
·Basic Research·
Tocilizumab
promotes corneal allograft survival in rats by modulating Treg-Th17 balance
Xiao-Song Wu1, Xiao-Li Lu1, Jing Wu2,
Ming Ma1, Jian Yu1, Zhen-Yu Zhang3
1Department of Ophthalmology, Nanfang
Hospital, Southern Medical University, Guangzhou 510515, Guangdong Province,
China
2Department of Huiqiao Building,
Nanfang Hospital, Southern Medical University, Guangzhou 510515, Guangdong
Province, China
3Guangdong Women And Children
Hospital, Guangzhou 511400, Guangdong Province, China
Co-first authors: Xiao-Song Wu and Xiao-Li Lu
Correspondence to: Jing Wu. Department of Huiqiao
Building, Nanfang Hospital, Southern Medical University, Guangzhou 510515,
Guangdong Province, China. wujingsci@126.com; Ming Ma. Department of
Ophthalmology, Nanfang Hospital, Southern Medical University, Guangzhou 510515,
Guangdong Province, China. maming658587@163.com
Received: 2019-02-01
Accepted: 2019-06-27
Abstract
AIM: To examine the therapeutic effects of tocilizumab on experimental
corneal transplantation and its effect on Treg/Th17 balance.
METHODS: Allograft corneal graft was performed between host
Sprague Dawley and Wistar donor rats. The rats were randomly divided into four
groups: normal, autograft, allograft, and allograft treated with tocilizumab.
Kaplan-Meier was performed to draw the survival curve. The protein levels of
interleukin-17A (IL-17A), vascular endothelial growth factor
(VEGF), and forkhead box protein 3 (Foxp3) were measured by
immunohistochemistry. The mRNA levels of IL-17A, VEGF, retinoid-related orphan receptor gammat
(RORγt), interleukin-6 (IL-6) and
Foxp3 were detected by reverse transcription real-time polymerase chain
reaction (RT-PCR). The Treg and Th17 cells were investigated by flow cytometry.
RESULTS: The survival time of tocilizumab group was
(24±1.27d) longer than that of allograft group (10±0.55d). Moreover,
immunohistochemical examination revealed that IL-17A and VEGF protein levels in the allograft group
were significantly higher than that of tocilizumab group (P<0.01),
while Foxp3 levels in the allograft group was significantly lower than that of
the tocilizumab treated group (P<0.001). Flow cytometry showed that
the number of Th17 cells in allograft group was significantly higher than that
in tocilizumab group (P<0.001). Meanwhile, the number of Tregs was
significantly lower than in tocilizumab group (P<0.001).
Simultaneously, Foxp3 mRNA expression level in corneal tissues of tocilizumab treated
group was significantly higher than other groups (P<0.001).
CONCLUSION: These findings suggest that tocilizumab may promote
corneal allograft survival, possibly by modulating Treg-Th17 balance.
KEYWORDS: tocilizumab; corneal
transplantation; Th17/Treg; rats
DOI:10.18240/ijo.2019.12.02
Citation:
Wu XS, Lu XL, Wu J, Ma M, Yu J, Zhang ZY. Tocilizumab promotes corneal
allograft survival in rats by modulating Treg-Th17 balance. Int J Ophthalmol 2019;12(12):1823-1831
INTRODUCTION
Corneal
transplantation is the most commonly used treatment for end-stage corneal
diseases. Despite corneal immune privilege and anterior chamber-associated
immune deviation, immune rejection is still the major limiting factor for
corneal grafting. This is specifically seen in patients with high-risk corneal
transplantation during corneal vascularization, repeated transplantation,
alkali burn, which had almost less than 54.2% long-term survival rate[1-3]. Therefore, prevention and
treatment of corneal graft rejection is still an ongoing and important issue in
clinical research. Clinically, medicines that suppress the rejection have been
applied to improve corneal transplantation success rate and long-term survival
rate of the graft, but are still limited due to drug tolerance in certain
patients, recurrence of rejection, high costs and also severe toxicity and side
effects. Thus, there is a pressing need to screen for immunosuppressive agents
with high efficiency and low toxicity. Recently, interleukin-6 (IL-6) has been
considered as a key mediator in the pathogenesis of graft-versus-host diseases (GVHD)[4-5]. The pathway of IL-6/IL-6 receptor
(IL‑6/IL‑6R) signaling regulates various biological process, including cell
growth and differentiation, as well as immune and hematopoietic systems[6]. Tocilizumab is a recombinant monoclonal antibody
specific to IL-6R, and is originally used for the treatment of rheumatoid
arthritis[7]. According to previous studies,
tocilizumab could reduce Th17 cell proportion, and increase Treg cell
proportion, thus changing the Th17/Treg balance in patients with rheumatoid
arthritis[8-10]. Recent reports
have demonstrated that tocilizumab may reduce the severity of GVHD in
steroid-refractory GVHD[11-12].
A previous study demonstrated
that tocilizumab
could prolong the survival time of islet transplantation and protect the
function of islet after transplantation[13]. Some
ophthalmological studies also reported that tocilizumab
could reduce the formation of corneal neovascularization[14-15]. However, whether tocilizumab could affect the immune
mediated corneal graft rejection is unknown. Hence, this study was conducted to
explore this question.
MATERIALS AND METHODS
Ethical Approval All rats were housed in a specific
pathogen-free (SPF) environment. Animal experiments were approved by the
Nanfang Hospital Animal Ethics Committee and adherence to the ARVO Statement
for the Use of Animals in Ophthalmic and Vision Research.
