
·Brief Report·
The
expression of MAPK signaling pathways in conjunctivochalasis
Yuan-Ling Jia, Xiao-Jing Liu, Hang Wen, Yue-Ping Zhan,
Min-Hong Xiang
Department of Ophthalmology, Putuo Hospital,
Shanghai University of Traditional Chinese Medicine, Shanghai 200062, China
Co-first authors: Yuan-Ling Jia, Xiao-Jing Liu, and
Hang Wen
Correspondence to: Min-Hong Xiang and Yue-Ping Zhan.
Department of Ophthalmology, Putuo Hospital, Shanghai University of Traditional
Chinese Medicine, 164 Lanxi Road, Shanghai 200062, China.
xiangminhong@sohu.com; xiangminhong1977@126.com
Received:
Abstract
This study investigated the potential role of MAPK
signaling pathways in conjunctivochalasis (CCH). Twenty loose conjunctival
biopsy samples from 20 CCH and 15 conjunctival biopsy samples from 15 normal
controls (CON) were collected. The conjunctival fibroblasts were cultured in
vitro. Immunofluorescence, ELISA, Western blot and reverse
transcription-polymerase chain reaction (RT-PCR) were used. Our results showed
that the expression of p-ERK, p-JNK, and p-p
KEYWORDS: ERK; JNK;
p38 MAPK; conjunctivochalasis; phosphorylation level
DOI:10.18240/ijo.2019.11.21
Citation: Jia
YL, Liu XJ, Wen H, Zhan YP, Xiang MH. The expression of MAPK signaling
pathways in conjunctivochalasis. Int J Ophthalmol
2019;12(11):1801-1806
INTRODUCTION
Conjunctivochalasis (CCH) is one of
the most common clinic eye diseases in elderly people. Epidemiological studies
in China have indicated a 44.8% prevalence of CCH in people older than 60y. The
incidence increases with age, reaching 89% in people older than 70y. CCH
induces dry eye, epiphora, and other uncomfortable symptoms, thus severely
affecting patients’ life quality[1-2].
Traditional treatment mainly comprises surgical therapies, including resection
of crescent shaped conjunctiva, conjunctival limbal trapezoid excision,
conjunctiva suture fixation and so on. However, the operation outcomes are not
ideal with recurrence rate[3-4].
The main pathological changes in CCH are the decreasing of elastic fibers and
dissolution of collagen fibers, which lead to excessive degradation of the
stroma and Tenon’s capsule in the conjunctiva[5-7]. Recent studies have shown that the main reason for the
dissolution of collagen fibers is over-expression of matrix
metalloproteinases-1 (MMP-1), MMP-3, and MMP
In recent years, the mitogen
activated protein kinase (MAPK) signaling pathways have been found to
up-regulate the expression of MMPs[10]. Members
of the MAPK family play vital roles in many cellular processes, including
proliferation, apoptosis, differentiation, metabolism, senescence and survival.
In mammals, the MAPK signaling pathways divide into four groups: ERKs
(extracellular signal-regulated kinases), JNKs (c-Jun N-terminal kinases), p38
MAPKs (α, β, γ, and δ), and ERK5. They are activated by diverse extracellular
stimuli, such as growth factors, cytokines, mitogens, hormones, and other
various cellular stresses including oxidative stress, heat shock, hypoxia,
ischemia, ultraviolet irradiation, and DNA-damaging agents[11].
Although the MAPK signaling pathways
have been found to up-regulate the expression of MMPs in many cells, it has not
been demonstrated to have the same function in CCH. Therefore, we hypothesized
that ERK, JNK, and p38 MAPK might accelerate the occurrence of CCH through
up-regulating the expression of MMPs.
SUBJECTS AND METHODS
Ethical Approval The sample collection protocol was
approved by the ethics committee of Putuo Hospital Affiliated to Shanghai
University of Traditional Chinese Medicine. Informed consents were obtained
from all the CCH patients and control individuals before participation.
Specimen Collection Loose conjunctival tissue from 20 CCH
patients (9 males, 11 females; mean age: 70.43±8.89y; CCH group) were collected
between October 2016 and December
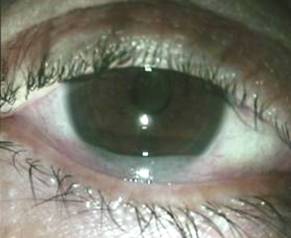
Figure 1 Slit lamp photograph
showing CCH.
