
·Basic
Research·
High
glucose causes apoptosis of rabbit corneal epithelial cells involving
activation of PERK-eIF2α-CHOP-caspase-12 signaling pathway
Pan-Pan Yao1, Min-Jie Sheng1,
Wen-Hao Weng2, Yin Long2, Hao Liu1, Li Chen1,
Jia-Jun Lu3, Ao Rong3, Bing Li1
1Department of Ophthalmology, Yangpu
Hospital, Tongji University School of Medicine, Shanghai 200090, China
2Department of Clinical Laboratory,
Yangpu Hospital, Tongji University School of Medicine, Shanghai 200090, China
3Department of Ophthalmology, Tongji
Hospital, Tongji University School of Medicine, Shanghai 200065, China
Co-first authors: Pan-Pan Yao and Min-Jie Sheng
Correspondence to: Bing Li. Department of
Ophthalmology, Yangpu Hospital, Tongji University School of Medicine, No.450,
Tengyue Road, Shanghai 200090, China. bing.li@tongji.edu.cn
Received:
Abstract
AIM: To investigate the effect of high concentration of glucose (HCG) on
double stranded RNA-activated protein kinase-like ER kinase (PERK)-eukaryotic
initiation factor-2α (eIF2α)-transcription
factor C/EBP homologous protein (CHOP)-cysteine aspartate specific proteinase
(caspase-12) signaling pathway activation and apoptosis in rabbit corneal
epithelial cells (RCECs).
METHODS: RCECs were treated by different concentrations of
glucose for 0-48h. The expressions of PERK, p-PERK, eIF2α, p-eIF2α, 78 kDa glucose-regulated
protein 78 (GRP78), CHOP, B-cell lymphoma 2 (Bcl-2), B-cell
lymphoma-2-associated X protein (Bax) and caspase-12 were determined by Western
blot. Apoptosis was detected by TUNEL assay. Meanwhile, the function of
PERK-eIF2α-CHOP-caspase-12
signaling pathway activation in high glucose-induced apoptosis was evaluated
using PERK inhibitor, GSK2606414.
RESULTS: HCG significantly promoted the expression of
p-PERK, p-eIF2α,
GRP78, CHOP, Bax and cleaved caspase
CONCLUSION: HCG activates PERK-eIF2α-CHOP-caspase-12 signaling
pathway and promotes apoptosis of RCECs.
KEYWORDS: high glucose; rabbit corneal
epithelial cells; PERK-eIF2α-CHOP-caspase-12 pathway; apoptosis
DOI:10.18240/ijo.2019.12.01
Citation: Yao
PP, Sheng MJ, Weng WH, Long Y, Liu H, Chen L, Lu JJ, Rong A, Li B. High glucose
causes apoptosis of rabbit corneal epithelial cells involving activation of
PERK-eIF2α-CHOP-caspase-12 signaling pathway. Int J Ophthalmol
2019;12(12):1815-1822
INTRODUCTION
Dry eye is an irreversible and
chronic progressive ocular surface disease characterized by tear film
instability and ocular surface damage, accompanying by visual dysfunction and
ocular surface discomfort, which seriously affect life quality of the patients[1]. Tear glucose in diabetic patients was relatively
higher (about 5-fold than normal). Moreover, tear glucose was positively
correlated with blood glucose in diabetic patients[2].
Till now, it is yet to cure this disease completely, especially diabetes-related
dry eye. Therefore, finding the molecular mechanism between diabetes and dry
eye is the key to prevent and treat diabetes-related dry eye.
Hyperglycemia impacts healthy
condition of tear film. Once the stability of its inner environment cannot be
compensated, dry eye might occur and develop. Diabetes-related dry eye includes
decreased tear secretion, change of tear composition, decreased corneal
sensitivity, and prevented corneal epithelial regeneration[3].