Animals and Materials Wistar rats served as hosts, and
accepted corneal grafts from either Sprague-Dawley (SD) rats (autograft) or
Wistar rats (allograft). Female rats, aged 6-8wk and weighing 180-220 g, were purchased from the Experimental
Animal Centre of Southern Medical University. There are 9 Wistar rats in normal
group. In each of the other 3 groups, there are 24 Wistar rats in each group
respectively.
Anesthesia
was carried out by injecting 3% thioethamyl (1.5 mL/kg). Tropicamide, a
compound of mydriatics, eye drops and 10-0 nylon line was purchased from
Ethicon (USA). Tocilizumab was purchased from Roche (Switzerland). The
antibodies for immunohistochemistry were purchased from Abcam (UK) and Santa
Cruz (USA). The antibodies for flow cytometry were purchased from Ebioscience
(USA). RNA extraction, reverse transcription real-time polymerase chain reaction (RT-PCR) kits were
purchased from Takara (Japan).
Corneal
Transplantation and Post-transplant Therapies Penetrating orthotopic corneal
transplantation was performed as previously described[16].
Briefly, a 3.5-mm central area of the cornea was excised from the donor and
secured in the host graft bed of 3.0-mm diameter with 8-10 interrupted 10-0
nylon sutures. The occurrence of hyphema, synechia and cataract in rats during
the operation were regarded as failed cases. The failed experimental animals
were removed and supplemented with new ones. Four groups were included: normal,
autograft, allograft, allograft treated with tocilizumab by tail vein injection
intravenously, 2 mg (0.1 mL) (as group tocilizumab). In the allograft rats group, same amount of 0.9% saline was
injected (as group allograft). In Wistar rats group, there was no intervention conducted,
and served as normal controls. Specific grouping and schematic representation of
the experiment were shown in Figure 1.
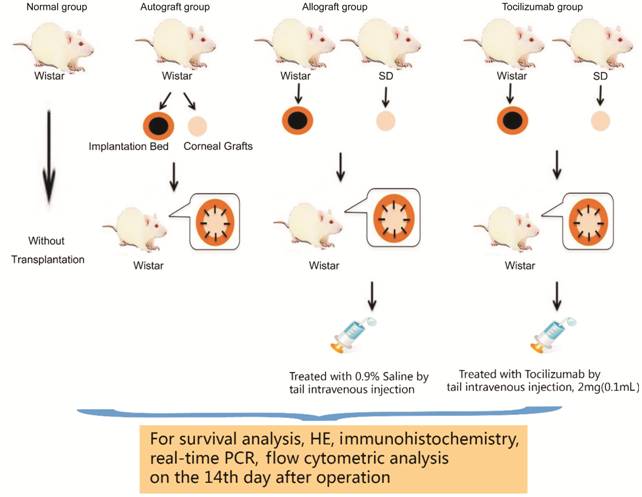
Figure 1 Schematic representation
of the experiment.
Rejection
Observation and Judgment Standard After the
operation, 15 grafts from each group were randomly selected for clinical
evaluation of rejection. According to the scoring method of Larkin[17], the corneal rejection score was recorded. Rejection
was defined by a total score of not less than 5 points or an opacity score of
over 3 points. Long-term survival was defined by no sign of rejection for more
than 100d.
Hematoxylin-eosin
Staining and Immunohistochemistry The eyeballs (3 rats for each
group) with transplantation were taken out after the rats were sacrificed.
These were then fixed with 4% polyformaldehyde solution, a gradient dehydrated
with alcohol, followed by embedding in paraffin, and then were cut into 4 μm
slices. Some slices were selected for staining by hematoxylin-eosin (H&E) and the
corneal thickness and inflammatory cell infiltration under microscopy were
observed. Some slices were used for immunohistochemistry by using rabbit
anti-rat IL-17A antibody
(sc7927, Santa Cruz), mouse anti-rat VEGF antibody (ab22510, abcam), mouse
anti-rat Foxp3 antibody (ab22510, abcam) as primary antibodies. Goat anti-mouse
antibody (ab6788, abcam), goat anti-rabbit antibody (ab97049, abcam) were used
as secondary antibodies. Experiments that used phosphate buffer solution (PBS),
instead of primary antibody, were set as negative controls. Using SP three step
method, antigen repair by heat was performed. The specimens were observed under
microscopy after BAD staining and mounting, and then photographs were taken by
using the 400× field of vision (Olympus digital camera, Japan). Using
Imagepro-Plus software, the cumulative integrated optical density [IOD(sum)]
and corneal tissue area were calculated by the formula:
IOD(mean)=IOD(sum)/area.
Quantitative Reverse Transcription
Polymerase Chain Reaction Corneal
grafts (3 rats for each group) were
taken out after the rats were sacrificed, then total RNA were extracted from
the grafts by Trizol and RNA concentration was measure by D260/D280 value using
Nanodrop. RNA were then used for cDNA synthesis using reverse transcriptase kit
(Cat: RR037A,
Takara). Rat GAPDH was used as
endogenous control. The primer pair sequences used for the PCRs were: 5’-ACCACAGTCCATGCCATCAC-3’, and 5’-TCCACCACCCTGTTGCTGAT-3’
for rat GAPDH, 5’-TGCTGCTACTGAACCTGGAG-3’, and 5’-GCGTTTGGACACACTGAACT-3’
for rat IL-17A, 5’-GACAGGGCCCCACAGAGA-3’, 5’-TTTGTGAGGTGTGGGTCTTCTTT-3’
for rat RORγt, 5’-AGTGGCAGGGAAGGAGTGTC-3’, and 5’-TTCCAAGTCTCGTGTGAAGGC-3’
for rat Foxp3, 5’-GGCCTCTGAAACCATGAACT-3’, and 5’-TGAACTTCACCACTTGGCAT-3’
for rat VEGF, 5’-ATTCTGTCTCGAGCCCACCA-3’, and 5’-GGAAGGCAGTGGCTGTCAAC-3’
for rat IL-6. The PCR reaction solution was prepared according to the instructions
of the qPCR kit (Cat: RR420A,
Takara) and PCR analysis were carried out with 7500 PCR instrument (ABI, USA). Transcript quantification was
performed in triplicate for each sample.