Cell Culture Conjunctival fibroblasts were
obtained from primary cell culture. Primary and passaged cultures of fibroblast
cells were grown under Xiang et al’s[8]
fibroblasts culturing methods. The specimens were rinsed with 0.9% saline three
times to remove blood, then were cut into 0.5
Then, each conjunctival tissue
sample was placed at the bottom of a 75-cm2 cell culture flask. The
3 mL Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA, USA)
medium containing 10% fetal bovine serum (FBS), 1% double antibody, 1%
fibroblast growth supplement (FGS), 1% penicillin, and 1% streptomycin was then
slowly added to avoid tissue floating, and the flasks were placed in a humidified
atmosphere of 5% CO2. When the cells overflowed from the
conjunctival tissue and 80% confluency was reached under inverted microscope,
the cells were routinely subcultured.
Immunofluorescence Staining The conjunctival tissues were fixed
in 10% formalin for 48h, then rinsed with water, dehydrated with different
concentrations of ethanol, and made transparent with ethanol and xylene. The
tissues were then dipped in wax, then embedded and sliced to a thickness of 4-7
μm. After dewaxing, the slices were repaired with 0.01 mol/L sodium citrate
buffer for 15min, and then incubated with primary antibody (dilution, 1:150) at
Enzyme-linked Immunosorbent
Assay Cells at generation 3-6 were
selected for the logarithmic growth period. After the cells grew to 90% in the
culture medium, they were washed with PBS two times, and 500 μL lysate liquid
was added. After centrifugation, the supernatant was placed in an aseptic EP
tube. The relative concentration was detected with ELISA kits (R&D,
Minneapolis, MN, USA). The enzyme labeling was detected at a wavelength of 450
nm. The absorbance (A450) OD value of each well was measured according to the
manufacturer’s protocol.
Western Blotting Analysis Total
protein was isolated from the cells lysed with cell lysis reagent (Cell
Signaling Technology, US). Protein quantification was performed using BCA
assays. Twenty micrograms of total protein were subjected to 10% SDS
polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to
polyvinylidene difluoride membranes (PVDF; EMD Millipore, Bedford, MA, USA).
The PVDF membranes were blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich) and washed three times with Tris-buffered saline containing
Tween (TBST; Beyotime Institute of Biotechnology). The membranes were then
incubated with specific primary antibodies anti-human p38 MAPK, p-p38 MAP, ERK,
p-ERK, JNK, p-JNK, and rabbit monoclonal anti-human GAPDH antibodies (Santa
Cruz Biotechnology) overnight at
RT-PCR Total RNA was extracted from the
fibroblasts of the normal control conjunctiva and CCH samples with TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific). RNA was then converted to
cDNA with a Prime Script RT Master Kit (Invitrogen; TaKaRa, Tokyo, Japan).
PT-PCR was performed with SYBR Premix Ex Taq II (Invitrogen; TaKaRa) on an ABI
Real-Time PCR system (Applied Biosystems, Thermo Fisher Scientific). The
relative expression levels of each cell line in each group were measured using
the 2-ΔΔCt methods.
Statistical Analysis All statistical analyses were performed
using the Statistical Package of Social Sciences software (version 23.0; SPSS,
Chicago, IL, USA). The data were presented as means±standard deviations (SD)
for all patients. Groups were tested for normal distribution with the
Shapiro-Wilk test and for variance homogeneity with Levene’s test. Differences
between two paired data sets were compared with a two-sample t-test. All
statistical significance were considered a value of P<0.05.
RESULTS
The green fluorescence area of p-ERK
in CCH group (677.70±49.97 μm2) was significantly larger than that
in CON group (448.47±86.97 μm2; P=0.0146). The green
fluorescence area of p-JNK in CCH group (730.85±45.16 μm2) was
significantly larger than that in CON group (353.73±76.54 μm2; P=0.0340).
The green fluorescence area of p-p
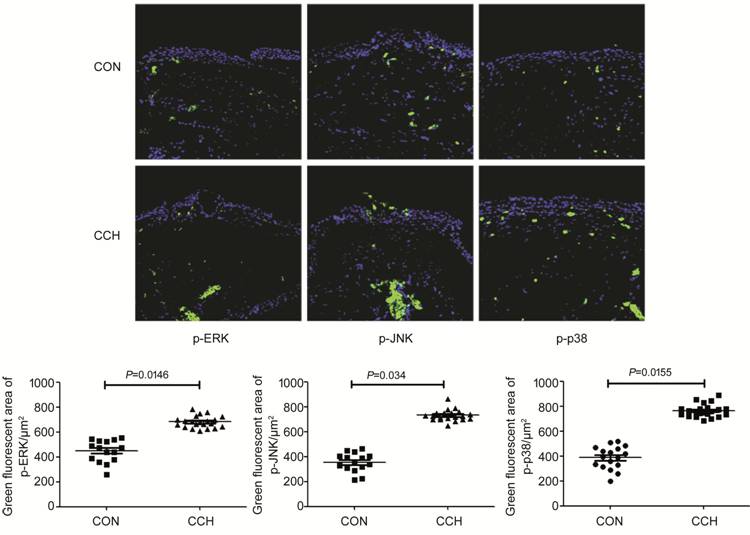
Figure 2 Immunofluorescence staining
of conjunctival tissue.