At the same time, tear osmotic pressure is promoted due to the high
concentration of glucose (HCG), eliciting ocular surface damage[4]. The incidence of diabetes-related dry eye accounts for
about 52.8%-70% at present and high glucose condition was thought to be primary
reason for dry eye[5].
Histochemical analysis revealed that
goblet cell loss and corneal epithelial cell injury in patients with dry eyes
correlate with apoptosis[6], while endoplasmic
reticulum stress (ER stress) is an important pathway closely related to the
functional failure and apoptosis complicated by various diseases, including
diabetes[7]. The accumulation of glycotoxic
metabolites in the tears of diabetic patients may lead to emergence of a series
of damage factors, such as oxygen free radicals and calcium ion overloading,
which may trigger severe ER stress and even final apoptosis of the ocular cells[8]. The conditions within the endoplasmic reticulum are
monitored by the unfolded protein response (UPR) signaling pathway[9], and activation of the UPR restores protein folding
homeostasis by reducing protein translation, increasing endoplasmic reticulum
chaperone expression, and degrading misfolded proteins. However, prolonged ER
stress fails to repair protein homeostasis and activates apoptotic signaling
pathway once UPR does not sustain the balance of protein production and calcium
in the endoplasmic reticulum[10].
Double stranded RNA-activated
protein kinase-like ER kinase (PERK)-eukaryotic initiation factor-2α
(eIF2α)-transcription factor C/EBP homologous protein (CHOP)-cysteine aspartate
specific proteinase (caspase-12) signaling pathway is the key branch of ER
stress to activate downstream signal transduction of apoptosis. In this study,
we used an in vitro model to explore the activation of
PERK-eIF2α-CHOP-caspase-12 signaling pathway in high glucose condition and
investigate its role in the apoptosis of rabbit corneal epithelial cells
(RCECs).
MATERIALS AND METHODS
Ethical Approval The experimental protocol was
approved by the Ethics Committee of the Yangpu Hospital, Tongji University
Medical School. All animal procedures and experiments were approved by the
Animal Care and Use Committee in Tongji University. All animals were cared
according to the Association for Research in Vision and Ophthalmology Statement
for using animals in ophthalmic and vision research. Surgeries were performed
under anesthesia by sodium pentobarbital, and all efforts were made to minimize
animal suffering.
Cell Culture Totally forty New Zealand rabbits
(weight: 2.25±
Groups and Treatments RCECs were seeded at 4×105/well
into a 6-well plate and maintained in medium as described previously for
different treatments[11]. The cells were treated
with different concentrations of glucose for different periods (0-48h). The
osmotic pressure was equalized using different concentrations of D-mannitol.
The function of PERK-eIF2α-CHOP-caspase-12 signaling pathway activation in high
glucose-induced apoptosis was evaluated using PERK inhibitor, GSK2606414
(Millipore Corporation, Billerica, MA, USA). Tunicamycin (Tm; Abcam, Cambridge,
MA, USA) was used as the positive agent to induce ER stress. Therefore, the
experiments were divided into blank control (BC), normal concentration of
glucose (NCG, 5.5 mmol/L glucose), osmotic pressure control (OPC, 27.5 mmol/L
D-mannitol+5.5 mmol/L glucose), HCG (33 mmol/L glucose) and Tm (100 nmol/L)
groups (Table 1). Additionally, in each group, a subgroup with GSK2606414 (10
nmol/L) was set. To observe the concentration-dependent effect of glucose, the
experiment was also divided into 0, 5, 15, 25, 35 and 45 mmol/L glucose. To
observe the time-dependent effect of glucose, the cells were also treated by 33
mmol/L glucose for 0, 12, 24, 36 and 48h respectively.
Table 1 Cell groups and treatment
|
Groups |
Treatments |
|
BC |
— |
|
NCG |
5.5
mmol/L glucose |
|
OPC |
27.5
mmol/L D-mannitol+5.5 mmol/L glucose |
|
HCG |
33
mmol/L glucose |
|
Tm |
100
nmol/L Tm |
BC: Blank control; NCG: Normal
concentration of glucose; OPC: Osmotic pressure control; HCG: High concentration
of glucose; Tm: Tunicamycin.