Flow
Cytometric Analysis Fourteen
days after operation, 3 rats were randomly selected from each group,
anaesthetized by 3% thioethamyl (1.5 mL/kg) intraperitoneally, and then 5 mL
blood sample was collected from the heart by blood collection tube containing
heparin. Lymphocyte was separated in the ultra clean cabinet by lymphocyte
separation medium (LTS1083, TBD, China). Each lymphocyte was evenly divided
into 2 samples (samples A and B). Sample A was placed in a 1640 culture medium
containing 10% fetal bovine serum and 4 μL (2 μL/mL) cell stimulation cocktail
(plus protein transport inhibitors; 85-00-4975-93, eBioscience, USA). The
lymphocyte was cultured in cell incubation box at 37°C with 5% CO2 for 6h. Subsequently, the
lymphocytes were collected by centrifugation at 2000 rpm and were divided into
3 tubes (experimental tube lymphocyte, ISO control tube lymphocyte and empty
tube lymphocyte), followed by the addition of anti-rat CD4 FITC (empty tube
lymphocyte were added nothing) and then placing at 4°C environment for 30min in dark. After that,
Foxp3/transcription factor staining buffer (85-00-5523-00, eBioscience, USA)
was added to the lymphocytes. After maintaining for 30min, the anti-mouse/rat
IL-17A PE were added in the
experimental tube lymphocytes, rat IgG2a
K isotype control PE were added in the ISO control tube, and nothing in the
empty tube lymphocytes. After 30min, the unbinding antibodies were washed by
PBS. Finally, the percentage of Th17 (CD4+/IL-17A+) in CD4+ cells
was tested by BD FACSCalibur (BD, USA). Sample B was also divided into 3 tubes
(experimental tube lymphocyte, ISO control tube lymphocyte and empty tube
lymphocyte) and no stimulation and culturing were performed. Anti-rat CD4 FITC
was added into experimental tube lymphocytes and anti-rat CD4 FITC and mouse
IgG1 K isotype control APC were added into anti-rat CD25 APC, ISO control tube
lymphocytes, and nothing in the empty tube lymphocytes. All the tubes in sample
B were maintained at 4°C
for 30min in dark. Then, Foxp3/transcription factor staining buffer was added
to the lymphocytes. After 30min, the anti-mouse/rat Foxp3 PE were added in the
experimental tube lymphocytes, rat IgG2a
K isotype control PE were added in the ISO control tube lymphocytes, and
nothing in the empty tube lymphocytes. After 30min, the unbinding antibodies
were washed away by PBS. Finally, the percentage of Treg (CD4+/CD25+/Foxp3+)
in CD4+ cells was measured by BD FACSCalibur. All antibodies in flow
cytometric analysis were purchased from eBioscience company (USA).
Statistical
Analysis The
experimental data were analyzed by IBM SPSS Statistics 20 and GraphPad Prism software.
One-way ANOVA was performed to test the variation of corneal neovascularization
area, mRNA and protein expression levels, as well as the ratio of Th17/Treg in
each group.
RESULTS
Clinical
Observation of Rejection and Draw Survival Curve
After
operation, all rats were examined by slit-lamp microscopy on alternate days to
calculate the rejective indices (RIs) according to opacity, edema, and
neovascularization of grafts. According to the survival curve (Figure 2C), the survival time of tocilizumab group
was 24±1.27d, while the survival time of allograft was 10±0.55d. After
mydriasis (Figure 2A), the
results showed that the neovascularization area in autograft and allograft
groups were 2.21±0.19 mm2
and 3.31±0.66 mm2,
respectively with Imagepro-Plus software, and were significantly higher than
that of tocilizumab
group (0.33±0.17 mm2,
P<0.01; Figure 2B). On day 14 after operation, the intact cornea was cut from the limbus by
puncturing the limbus with a bayonet, and stored at -80°C.
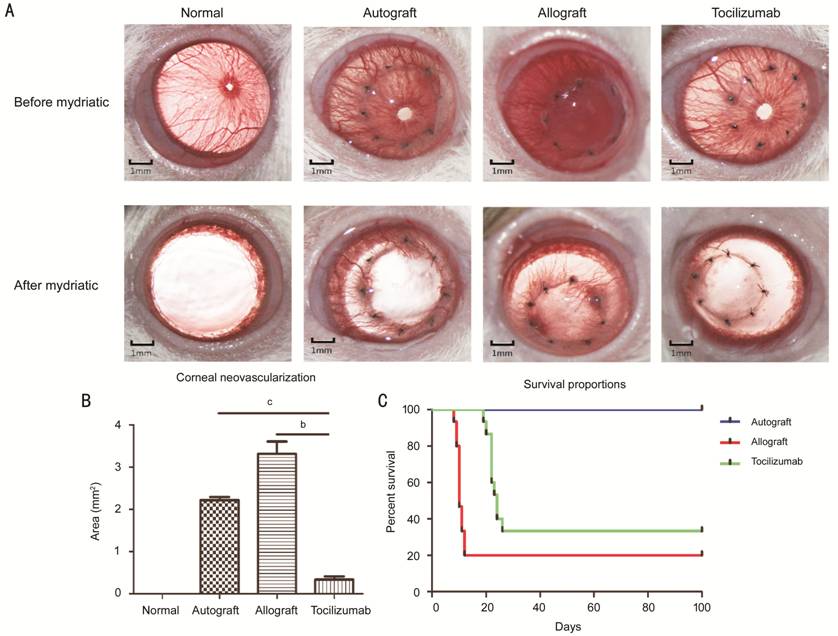
Figure 2
Clinical observation of corneal graft after corneal transplantation A: The
appearance of corneal grafts after transplantation. Fourteen days after transplantation,
the opacity, area of neovascularization and edema were observed under the
microscopy before and after mydriasis. B: Corneal neovascularization area in
each group after mydriasis (n=15). bP<0.01, cP<0.001.