To investigate the expression of
MAPKs protein in the conjunctival fibroblasts, we used ELISA to detect the
protein levels. The OD values of p38 MAPK, p-p38 MAPK, JNK, p-JNK, ERK, and
p-ERK in the conjunctival fibroblasts were significantly higher in CCH group
than those in CON group (p38 MAPK: P=0.047, p-p38 MAPK: P=0.016,
JNK: P=0.047, p-JNK: P=0.009, ERK: P=0.047, p-ERK: P=0.028;
Table 1). The expression of p-p38 MAPK, p-JNK, and p-ERK protein was
significantly higher in fibroblasts of CCH group than that of CON group (p-p38
MAPK: t=-2.809, P=0.048; p-JNK: t=-5.470, P=0.005;
p-ERK: t=-2.891, P=0.045). The expression of p38 MAPK, JNK, and
ERK proteins in fibroblasts was higher of CCH group than that of CON group
without statistically significance (p38 MAPK: t=-1.321, P=0.257;
JNK: t=-1.582, P=0.189; ERK: t=-1.481, P=0.213;
Figure 3).
Table 1 OD values of MAPKs and their
phosphorylation levels in fibroblasts mean±SD,
OD450 nm
|
Group |
n |
p38 |
p-p38 |
JNK |
p-JNK |
ERK |
p-ERK |
|
CON |
6 |
2.51±1.21 |
0.09±0.02 |
1.44±0.44 |
0.21±0.06 |
1.56±0.51 |
1.02±1.04 |
|
CCH |
6 |
3.98± |
0.15± |
1.95± |
0.33± |
2.40± |
2.87± |
|
Z |
|
-1.984 |
-2.402 |
-1.984 |
-2.611 |
-1.984 |
-2.193 |
|
P |
|
0.047 |
0.016 |
0.047 |
0.009 |
0.047 |
0.028 |
CCH
vs CON group, aP<0.05.
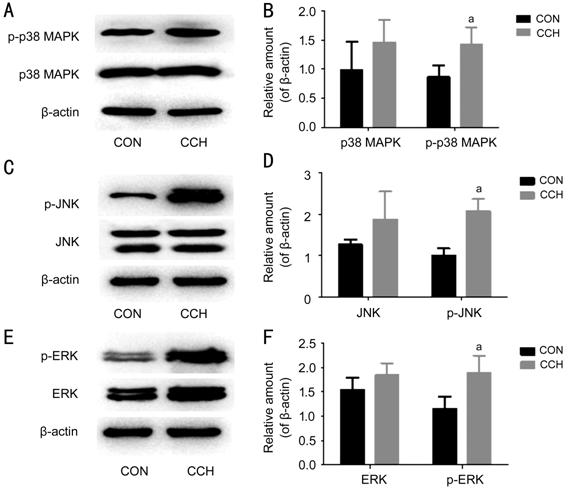
Figure 3 Expression of MAPKs protein
in fibroblasts CCH vs CON group, aP<0.05.
The total expression of MAPKs mRNA in
fibroblasts of CCH group was significantly higher than that of CON group (p38
MAPK: P=0.019; JNK: P=0.010; ERK: P=0.028; Table 2).
Table 2 Expression of MAPKs mRNA in
fibroblasts
mean±SD,
normalized to GAPDH
|
Group |
n |
p38 MAPK |
JNK |
ERK |
|
CON |
6 |
1.00±0.00 |
1.00±0.00 |
1.00±0.00 |
|
CCH |
6 |
7.41± |
1.98± |
4.17± |
|
t |
|
-3.806 |
-4.632 |
-3.358 |
|
P |
|
0.019 |
0.010 |
0.028 |
CCH vs CON group, aP<0.05.
DISCUSSION
CCH is an ocular surface disease accompanied
by redness, dryness, irritation, epiphora and blurry vision[13].
The tear microenvironment then changes, owing to excessive conjunctival
relaxation with or without high tension of the lower eyelid[14].