Western Blotting Cell lysates of each well were
collected directly using 120 μL 1×sodium dodecyl sulphate (SDS) loading buffer
(Proteintech, Rosemont, IL, USA) and then sonicated. After heating at
TUNEL Assay RCECs were cultured on sterile
coverslips placed in 6-well plates. After 24h treatment, the cell slides were
washed with PBS once and fixed with 4% paraformaldehyde (PFA) for 30min. And
then, the cell slides were incubated with 0.3% Triton X-100 for 30min at room
temperature after washing 3 times. Apoptosis was detected using the TUNEL kit
(Roche Molecular Biochemicals, Mannheim, Germany) according to the
manufacturer’s instruction. The cell nucleus were staining with
Statistical Analysis All data were presented as means±
standard deviation (SD). Statistical and image analysis was performed with
GraphPad Prism version 7.00 (GraphPad Software; San Diego, CA, USA) and Image-J
program. One-way analysis of variance (ANOVA) with multiple comparisons was
used to detect differences between groups. For all tests, a difference was
considered to be significant at P<0.05.
RESULTS
High Glucose Activated
PERK-eIF2α-CHOP-caspase-12 Signaling Pathway RCECs
were treated with different concentration of glucose and expression of
PERK-eIF2α-CHOP-caspase-12 signaling pathway-related protein was measured by
Western blot. As shown in Figure 1, HCG group remarkably increased the
expression of p-PERK and p-eIF2α compared with NCG group and OPC group (P<0.05),
whereas the increase was partially blocked by PERK inhibitor, GSK2606414 (P<0.05).
As the positive control, Tm also increased the expression levels of p-PERK and
p-eIF2α (P<0.05), which were also inhibited by GSK2606414 (P<0.05).
In addition, the expressions of p-PERK and p-eIF2α increased significantly in
the OPC group than those in the NCG group (P<0.05), while their
expression decreased significantly in the OPC+GSK group (P<0.05). The
PERK and eIF2α expression were not altered in different groups and by
GSK2606414 (P>0.05).
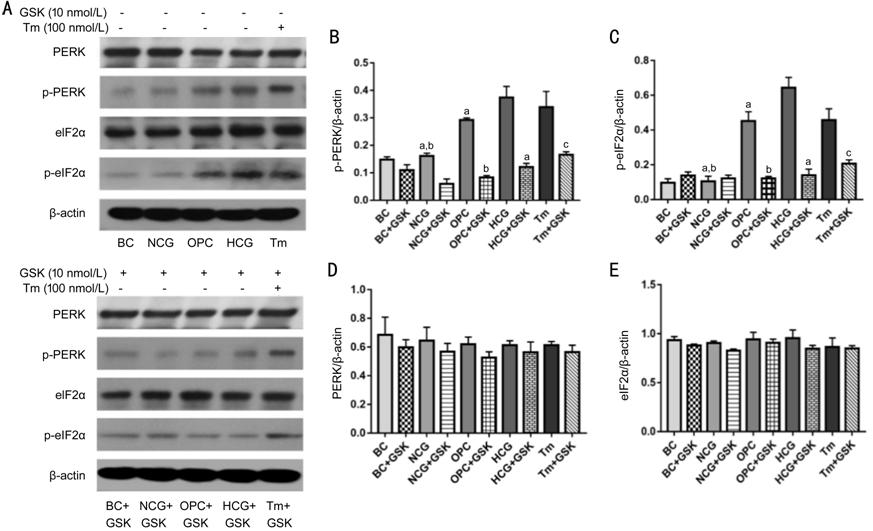
Figure 1 High glucose activated
PERK-eIF2α-CHOP-caspase-12 signaling pathway A: Blots of different proteins; B:
Quantification of p-PERK; C: Quantification of p-eIF2α; D: Quantification of
PERK; E: Quantification of eIF2α. Compared with NCG group and OPC group, the
expression level of p-PERK and p-eIF2α in corneal epithelial cells of HCG group
was significantly up-regulated, which was reduced by application of GSK2606414
(aP<0.05 vs HCG); compared with NCG group, the
expression of p-PERK and p-eIF2α in OPC group was up-regulated, and GSK2606414
treatment down-regulated the expression of p-PERK and p-eIF2α (bP<0.05
vs OPC); GSK2606414 treatment also reduced the expression level of p-PERK
and p-eIF2α in Tm condition (cP<0.05 vs Tm); while
PERK and eIF2α expressions have no statistically significant difference among
groups (P>0.05). Statistical analysis of Western blot was represented
as mean±SD. Tm: Tunicamycin.