C: Survival percentages after surgery. On postoperative day 100, the corneal
graft and the rejection score were recorded for drawing the survival curves (n=15).
Histopathological
and Immunohistochemical Analysis In the
normal group, the structure of the cornea was clear, and there was no blood
vessel and lymphocyte infiltration. As shown in Figure 3A, 14d after surgery, the corneal structures
in the autograft group were clear, but showed a few new blood vessels and
slight inflammatory cell infiltration in the stroma. In the allograft group,
the corneal graft was thickened with a large number of lymphocytes infiltrated,
and new blood vessels were observed in the stroma. More importantly, the
corneal structures in the tocilizumab group were regular with individual
lymphocytes, but without any obvious vascular cavity. Immunohistochemistry
revealed that IL-17A and VEGF
were mainly expressed in the corneal epithelial and stromal layers, and Foxp3
was predominantly expressed in the nucleus of stromal layer cells. The
expressions of IL-17A and VEGF
in the cornea of allograft group were significantly higher than that of the tocilizumab
group, while the expression of Foxp3 was lower than that of the tocilizumab
group. The corneal grafts of autograft group also exhibited high levels of VEGF
expression. The results of mean IOD detected by Imagepro-Plus software were
presented in Figures3B-3D, showing variations in similar expression pattern by
visual observation. The mean IL-17A
IOD of the allograft
group and tocilizumab
group were 0.026±0.002 and 0.005±0.001, respectively (P<0.05).
Moreover, the mean VEGF IOD of autograft group and allograft group were
0.022±0.001 and 0.021±0.002, respectively, while that of the tocilizumab
group was 0.009±0.001 (P<0.05). The mean Foxp3 IOD of allograft group
was 0.006±0.006, while that of tocilizumab group was as high as 0.112±0.032 (P<0.05).
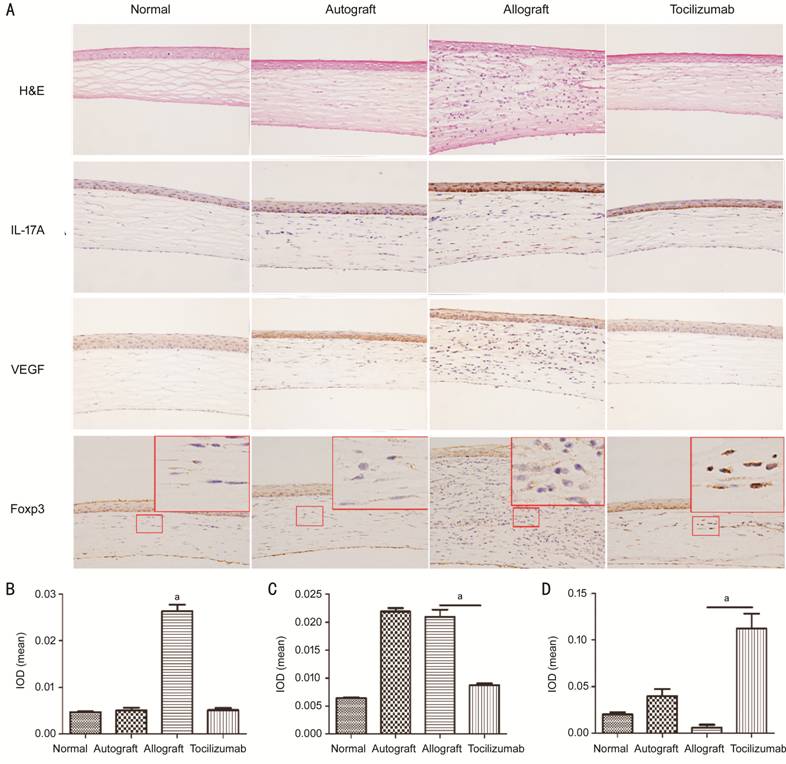
Figure 3 H&E staining and
immunohistochemistry analysis of gene expression A: H&E staining and
immunohistochemistry paraffin sections under 400× microscopy. The red box in
Foxp3 immunohistochemistry means that the local areas were enlarged by ten
times, showing better nuclear staining. B-D: The cartogram of mean IOD values
of IL-17A, VEGF and
Foxp3 expressions (n=3). aP<0.05.
The Relative
mRNA Levels of IL-17A, RORγt,
VEGF, IL-6 and Foxp3 in Corneal
Grafts The
expression levels of IL-17A,
RORγt, VEGF and IL-6 in the
corneal grafts of allograft group were significantly elevated when compared
with those of the normal group, and autograft group also showed VEGF
accumulation in the cornea. Compared to allograft group, the expressions of IL-17A, RORγt, VEGF and IL-6 in tocilizumab
group were all decreased to different extents. The expression of Foxp3 gene in tocilizumab
group was remarkably higher than that of the other three groups (P<0.001;
Figure 4).
