
·Letter to the Editor·
Primary
implantation of non-valved glaucoma-drainage-device in sulcus in iridocorneal
endothelial syndrome
Vanita Pathak Ray1, Divya P Rao2,
Isha Gulati2
1Centre For
Sight, Banjara Hills, Hyderabad 500034, India
Correspondence
to: Vanita
Pathak Ray. Centre For Sight, Road No.2, Banjara Hills, Hyderabad 500034,
India. vpathakray@gmail.com
Received:
DOI:10.18240/ijo.2019.11.23
Citation: Pathak
Ray V, Rao DP, Gulati I. Primary implantation of non-valved
glaucoma-drainage-device in sulcus in iridocorneal endothelial syndrome. Int
J Ophthalmol 2019;12(11):1809-1811
Dear Editor,
Iridocorneal
endothelial syndrome (ICE) is a rare, usually unilateral, acquired condition,
hypothesized to be secondary to a viral etiology[1].
It affects females more often than males and comprises of three distinct
clinical types related to endothelial proliferation and its structural
abnormalities. Proliferation of endothelium over the iridocorneal angle leads
to progressive secondary angle closure and that over the iris leads to typical
changes of polycoria and atrophy. Three clinical entities included in the
syndrome are Chandlers (predominant corneal involvement), progressive iris
atrophy (predominant iris involvement with polycoria and ‘holes’) and Cogan
Reese (iris nodules with loss of stromal features). No matter what the clinical
type, it is a progressive condition and controlling intraocular pressure (IOP)
and maintaining corneal clarity in the long term is usually a challenge.
When
glaucoma becomes medically refractory, as it frequently does, then surgical
management is indicated. Trabeculectomy with anti-fibrotics have been tried;
one study reported failure in one-third and loss of corneal clarity in half of
the cohort consisting of 16 eyes, with an average of 1.3 (SD 0.5) glaucoma
surgeries per eye[2]. Yet others have met with a
little more success (8 out of 10 eyes)[3].
Nonetheless Doe et al[4] have reported a
decline in success rate to 29% at 5y (vs 73% at 1y) in the group
receiving trabeculectomy with anti-fibrotics; in the same study success rate
was almost double in the group that had a glaucoma drainage device (GDD)
implanted (53% at 5y).
Each eye in
our small series presented with uncontrolled IOP and corneal edema and were
referred for combined glaucoma and corneal surgery. Presence of corneal edema
precluded acquisition of specular image of the endothelium. Nonetheless, we
chose to use our above-mentioned approach (primary non-valved GDD surgery, tube
implanted in the sulcus), having counselled each patient for possible need of a
second procedure (keratoplasty). We implanted Aurolab Aqueous Drainage Implant
(AADI, Aurolab, India) which is a relatively new, affordable non-valved GDD,
the design inspiration of which is the Baerveldt Glaucoma Device
Routine
non-valved AADI surgery was performed[5]; notably
tube was occluded with non-permanent 6/0 polyglactin suture and 4 pairs of
fenestrating vents were made anterior to the occlusive ligature. All plates
were positioned in the supero-temporal pocket and fixed to the sclera
A total of 7
eyes of 6 patients of ICE with corneal edema and uncontrolled IOP, underwent
AADI with sulcus placement of tube in the study period of July 2014 to January
2017. However, 3 eyes had previous filtration surgery and were therefore
excluded. Four eyes of 3 patients with primary AADI surgery with tube placed in
sulcus, were included with a median follow up of 14mo. All were females and one
had bilateral ICE (Figure
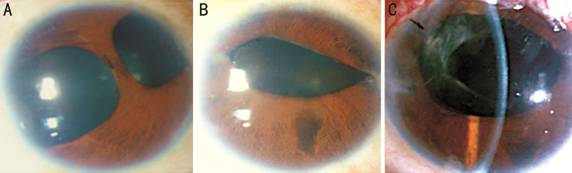
Figure 1
Bilateral ICE with bilateral corneal edema, more in left eye (A, B);
postoperative (3wk) clear cornea of same eye as 1B, with AADI tube in sulcus
(C).