Osmotic pressure was then set
equally using D-mannitol to assess the concentration-dependent effect of
glucose on the expression of PERK-eIF2α-CHOP-caspase-12 signaling
pathway-related protein in RCECs. As shown in Figure 2, the protein
level of p-PERK and p-eIF2α increased firstly then decreased as the
concentration of glucose increased (P<0.05). In this condition,
expression of GRP78, CHOP, Bax, cleaved-caspase-12 kept increasing (P<0.05),
while expression of Bcl-2 and caspase-12 decreased with the increase of glucose
concentrations (P<0.05).
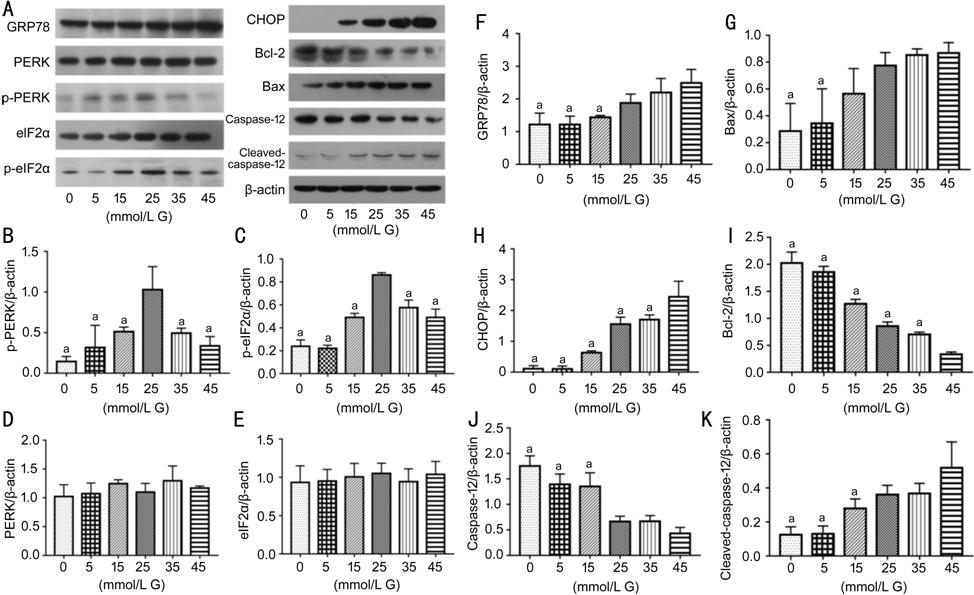
Figure 2 The effects of different
concentrations of high glucose on PERK-eIF2α-CHOP-caspase-12 signaling pathway and
apoptosis-related proteins A:
Blots of different proteins; B: Quantification of p-PERK; C: Quantification of
p-eIF2α; D: Quantification of PERK; E: Quantification of eIF2α; F:
Quantification of GRP78; G: Quantification of Bax; H: Quantification of
CHOP; I: Quantification of Bcl-2; J: Quantification of caspase-12; K:
Quantification of cleaved-caspase-12. As glucose concentration increase but not
osmotic pressure, the expression of p-PERK and p-eIF2α increased firstly but
then decreased, and their expressions peaked when RCECs treated with 25 mmol/L
glucose for 24h (aP<0.05 vs 25 mmol/L G). PERK and
eIF2α expressions have no statistically significant difference among groups (P>0.05).
