluorescein sodium ophthalmic strip (Liaoning
Meizilin Pharmaceutical Co., Ltd., China). TBUT was measured three times for
each patient and made an average[5]. 3) CFS score was quantified according to the system
provided by National Eye Institute[29]. 4) The central glands of eyelid were pressed to
enumerate meibomian gland expressibility (MGE) score. It was scored according
to the number of the five glands from which a meibum secretion could be
expressed (0=5 glands expressing, 1=3 to 4 glands expressing, 2=1 to 2 glands
expressing and 3= none gland expressing)[30]. MGE of the upper and lower
eyelids should be scored respectively and then the two scores were added. 5)
Meibums quality from the upper and lower eyelids were scored respectively (0=
clear and fluid-like, 1= cloudy and fluid-like, 2= cloudy and granular, and 3=
whitish, toothpaste-like)[31],
and then the two scores were added as a meibum quality score. 6) The severity
of gland dropout was scored by observing the morphology of meibomian glands
with infrared meibography system (Topcon, Japan). Magnification was set at 10×
and image resolution at 640×480. The upper and lower eyelids were scored
respectively (0= normal, 1= dropout <1/3, 2= dropout between 1/3-2/3, and 3=
dropout >2/3)[30],
and then the two scores were added.
Tear Sample Collection and
Analysis Tear collection was performed before any other test at
baseline, 1wk and 1mo after treatment. Tear samples were collected
non-traumatically from the inferior tear meniscus. Glass capillary
micropipettes (Drummond Scientific, Broomall, PA, USA) were used to collect 5
μL of tears. Tear samples were fully eluted into a sterile collection tube
(Sigma-Aldrich, St. Louis, MO, USA) at once. Tubes with tear samples were kept
cold (
Statistical Analysis SPSS 23 was used to analyze the data. Data were expressed
as mean±standard error of the mean (SEM). As the concentrations of IL
RESULTS
Patients and Clinical Outcomes Eighty-two patients were included in this study.
Forty-one patients were analyzed in Group A (10 males and 31 females), with a
mean age of 54.44±16.19 (range 22-80)y. Forty-one patients were analyzed in
Group B (11 males and 30 females), with a mean age of 55.22±16.71 (range
23-86)y. The visual acuity and intraocular pressure of patients were stable
during treatment in both groups. Compared Group A with Group B, there was no
difference in OSDI, TBUT, CFS, MGE, meibum quality, gland dropout and levels of
IL-6, IL
OSDI, CFS, TBUT and MGE scores were
improved in both Group A and Group B at 1wk and 1mo after treatment compared
with baseline, which were of statistically differences (all P<0.05).
However, there was no significant difference in meibum quality scores and gland
dropout scores between each time point and baseline in both groups (all P>0.05;
Table 1).
Table 1 Clinical outcomes in Group A
and Group B at baseline, 1wk and 1mo
|
Parameters |
Group |
Baseline |
1wk |
1mo |
|
OSDI |
A |
38.92±2.59 |
29.98± |
25.72±4.52b |
|
B |
38.14±2.39 |
31.07±2.44b |
21.48±4.79b |
|
|
TBUT(s) |
A |
4.17±0.31 |
5.34±0.37b |
5.87±0.44b,d |
|
B |
3.80±0.28 |
4.71± |
4.63± |
|
|
CFS |
A |
2.24±0.42 |
1.39± |
1.18± |
|
B |
2.85±0.49 |
1.68±0.41b |
1.24± |
|
|
MGE |
A |
3.71±0.20 |
2.63± |
1.61±0.15b,e |
|
B |
3.80±0.21 |
3.12±0.22b |
2.61±0.23b |
|
|
Meibum quality |
A |
2.22±0.22 |
2.00±0.20 |
2.53±0.32 |
|
B |
2.54±0.22 |
2.15±0.20 |
2.94±0.33 |
|
|
Gland dropout |
A |
3.80±0.17 |
3.80±0.13 |
4.18±0.21 |
|
B |
3.87±0.13 |
3.70±0.11 |
4.12±0.19 |
OSDI: Ocular surface disease index;
TBUT: Tear film breakup time; CFS: Corneal fluorescein staining; MGE: Meibomian
gland expressibility. aP<0.05, bP<0.01,
cP<0.001, comparing with baseline. dP<0.05,
eP<0.01, comparing Group A with Group B.