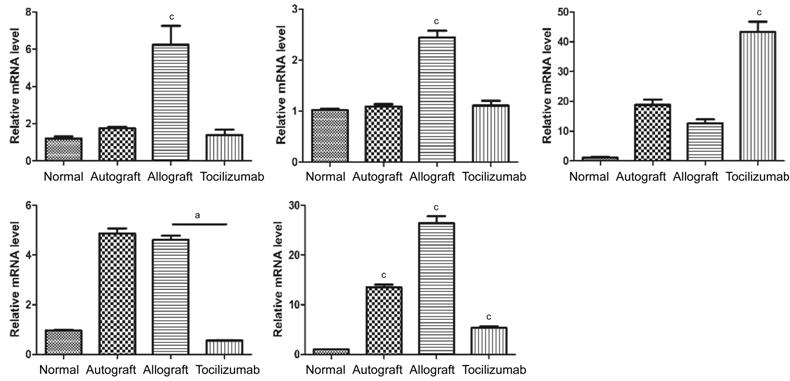
Figure 4
Expression levels of IL-17A,
RORγt, Foxp3, VEGF and IL-6 in
corneal graft Fourteen
days after transplantation, the relative expression levels of IL-17A, RORγt, Foxp3, VEGF and IL-6 genes in
each corneal graft group (n=3). aP<0.05, cP<0.001.
Flow
Cytometry Analysis On day 14
after transplantation, the percentages of Th17 cells in CD4+ cells
of rat blood between the normal group and autograft group showed no significant
difference, which were 1.20%±0.19% and 1.40%±0.66% respectively. In the
allograft group, it was found to be 7.32%±1.33%, and was significantly higher
than that in normal group (P<0.001), while it was only 2.05%±0.29% in
tocilizumab group, showing no significant elevation (P=0.21). The
tocilizumab group had the highest percentage of Treg cells in CD4+
cells of rat blood (7.27%±0.21%), followed by normal group (6.96%±0.47%) and
autograft group (5.80%±0.95%). The allograft group had the lowest percentage
(4.60%±0.20%), showing significant differences when compared with the
tocilizumab group (P<0.001). The percentage of CD4+/CD25high/Foxp3+
cells in CD4+/CD25+/Foxp3+ cells was also
different from each group with the tocilizumab group, exhibiting the highest
(84.40%±1.91%), and are higher than those in the normal group (80.83%±0.89%, P<0.05)
and the allograft group (76.30%±0.91%, P<0.001; Figure 5).
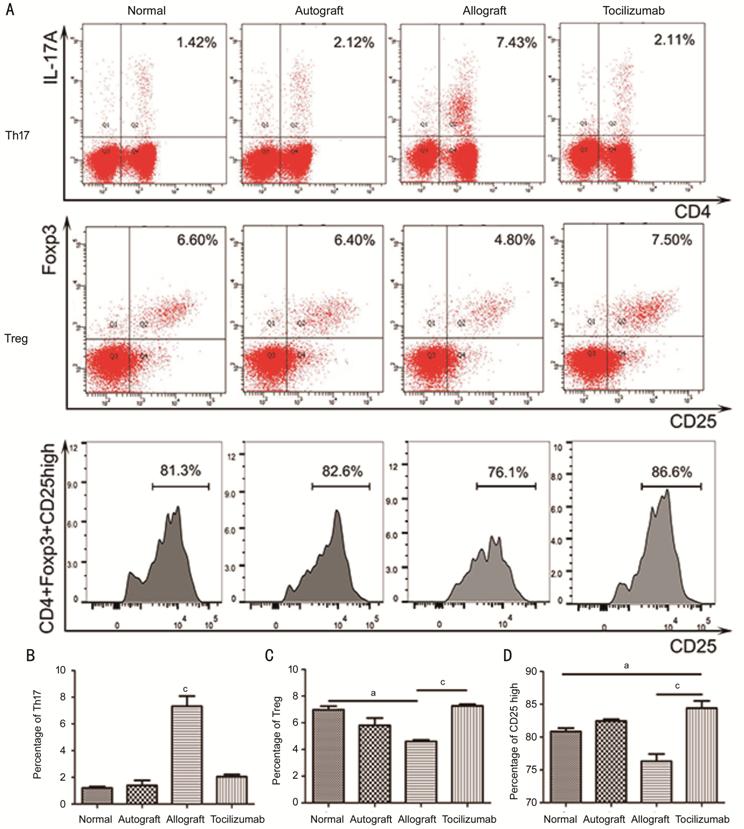
Figure 5
Analysis of blood lymphocytes by flow cytometry on postoperative day 14 A: Flow type scatter plot of Th17 (CD4+/IL-17A+) and Treg (CD4+/CD25+/Foxp3+)
and the cell count of CD4+/CD25high/Foxp3+ Tregs;
B: Percentages of CD4+/IL-17A+
Th17 cells in CD4+ cells (n=3); C: Percentages of CD4+/CD25+/Foxp3+
Treg cells in CD4+ cells (n=3); D: Percentages of CD4+/CD25high/Foxp3+
Treg cells in CD4+/CD25+/Foxp3+ Treg cells (n=3).
aP<0.05, cP<0.001.
DISCUSSION
The immune
response following corneal transplantation is a complicated process, and
infiltration of inflammatory cells and neovascularization at the implantation
site after transplantation are major risk factors of corneal graft rejection[3]. Common
immunosuppressive drugs may effectively inhibit rejection, but are associated
with side effects such as renal and liver toxicities. Tocilizumab is a
recombinant humanized monoclonal antibody against IL-6R that specifically binds
with IL-6R and blocks signal transduction of IL-6R to signal transducers and
activators of transcription 3 (STAT3). Besides, it is safe with less toxicity
and side effects, no immunogenicity and no induction of immune response[7].