While the expression of GRP78, CHOP, Bax and cleaved-caspase-12 increased after
glucose treatment in a concentration-dependent manner (aP<0.05
vs 45 mmol/L G); Bcl-2 and caspase-12 expressions declined, their
changes were correlated negatively with the concentrations of glucose (aP<0.05
vs 45 mmol/L G). Date were represented as mean±SD. G: Glucose.
We then investigated the
time-dependent effect of glucose on the expression of
PERK-eIF2α-CHOP-caspase-12 signaling pathway-related protein in RCECs. As shown
in Figure 3, 33 mmol/L glucose elevated the protein level of p-PERK and p-eIF2α
with time from 0 to 48h (P<0.05). In this condition, expression of GRP78,
CHOP, Bax, cleaved-caspase-12 kept increasing (P<0.05), while
expression of Bcl-2 and caspase-12 decreased with the prolonged treatment time
(P<0.05). These results revealed that the expression of
PERK-eIF2α-CHOP-caspase-12 pathway-related molecules in RCECs was significantly
promoted or inhibited in a dose- and time-dependent manner.
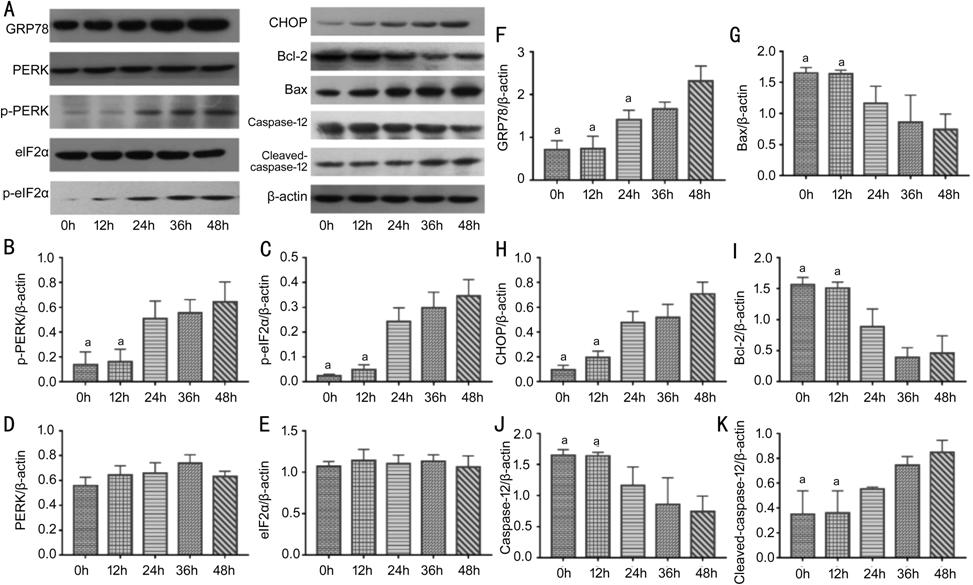
Figure 3 The effects of high glucose
on PERK-eIF2α-CHOP-caspase-12 signaling pathway and apoptosis-related proteins after treatment for
different periods A:
Representative blots of different proteins; B: The expression of p-PERK; C: The
expression of p-eIF2α; D: The expression of PERK; E: The expression of eIF2α;
F: The expression of GRP78; G: The expression of Bax; H: The expression
of CHOP; I: The expression of Bcl-2; J: The expression of caspase-12; K: The
expression of cleaved-caspase-12. The expression of p-PERK, p-eIF2α, GRP78,
CHOP, Bax and cleaved-caspase-12 increased with time and there was a positive
correlation between these molecular expression and time (aP<0.05
vs 48h); while Bcl-2 and caspase-12 expression declined, which have a
negative correlation with time (aP<0.05 vs 48h); PERK
and eIF2α expressions have still no statistically significant difference among
groups (P>0.05). The data were represented as mean±SD.