Compared Group A with Group B, there
was no difference in TBUT and MGE score at 1wk (P>0.05). Compared
with Group B, TBUT in Group A was higher than that in Group B at 1mo (P=0.035),
and MGE score in Group A was lower than that in Group B at 1mo (P=0.001).
However, there was no significant differences between Group A and Group B on
OSDI, CFS, meibum quality scores and gland dropout scores at 1wk or 1mo (all P>0.05).
Changes of Tear Cytokine Levels The concentrations of IL-6, IL
Table 2 Concentrations of tear cytokines in Group A and
Group B at baseline, 1wk and 1mo
pg/mL
|
Parameters |
Group |
Baseline |
1wk |
Change |
1mo |
Change |
|
IL-6 |
A |
126.90±39.68 |
42.96±7.99 |
-83.94±36.55 |
65.16±18.71 |
-61.74±35.94 |
|
B |
129.21±27.21 |
56.52±12.8 |
-72.68±23.39 |
32.40±7.14 |
-84.16±23.87 |
|
|
IL |
A |
17.31±2.09 |
15.35±1.98 |
-1.96±1.52 |
17.49±2.17 |
0.18±1.77 |
|
B |
15.81±1.89 |
18.11±2.28 |
2.30±1.68 |
14.74±1.87 |
-1.07±1.35 |
|
|
IL-1β |
A |
3.62±0.34 |
3.01±0.39 |
-0.61±0.26 |
3.55±0.35 |
-0.07±0.33 |
|
B |
3.18±0.33 |
3.53±0.34 |
0.35±0.26 |
3.57±0.56 |
0.39±0.44 |
The changes of IL
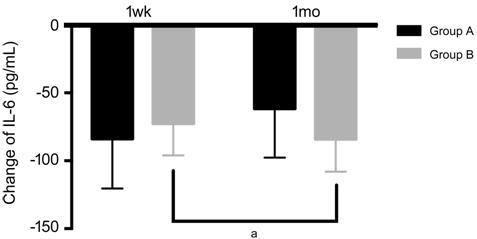
Figure 1 Changes of IL-6 at 1wk and
1mo in Group A and Group B IL: Interleukin. Change of IL-6: The
concentration of IL-6 at 1wk or 1mo minus the concentration of IL-6 at
baseline. aP<0.05 comparing 1wk with 1mo in Group B.
The changes of IL
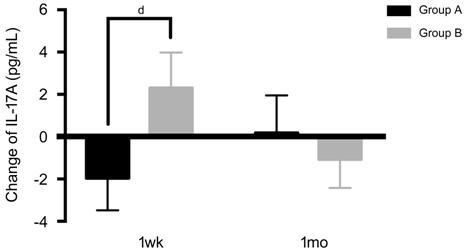
Figure 2 Changes of IL
The changes of IL-1β in Group A were
-0.61±0.26 pg/mL at 1wk and -0.07±0.33 pg/mL at 1mo. The changes of IL-1β in
Group B were 0.35±0.26 pg/mL at 1wk and 0.39±0.44 pg/mL at 1mo. In Group A,
change of IL-1β was lower at 1wk than that at 1mo (P=0.027). In Group B,
there was no significant difference compared change of IL-1β at 1wk with that
at 1mo (P=0.224). Compared with Group B at 1wk, the change of IL-1β in
Group A was lower, which differed significantly (P=0.005). Compared with
Group B at 1mo, the change of IL-1β in Group A did not differ statistically (P=0.626;
Figure 3).