The present
study found that tocilizumab could significantly prolong the survival time of
corneal grafts, shifting the Th17/Treg balance. Th17 cells, which are a subset
of CD4+ T cells with a role in autoimmunity, have been implicated as
main players in the acute phase of allograft rejection[18-19]. Conversely, CD4+/CD25+/Foxp3+
regulatory T cells (Treg), which maintain immune homeostasis, play a vital role
in protecting grafts from immune rejection[20].
Studies have shown that IL-6 and transforming growth factor β (TGF-β) regulate
the differentiation of T helper cell precursors (Thp) into Th17. When IL-6
levels are low, TGF-β induces differentiation of Thp into Treg[21]. Meanwhile, due to blockage of IL-6 signaling pathway
and unaffected TGF-β levels, Thp cells were inclined to differentiate into
Tregs[18,21]. Therefore, we
hypothesized that tocilizumab might induce the biological activity loss of
IL-6R by specifically binding with IL-6R, thus blocking the IL-6 signaling
pathway. It has been observed that interruption of signalling transduction
might induce downstream phosphorylation of STAT3, decrease in RORγt expression,
eventually decreasing IL-17A
expression and reducing Th17 cell number and activity[22-25]. Therefore, tocilizumab could prolong the survival
time of the graft by shifting the balance of Th17/Treg cells[18].
Corneal
neovascularization is an important risk factor of rejection after corneal
transplantation[26]. According to a previous
study, IL-17A played an
important role in the formation of corneal neovascularization, as it could
promote the growth of corneal neovascularization by destroying the
VEGF-A/sVEGFR-1 balance in the cornea, and blocking of IL-17A could suppress corneal neovascularization
and inflammatory cell infiltration as well[27].
Another study showed that tocilizumab could affect the expression of matrix
metalloproteinases and basic fibroblast growth factor by reducing the
phosphorylation of STAT3, and then by down-regulating the content of VEGF in
the cornea, thus suppressing the formation of corneal neovascularization[14]. Therefore, the use of tocilizumab
also prevents corneal graft rejection by reducing corneal neovascularization.
Interestingly,
we observed that the expression of corneal IL-6 was also reduced when treated
with tocilizumab
to block IL-6R in this experiment. This may be due to that the Th17 cells could
secrete IL-6, and so tocilizumab could reduce the secretion of IL-6 by
inhibiting Th17[21]. The reduction of IL-6 in turn reduces the rejection of graft and
formation of corneal neovascularization. The results showed that tocilizumab
can prolong corneal allograft survival by increasing the proportion of CD4+/CD25high/Foxp3+
Treg cells.
It has been
widely acknowledged that IL-17A
secreted by Th17 plays a partial role in rejection of liver, kidney and other
organs transplantation[28-31].
But in corneal transplantation, the role of IL-17A still remains controversial. Our study observed
that IL-17A
expression was increased in allograft group, while it decreased in tocilizumab
group. Hence we supposed that IL-17A
could promote corneal graft rejection. Some previous studies have claimed that
IL-17A promotes transplant
rejection, and anti-IL-17
therapy restricts and reverses late-term corneal allo-rejection[18,32-34]. However,
some studies have demonstrated that IL-17A could promote graft survival via promoting
immune privilege and anterior chamber associated immune deviation[35-36]. Since tocilizumab can effect
numerous cytokines except IL-17A
in this study, further studies are
required to clarify its mechanism.
In
conclusion, our findings
provide experimental evidences for potential clinical application of tocilizumab in corneal graft. However, the effects
of tocilizumab
on Th17/Treg balance in vitro were not tested, which should done in
future studies.
In summary, tocilizumab may promote
corneal allograft survival, possibly by modulating Treg-Th17 balance. This may
be a novel approach for inhibiting transplant rejection.
ACKNOWLEDGEMENTS
Foundations: Supported
by Science and Technology Planning Project of Guangdong Province (No.2017A020211005); Science and
Technology Programme of Guangzhou, China 2016 (No.201607010386).
Conflicts of Interest: Wu XS, None; Lu XL, None; Wu J, None; Ma M, None; Yu J, None; Zhang ZY, None.
REFERENCES
|
1 Qazi Y, Hamrah P. Corneal allograft
rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol
2013;2013(Suppl 9):006.
|
|
|
|
2 Niederkorn JY. Corneal transplantation
and immune privilege. Int Rev Immunol 2013;32(1):57-67.
https://doi.org/10.3109/08830185.2012.737877
PMid:23360158 PMCid:PMC3885418
|
|
|
|
|
3 Yu T, Rajendran V, Griffith M, Forrester
JV, Kuffová L. High-risk corneal allografts: a therapeutic challenge. World J
Transplant 2016;6(1):10-27.
https://doi.org/10.5500/wjt.v6.i1.10
PMid:27011902 PMCid:PMC4801785
|
|
|
|
|
4 Chen X, Das R, Komorowski R, Beres A,
Hessner MJ, Mihara M, Drobyski WR. Blockade of interleukin-6 signaling
augments regulatory T-cell reconstitution and attenuates the severity of
graft-versus-host disease. Blood 2009;114(4):891-900.
https://doi.org/10.1182/blood-2009-01-197178
PMid:19491393 PMCid:PMC2716024
|
|
|
|
|
5 Tawara I, Koyama M, Liu C, Toubai T,
Thomas D, Evers R, Chockley P, Nieves E, Sun YP, Lowler KP, Malter C,
Nishimoto N, Hill GR, Reddy P. Interleukin-6 modulates graft-versus-host
responses after experimental allogeneic bone marrow transplantation. Clin
Cancer Res 2011;17(1):77-88.