High Glucose Elicited Apoptosis
Through Activating PERK-eIF2α-CHOP-caspase-12 Signaling Pathway The ratio of TUNEL-positive RCECs
was both very low in the BC and NCG group basically (Figure 4). Compared
with OPC group, the apoptotic rate in HCG group increased remarkably (P<0.05).
However, GSK2606414 reduced high glucose-induced apoptosis (P<0.05).
RCECs in OPC and Tm group have the same change after adding GSK2606414 (P<0.05).
The percentage of TUNEL-positive RCECs in the OPC group increased significantly
than that in the NCG group (P<0.05). No significant difference in the
percentage of apoptotic RCECs were observed among the BC, NCG, BC+GSK and
NCG+GSK group (P>0.05).
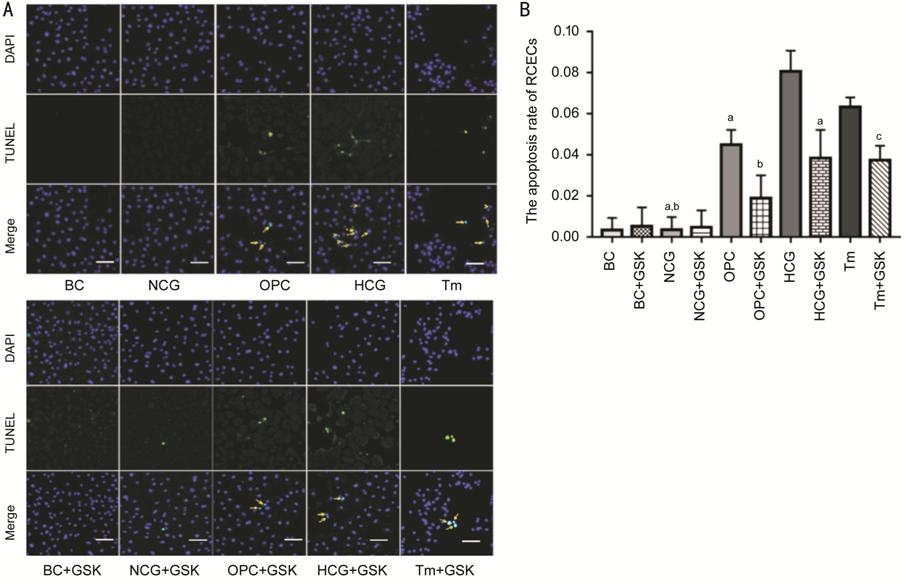
Figure 4 The apoptosis rate of RCECs
was evaluated by TUNEL assay A: Representative images of TUNEL
staining. The magnification of images were 200×. B: The percentage of apoptotic
RCECs in the HCG group was significantly higher compared to those in the NCG
group and the OPC group, but it significantly decreased in the HCG+GSK group (aP<0.05
vs HCG). The percentage of TUNEL-positive RCECs in the OPC group was
higher compared with NCG and OPC+GSK groups (bP<0.05 vs
OPC). The percentage of apoptotic RCECs in the Tm group was significantly
higher than those in the Tm+GSK group (cP<0.05 vs Tm).
Data were represented as mean±SD. Tm: Tunicamycin.
The percentage of TUNEL-positive
RCECs increased as concentration of glucose increased when the osmotic pressure
was set equally in all group (P<0.05; Figure 5). These data furtherly
revealed that HCG elicited apoptosis through activating
PERK-eIF2α-CHOP-caspase-12 signaling pathway.