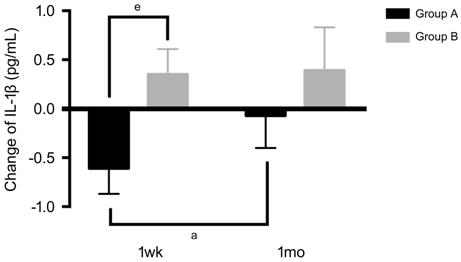
Figure 3 Changes of IL-1β at 1wk and
1mo in Group A and Group B Change of IL-1β: The concentration
of IL-1β at 1wk or 1mo minus the concentration of IL-1β at baseline. aP<0.05
comparing 1wk with 1mo in Group A. eP<0.01 comparing Group
A with Group B at 1wk.
DISCUSSION
IPL is a new treatment for patients
with DED associated MGD. However, the mechanisms of IPL to treat DED associated
MGD still remain uncertain currently. The probable mechanisms included heat
transfer, antibiotic effect and anti-inflammatory effect. The light emitted
from IPL device was selectively absorbed by chromophores in hemoglobin,
subsequently releasing thermal energy, which heated and destructed the abnormal
vasculature in the eyelid margin and adjacent conjunctiva, thus preventing
inflammatory mediators from the meibomian glands[24]. The probable mechanisms of IPL
covered almost all the principles to treat DED associated MGD in classical
therapy. Tobramycin/dexamethasone is widely used as an antibacterial and
anti-inflammatory combination by ophthalmologist. Dexamethasone is a pure
glucocorticoid agonist. It’s already known that therapeutic doses of
dexamethasone have been shown to inhibit influx of macrophage and neutrophil,
accompanied by a substantial downregulation of inflammatory cytokine production
such as IL-6[32-33]. Several studies
have reported the improvements of symptoms and signs of DED associated MGD
after IPL[19-20]. Some studies
indicated that IPL treatment could downregulate levels of IL-6 and IL
The clinical symptoms and signs for
DED associated MGD after IPL were compared with tobramycin/dexamethasone plus
warm compress in our study. OSDI, TBUT, CFS and MGE scores were all improved
after treatment in both Group A and Group B, manifesting the clinical effects
of both IPL and tobramycin/dexamethasone plus warm compress. These results
coincided with previous reports[19,22,34]. Our study
manifested that IPL improved TBUT and MGE more than tobramycin/dexamethasone
plus warm compress at 1mo after treatment.
The changes of tear cytokine levels
after IPL were compared with tobramycin/dexamethasone plus warm compress in
order to evaluate their anti-inflammatory effects. As proved in many studies,
hyperosmolar stress could activate mitogen-activated protein kinases (MAPKs) on
the ocular surface epithelium and stimulate secretions of IL-1β and IL-6[8]. IL-6 and IL-1β are
pro-inflammatory cytokines. IL-1β stimulates the production of other
inflammatory cytokines, and then lyse the tight junctions in the superficial
corneal epithelium[35].
IL-6 drive the production of IL
In this study, the effects of IPL
and tobramycin/dexamethasone plus warm compress on the changes of IL-6, IL
Interestingly, changes of IL-6, IL
The study also had some limitations.
For the safety, tobramycin/dexamethasone ointment cannot be used consecutively
because of its potential side effects such as ocular hypertension and cataract.
Thus, Group B in this study was treated with tobramycin and dexamethasone
ointment for only one month. It could not be deduced from this study whether
further benefit would be realized if tobramycin/dexamethasone was used in Group
B for a longer time.
In conclusion, our study suggested
that treatment with IPL could improve TBUT and MGE and downregulate levels of
IL
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural
Science Foundation of China (No. 81570813); the Lin Hu Scientific Research
Foundation of Department of Ophthalmology, Peking University Third Hospital;
the Scientific Research Foundation for the Excellent Returned Overseas Chinese
Scholars, Peking University Third Hospital; the Scientific Research Foundation
for the Returned Overseas Chinese Scholars, State Education Ministry.
Conflicts of Interest: Gao YF, None; Liu RJ, None; Li
YX, None; Huang C, None; Liu YY, None; Hu CX, None; Qi
H, None.
REFERENCES