CCH is generally considered a condition affecting the older population with CCH
severity increasing with age[15]. Mimura et al[16] have reported a higher prevalence of 75.5% in a
hospital-based Japanese population. CCH is most often located in the nasal and
temporal regions of the inferior conjunctiva[17].
Although CCH will not cause blindness, it strongly affects the vision-related
quality of life. So it is necessary to clarify the mechanism of CCH and improve
treatment methods.
Recent studies have shown that
redundant conjunctiva results in the instability of tear film and dysfunction
of tear meniscus, which cause disrupted tear flow, delayed tear clearance and
increased tear osmotic pressure. All the above factors can cause ocular surface
inflammation, leading to dry eye[18]. Despite the
high prevalence of CCH, particularly among the elderly[1-2,16], the exact mechanisms involved in
CCH pathogenesis have remained unknown. The gradual dissolution of Tenon’s
capsule is widely expected to lead to the loss of subconjunctival adhesion and
conjunctival thinning and stretching, which is the main pathological mechanism
of CCH[6-7]. Moreover, the
degradation of collagen fibers and fibroblast apoptosis, which cause decreased collagen
synthesis, lead to thinning of the conjunctival tissue together. Zhang et al[7] have reported decreased elastic fiber and chronic
inflammation lead to CCH.
MMPs play a critical role in wound
healing, tissue remodeling, and many diseases, including ocular surface
diseases. Acera et al[19] have reported
that pro-MMP-9 levels are significantly higher in CCH and then decrease
significantly after resection of loose conjunctiva. MMP-1, MMP-3, and MMP-9 were
thought to participate in the CCH pathogenesis. Li et al[9] have reported up-regulation of MMP-1 and MMP
Recently MAPKs have been reported to
increase the levels of MMPs in some diseases[20].
ERKs function is to control cell division, whereas JNKs are key regulators of
transcription. The p38 MAPKs are activated by inflammatory cytokines and
environmental stresses and involved in cell apoptosis and senescence[21]. Yang et al[22]
have reported that activation of the ERKs/JNKs signaling pathways contributes
to the up-regulation of MMP-9. Moreover, MMP-9 may be one of the most important
molecules in cancer cell metastasis. Aroui et al[23]
have shown that naringin could attenuate the MAPK signaling pathways, such as
the ERKs, JNKs and p38 MAPKs, decrease the expression and enzymatic activities
of MMP-2, MMP-9, thus inhibiting the metastasis of U87 cells. Simon et al[24] have found that p38 and MKK-6 isoform mutants
decrease the MMP-9 levels in vitro in UM-SCC-1 cells.
However, previous studies have not
elucidated the MAPKs expression and the relationship between MAPKs and MMPs in
CCH. Our study showed that the expression of p-ERK, p-JNK, and p-p38 of
conjunctival tissue in CCH group was apparently higher than that in CON group.
In cultured conjunctival fibroblasts, the OD values of p38 MAPK, JNK, ERK, and
their phosphorylation in CCH group were apparently higher than those in CON
group (P<0.05). The protein expression of phosphorylated p38 MAPK,
JNK, and ERK in CCH group was apparently higher than that in CON group (P<0.05).
The total expression of p38 MAPK, JNK, and ERK mRNA of the fibroblasts in CCH
group was significantly higher than that in CON group (P<0.05). Thus,
the MAPKs expression of conjunctival tissue and human conjunctival fibroblasts
in CCH group were higher than those in CON group. These differences might also
cause up-regulation of MMPs expression. The MAPK signaling pathways regulate
the abundance and activity of MMP-9 by activating transcription factors such as
NF-κB and AP
In conclusion, we found the
up-regulation of p38 MAPK, JNK, ERK proteins and mRNA in CCH loose conjunctival
tissue and fibroblasts, which would also activate the expression of MMPs. This
up-regulation might cause the degradation of collagen fibers and elastic fibers
and promote CCH. Our results improve the understanding of the pathological
mechanism underlying CCH. But this study also has some limitations, and further
experiments are needed to establish a direct link between MAPKs and MMPs in
CCH. In addition, a larger sample size may be needed to adequately detect
differences in the future.
ACKNOWLEDGEMENTS
Foundations: Supported by the Research Project of
Health and Family Planning Commission in Shanghai (No.201840196); Yingcai
Program of Putuo Hospital (No.2017202B); Yuying Program of Putuo Hospital (No
Conflicts of Interest: Jia YL, None; Liu XJ, None; Wen H,
None; Zhan YP, None; Xiang MH, None.
REFERENCES