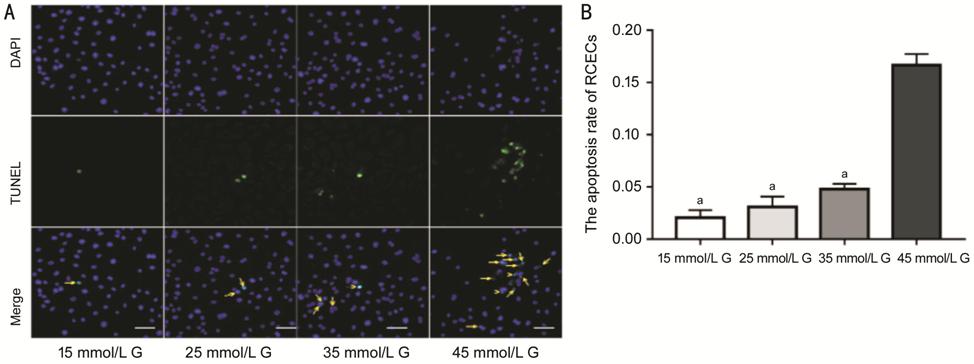
Figure 5 The apoptosis rate of RCECs
after treatment with different concentrations of glucose A: Representative images of TUNEL
staining. The magnification of images were 200×. B: With the increase of
glucose concentration, the percentage of apoptotic RCECs increased (aP>0.05
vs 45 mmol/L G). The data were expressed as mean±SD. G: Glucose.
DISCUSSION
Severe dry eye is featured by
delayed epithelial regeneration, persistent corneal epithelial defects, or
other complications after ocular surgery[13-14]. Dry eye in diabetic patients is probably more
related to diabetic neuropathy[2]. In this study,
we systemically assessed the potential effects of high glucose and hyperosmosis
on PERK-eIF2α-CHOP-caspase-12 signaling pathway and ER stress-dependent
apoptosis in RCECs. Our data revealed that high glucose more than the inner
hyperosmosis activated PERK-eIF2α-CHOP-caspase-12 signaling pathway to elicit
ER stress-dependent apoptosis of RCECs.
In this study, RCECs were treated
with different concentrations of glucose to verify that high-glucose with the
impact of inner osmotic pressure excluded can activate
PERK-eIF2α-CHOP-caspase-12 pathway but not normal glucose, which induce
apoptosis of RCECs. When ER suffers from stress, dissociation of the GRP78
triggers dimerization, oligomerization, autophosphorylation and activation of
PERK[15]. PERK phosphorylates the alpha subunit
of eIF2α, leading to inhibition of protein synthesis[16-17]. Our previous study demonstrated that p-PERK in high
glucose-treated RCECs is significantly higher than those in the normal or the
osmotic pressure control group. In the present study, we furtherly demonstrated
that the expressions of p-PERK and p-eIF2α increased in high glucose-treated
RCECs, which were reduced by PERK inhibitor, GSK2606414. Previous studies on
neurons have shown that the activation of PERK-eIF2α pathway can be blocked by
GSK2606414, which result in neurological rehabilitation of model animals with
neurodegenerative diseases[16-17].
The increase of glucose concentration promoted the expression of p-PERK and
p-eIF2α firstly but then turned down those protein expressions. In combination
with the time-dependent effect of glucose, these data revealed that the
PERK-eIF2α-CHOP-caspase-12 pathway was activated in high glucose-treated RCECs
and influenced by the concentration and action time of glucose.
CHOP is the downstream of p-PERK and
considered as a marker of cell death[18]. In
normal conditions, the expression of CHOP is quite low while the expression of
CHOP would be remarkably increased under the ER stress[19].
CHOP interferes the formation of protein disulfide bonds by inducing disorder
of the expression of endoplasmic reticulum oxidoreductases 1α (ERO1)[20], which reduce protein folding and lead to the
accumulation of unfolded proteins. Meanwhile, CHOP consumes glutathione and
promotes the production of reactive oxygen species furtherly[21].
Peroxide status of ER will affect the ion channel function on the membrane.