https://doi.org/10.1158/1078-0432.CCR-10-1198
PMid:21047980 PMCid:PMC3058832
|
|
|
|
|
6 Kishimoto T, Akira S, Narazaki M, Taga T.
Interleukin-6 family of cytokines and gp130. Blood 1995;86(4):1243-1254.
https://doi.org/10.1182/blood.V86.4.1243.bloodjournal8641243
PMid:7632928
|
|
|
|
|
7 Sebba A. Tocilizumab: the first interleukin-6-receptor
inhibitor. Am J Health Syst Pharm 2008;65(15):1413-1418.
https://doi.org/10.2146/ajhp070449
PMid:18653811
|
|
|
|
|
8 Samson M, Audia S, Janikashvili N, Ciudad
M, Trad M, Fraszczak J, Ornetti P, Maillefert JF, Miossec P, Bonnotte B.
Brief Report: Inhibition of interleukin-6 function corrects Th17/Treg cell
imbalance in patients with rheumatoid arthritis. Arthritis Rheum
2012;64(8):2499-2503.
https://doi.org/10.1002/art.34477
PMid:22488116
|
|
|
|
|
9 Pesce B, Soto L, Sabugo F, Wurmann P,
Cuchacovich M, López MN, Sotelo PH, Molina MC, Aguillón JC, Catalán D. Effect
of interleukin-6 receptor blockade on the balance between regulatory T cells
and T helper type 17 cells in rheumatoid arthritis patients. Clin Exp Immunol
2013;171(3):237-242.
https://doi.org/10.1111/cei.12017
PMid:23379428 PMCid:PMC3569529
|
|
|
|
|
10 Kikuchi J, Hashizume M,
Kaneko Y, Yoshimoto K, Nishina N, Takeuchi T. Peripheral blood
CD4(+)CD25(+)CD127(low) regulatory T cells are significantly increased by
tocilizumab treatment in patients with rheumatoid arthritis: increase in
regulatory T cells correlates with clinical response. Arthritis Res Ther
2015;17:10.
https://doi.org/10.1186/s13075-015-0526-4
PMid:25604867 PMCid:PMC4332922
|
|
|
|
|
11 Gergis U, Arnason J, Yantiss
R, Shore T, Wissa U, Feldman E, Woodworth T. Effectiveness and safety of
tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in a patient
with refractory GI graft-versus-host disease. J Clin Oncol
2010;28(30):e602-e604.
https://doi.org/10.1200/JCO.2010.29.1682
PMid:20713858
|
|
|
|
|
12 Drobyski WR, Pasquini M,
Kovatovic K, Palmer J, Douglas Rizzo J, Saad A, Saber W, Hari P. Tocilizumab
for the treatment of steroid refractory graft-versus-host disease. Biol Blood
Marrow Transplant 2011;17(12):1862-1868.
https://doi.org/10.1016/j.bbmt.2011.07.001
PMid:21745454 PMCid:PMC3716013
|
|
|
|
|
13 Sahraoui A, Kloster-Jensen
K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance
survival and function of human islets during culture: implications for
clinical islet transplantation. Cell Transplant 2014;23(10):1199-1211.
https://doi.org/10.3727/096368913X667529
PMid:23635711
|
|
|
|
|
14 Yoo AR, Chung SK. Effects of
subconjunctival tocilizumab versus bevacizumab in treatment of corneal
neovascularization in rabbits. Cornea 2014;33(10):1088-1094.
https://doi.org/10.1097/ICO.0000000000000220
PMid:25119962
|
|
|
|
|
15 Sari ES, Yazici A, Aksit H,
Yay A, Sahin G, Yildiz O, Ermis SS, Seyrek K, Yalcin B. Inhibitory effect of
sub-conjunctival tocilizumab on alkali burn induced corneal
neovascularization in rats. Curr Eye Res 2015;40(1):48-55.
https://doi.org/10.3109/02713683.2014.914541
PMid:24910898
|
|
|
|
|
16 Williams KA, Coster DJ.
Penetrating corneal transplantation in the inbred rat: a new model. Invest
Ophthalmol Vis Sci 1985;26(1):23-30.
|
|
|
|
|
17 Larkin DF, Calder VL,
Lightman SL. Identification and characterization of cells infiltrating the
graft and aqueous humour in rat corneal allograft rejection. Clin Exp Immunol
1997;107(2):381-391.
https://doi.org/10.1111/j.1365-2249.1997.279-ce1171.x
PMid:9030879 PMCid:PMC1904582
|
|
|
|
|
18 Wang X, Wang WT, Xu JJ, Wu
SQ, Le QH. All-trans retinoid acid promotes allogeneic corneal graft survival
in mice by regulating Treg-Th17 balance in the presence of TGF-Β. BMC Immunol
2015;16:17.
https://doi.org/10.1186/s12865-015-0082-3
PMid:25887926 PMCid:PMC4395899
|
|
|
|
|
19 Fan H, Li LX, Han DD, Kou
JT, Li P, He Q. Increase of peripheral Th17 lymphocytes during acute cellular
rejection in liver transplant recipients. HBPD INT 2012;11(6):606-611.
https://doi.org/10.1016/S1499-3872(12)60231-8
|
|
|
|
|
20 Hori S, Nomura T, Sakaguchi
S. Control of regulatory T cell development by the transcription factor
Foxp3. Science 2003;299(5609):1057-1061.
https://doi.org/10.1126/science.1079490
PMid:12522256
|
|
|
|
|
21 Afzali B, Lombardi G,
Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells
(Treg) in human organ transplantation and autoimmune disease. Clin Exp
Immunol 2007;148(1):32-46.