Median age
was 45y (Q1 32.7, Q3 48; IQR 15.3). One eye was pseudophakic, whereas the rest
presented with early cataract, so underwent routine phaco surgery with
in-the-bag IOL for unhindered positioning of the tube in the sulcus. The
pre-operative uncontrolled median IOP
Although,
median logMAR best corrected visual acuity (BCVA) remained unchanged (P=0.5)
both eyes of the patient with bilateral ICE (Figure 1) with very advanced
glaucoma had preservation of central vision (count fingers and hand movements
in right and left eye respectively), but BCVA of the rest two eyes improved.
BCVA of the
eye in Figure 2 improved from 20/30 to 20/20; contributory factors for
improvement were resolved corneal edema with controlled IOP and cataract
extraction. Humphrey Field Analysis (HFA) 24-2 was done pre-operatively and
Mean deviation (MD) was recorded as
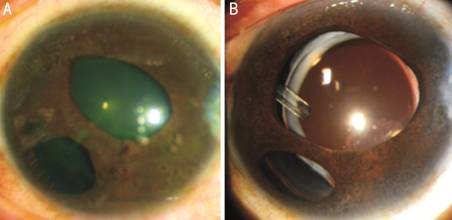
Figure 2
Essential iris atrophy of ICE A: Pre-operative corneal edema with
hazy details of the iris; B: AADI tube in sulcus and clear cornea (2y
postoperative).
We did not
encounter blockage, retraction or migration of tube and there were no
re-procedures or tube revision surgeries required in these cases. Most notably,
corneal edema resolved in all eyes (Figures
In the study
reported by Doe et al[4], the group that
received GDD had 6 eyes with primary tube surgery, and though not analysed
separately by the authors themselves, 5 eyes had IOP control with clear cornea
at last follow-up. Therefore, intuitively, it would appear that a GDD may be a
better option but success in terms of IOP control alone may not translate into
one that encompasses corneal clarity too. This was the experience of Kim et
al[6] who had 70% success rate at 55mo with
GDD surgery, albeit with a few tube revisions but they found that maintaining
corneal clarity was a challenge. The 60% of eyes in their series decompensated;
of a further 3 eyes which had grafts, one failed. All eyes in their series were
phakic and all tubes were placed in the anterior chamber (AC) and 80% patients
in their cohort had previous trabeculectomy, 60% with anti-fibrotics.
In view of
these findings we hypothesized that a primary implantation of GDD in sulcus may
not just have the advantage of IOP control but could also serve the following
purposes. It would be farthest away from the corneal endothelium, as far as
possible by an anterior approach, thereby retarding loss of endothelial cells
compared to one placed in the AC. Furthermore, in the PC, it would also be away
from the proliferating endothelium, minimizing any chance of significant
retraction or migration with subsequent need for repositioning, as reported by
Doe et al[4] and Kim et al[6].
As IOP rise
in ICE is chronic in nature, it is commonly believed that loss of corneal
clarity prior to any intervention is likely due to endothelial failure. Yet
with control of IOP alone we achieved and maintained clear corneas in all cases
till last follow-up. This not only helped avoid unnecessary surgery in the
first instance, but also deferred keratoplasty indefinitely. We, therefore,
strongly recommend surgical control of IOP first and foremost with primary GDD
surgery with tube in sulcus. The eye needs to be pseudophakic for this purpose
and presence of cataract in phakic eyes, in our series, aided the process. We
realise that this may become a contentious issue should cataract not be present.
Our series
of ICE eyes with uncontrolled IOP and corneal edema, referred for combined
glaucoma and corneal surgery, did well with control of IOP alone with
non-valved GDD in sulcus as primary surgery. We recognise that our series is
very small with limited follow-up. Nonetheless, it is a significant small and
successful step, hitherto unreported, in a relatively rare condition known to
be difficult-to-treat.
ACKNOWLEDGEMETNS
Conflicts of
Interest: Pathak Ray V,
None; Rao DP, None; Gulati I, None.
REFERENCES