Therefore, calcium inside the ER is released into cytoplasm, which will break
the balance of inner environment and activate the ER -associated calcium
ATPase, protease and nuclease[22]. The
permeability of mitochondrial membrane increases, and its internal components such
as cytochrome C, apoptotic protease activating factor 1 (APAF-1) and apoptosis
inducing factor (AIF) were released into the cytoplasm, triggering
caspase-dependent or independent apoptotic pathways[23].
Procaspase-12 is specifically generated and activated in ER. Calcium
homeostasis is broken and intracellular calcium drained away, then cytoplasmic
calcium-activated protease calpain cleave procaspase-12 to activate caspase-12
during ER stress. Cleaved-caspase-12 is transported from ER to cytoplasm in
order to activate caspase-9 that then lyses caspase-3. Caspase-3 cleaves
polyribose polymerase (PMuP) and multiple intracellular substrates, and the
fragmentation and inactivation of DNA eventually result in programmed cell
death[24-26]. Caspase-12 is
therefore considered as the marker molecule of ER dependent apoptosis.
Therefore, the apoptosis of high glucose-treated RCECs might result from
increasing expression of CHOP, Bax and cleaved-caspase-12 and decreasing
expression of Bcl
We found that the longer time RCECs
treated with high glucose or the higher the glucose concentration, the more
activation of apoptotic signaling pathway. p-PERK and p-eIF2α increased at low
concentration of glucose and began to decrease when it reached the peak. The
tendency was a bit different from others. Since PERK-eIF2α-CHOP-caspase-12 pathway
of ER stress is the upstream signal of cell apoptosis, and it plays a key role
in regulating the life and death of cells under stress, it might be that the
undue consumption of p-PERK and p-eIF2α in RCECs is not enough to resist the
excessive stress of high glucose, so that the expression of apoptotic signaling
protein is relatively more active, and triggers apoptotic signal transduction
finally.
Our previous study also found that
the apoptosis rate in early stage in the HCG group was higher than that in the
normal group assayed by flow cytometry[11]. This
present study revealed that the apoptotic ratio in high glucose was remarkably
higher than that in the normal or osmotic pressure group by TUNEL analysis, and
it was significantly reduced by GSK2606414. The results further proved that
high glucose condition could facilitate apoptosis of RCECs by activating
PERK-eIF2α-CHOP-caspase-12 pathway in profile. We also found that the
percentage of apoptotic RCECs increased as concentration of glucose increased
in dose-dependent manner. It corresponded to the results of Western blot
analysis. Apoptosis was divided into early apoptosis and late apoptosis. The
negative charge of phosphatidylserine in early apoptotic cells transfers from
the inside to the outside of the cell membrane, and then the surface of cell
membrane has changed. Once apoptotic signal transduction is initiated, it will
cause multiple morphological changes of cells by inducing genomic DNA
fragments, which triggers late apoptosis[27-28]. TUNEL assay detect DNA fragment directly, it was a
method to detect late stage of apoptosis. In our present study, there was a
significant difference among groups by TUNEL assay, but the apoptosis rate was
low in all groups. It was speculated that the HCG is prone to induce early
apoptosis of RCECs, which was consistent with the results of the previous study[11].
In summary,
PERK-eIF2α-CHOP-caspase-12 pathways were activated in high glucose-induced
RCECs and mainly involved in the early stages of cell apoptotic. This might
explain why the incidence of dry eye is high in diabetic and dry eye symptoms.
The injury severity of RCECs is related to the concentration or action time of
glucose. This work will help guide the early prevention and treatment of
diabetes-related dry eye.
ACKNOWLEDGEMENTS
Foundations: Supported by Shanghai Natural
Science Foundation (No.19ZR1450500); National Foundation Cultivation Project of
Tongji University (No.22120180285); the Good Physician Training Project of
Yangpu District, Shanghai.
Conflicts of Interest: Yao PP, None; Sheng MJ, None; Weng
WH, None; Long Y, None; Liu H, None; Chen L, None; Lu
JJ, None; Rong A, None; Li B, None.
REFERENCES