https://doi.org/10.1111/j.1365-2249.2007.03356.x
PMid:17328715 PMCid:PMC1868863
|
|
|
|
|
22 Ataie-Kachoie P, Pourgholami
MH, Morris DL. Inhibition of the IL-6 signaling pathway: a strategy to combat
chronic inflammatory diseases and cancer. Cytokine Growth Factor Rev
2013;24(2):163-173.
https://doi.org/10.1016/j.cytogfr.2012.09.001
PMid:23107589
|
|
|
|
|
23 Laurence A, Tato CM,
Davidson TS, Kanno Y, Chen Z, Yao ZJ, Blank RB, Meylan F, Siegel R,
Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5
constrains T helper 17 cell generation. Immunity 2007;26(3):371-381.
https://doi.org/10.1016/j.immuni.2007.02.009
PMid:17363300
|
|
|
|
|
24 Yang XP, Ghoreschi K,
Steward-Tharp SM, Rodriguez-Canales J, Zhu JF, Grainger JR, Hirahara K, Sun
HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of
the locus encoding IL-17 through direct, reciprocal actions of STAT3 and
STAT5. Nat Immunol 2011;12(3):247-254.
https://doi.org/10.1038/ni.1995
PMid:21278738 PMCid:PMC3182404
|
|
|
|
|
25 Yoon JH, Sudo K, Kuroda M,
Kato M, Lee IK, Han JS, Nakae S, Imamura T, Kim J, Ju JH, Kim DK, Matsuzaki
K, Weinstein M, Matsumoto I, Sumida T, Mamura M. Phosphorylation status
determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation.
Nat Commun 2015;6:7600.
https://doi.org/10.1038/ncomms8600
PMid:26194464 PMCid:PMC4518312
|
|
|
|
|
26 Dohlman TH, Omoto M, Hua J,
Stevenson W, Lee SM, Chauhan SK, Dana. VEGF-trap aflibercept significantly
improves long-term graft survival in high-risk corneal transplantation.
Transplantation 2015;99(4):678-686.
https://doi.org/10.1097/TP.0000000000000512
PMid:25606789
|
|
|
|
|
27 Suryawanshi A, Veiga-Parga
T, Reddy PB, Rajasagi NK, Rouse BT. IL-17A differentially regulates corneal
vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1
expression and promotes corneal angiogenesis after herpes simplex virus
infection. J Immunol 2012;188(7):3434-3446.
https://doi.org/10.4049/jimmunol.1102602
PMid:22379030 PMCid:PMC3311700
|
|
|
|
|
28 Chung BH, Kim KW, Kim BM,
Doh KC, Cho ML, Yang CW. Increase of Th17 cell phenotype in kidney transplant
recipients with chronic allograft dysfunction. PLoS One 2015;10(12):e0145258.
https://doi.org/10.1371/journal.pone.0145258
PMid:26717145 PMCid:PMC4696852
|
|
|
|
|
29 Ma L, Zhang HM, Hu KB, Lv G,
Fu YW, Ayana DA, Zhao PW, Jiang YF. The imbalance between Tregs, Th17 cells
and inflammatory cytokines among renal transplant recipients. BMC Immunol
2015;16:56.
https://doi.org/10.1186/s12865-015-0118-8
PMid:26400627 PMCid:PMC4581081
|
|
|
|
|
30 Serody JS, Hill GR. The
IL-17 differentiation pathway and its role in transplant outcome. Biol Blood
Marrow Transplant 2012;18(1 Suppl): S56-S61.
https://doi.org/10.1016/j.bbmt.2011.10.001
PMid:22226114 PMCid:PMC3588169
|
|
|
|
|
31 Kwan T, Chadban SJ, Ma J,
Bao S, Alexander SI, Wu H. IL-17 deficiency attenuates allograft injury and
prolongs survival in a murine model of fully MHC-mismatched renal allograft
transplantation. Am J Transplant 2015;15(6):1555-1567.
https://doi.org/10.1111/ajt.13140
PMid:25824574
|
|
|
|
|
32 Heidt S, San D, Chadha R,
Wood KJ. The impact of Th17 cells on transplant rejection and the induction
of tolerance. Curr Opin Organ Transplant 2010;15(4):456-461.
https://doi.org/10.1097/MOT.0b013e32833b9bfb
PMid:20616728 PMCid:PMC3095085
|
|
|
|
|
33 Chen HY, Wang WL, Xie HY, Xu
X, Wu J, Jiang ZJ, Zhang ML, Zhou L, Zheng SS. A pathogenic role of IL- 17 at
the early stage of corneal allograft rejection. Transpl Immunol
2009;21(3):155-161.
https://doi.org/10.1016/j.trim.2009.03.006
PMid:19358887
|
|
|
|
|
34 Yin XT, Zobell S, Jarosz JG,
Stuart PM. Anti-IL-17 therapy restricts and reverses late-term corneal
allorejection. J Immunol 2015;194(8): 4029-4038.
https://doi.org/10.4049/jimmunol.1401922
PMid:25754737 PMCid:PMC4390481
|
|
|
|
|
35 Cunnusamy K, Chen PW,
Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J
Immunol 2010;185(8):4651-4658.
https://doi.org/10.4049/jimmunol.1001576
PMid:20844197 PMCid:PMC3132880
|
|
|
|
|
36 Cunnusamy K, Chen PW,
Niederkorn JY. IL-17A-dependent CD4+CD25+ regulatory T cells promote immune
privilege of corneal allografts. J Immunol 2011;186(12):6737-6745.
https://doi.org/10.4049/jimmunol.1100101
PMid:21551366 PMCid:PMC3110606
|
|





