
·Basic Research·
The
role of mechanical stretch and TGF-β
Qian Cao1,2, Qu-Zhen Deji3,
Ya-Jun Liu4, Wei Ye5, Wang-Dui Zhaba3, Qin Jiang1,2, Feng Yan3
1Eye Hospital, Nanjing Medical
University, Nanjing 210002, Jiangsu Province, China
2The Fourth School of Clinical
Medicine, Nanjing Medical University, Nanjing 210002, Jiangsu Province, China
3Affiliated Jinling Hospital,
Medical School of Nanjing University, Nanjing 210002, Jiangsu Province, China
4Department of Ophthalmology,
Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University
Medical School, Nanjing 210008, Jiangsu Province, China
5School of Second Military
Medical University, Department of Ophthalmology, Jinling Hospital, Nanjing
210002, Jiangsu Province, China
Co-first authors: Qian Cao and
Qu-Zhen Deji
Correspondence to: Qin Jiang. Eye
Hospital, Nanjing Medical University, Nanjing 210002, Jiangsu Province, China;
The Fourth School of Clinical Medicine, Nanjing Medical University, Nanjing
210002, Jiangsu Province, China. jqin710@vip.sina.com; Feng Yan. Affiliated
Jinling Hospital, Medical School of Nanjing University, Nanjing 210002, Jiangsu
Province, China. yanfengdoctor@126.com
Received:
Abstract
AIM:
To explore the effects and mechanisms of mechanical stress and
transforming growth factor-beta2 (TGF-β2) on epithelial-mesenchymal transition
(EMT) in cultured human retinal pigment epithelial (RPE) cells.
METHODS: Human RPE
cells were inoculated on BioFex 6-well plates and RPE cells received 0, 1, 2,
3, or 4 mild stretch injuries delivered 3h apart after 24h of culture. The
device of mechanical stress parameters were set to sine wave, frequency 1 Hz,
stretch strength 20%. For treatment with TGF-β2, when the inoculated RPE cells
in 6-well plates were around 60% confluent, serum was reduced to 0 for 12h and
recombinant human TGF-β2 (0, 1, 5, 10 ng/mL) was added for 48h. α-SMA, Vimentin
and N-Cadherin, fibronectin proteins expressions were detected by Western
blotting, confocal cell immunofluorescence and quantitative real-time
polymerase chain reaction (qRT-PCR). Then we detected the change of miRNA-29b
and ascertained the changes of phosphatidylinositol 3-kinase-serine threonine
protein kinase (PI3K/Akt) pathway after RPE cells were stretched by the device
of mechanical stress and induced by TGF-β2 by Western blotting, confocal cell
immunofluorescence and qRT-PCR.
RESULTS:
Mechanical stress induce EMT and activate the PI3K/Akt pathway in
ways that lead to the EMT process. TGF-β2 induce RPE cells EMT and in a certain
range and TGF-β2 decrease the miRNA-29b expression in RPE cells, and the
inhibitory effect is more obvious with the increase of TGF-β2 concentration.
CONCLUSION:
Our findings are crucial steps in determining the critical roles
of the PI3K/Akt signaling pathway and miRNA-29b in pathogenesis of
proliferative vitreoretinopathy (PVR) which may be a potential target for
preventing or treating PVR.
KEYWORDS: mechanical
stress; transforming growth factor-beta2; microRNA 29b; epithelial-mesenchymal
transition; phosphatidylinositol 3-kinase-serine threonine protein kinase
pathway; proliferative vitreoretinopathy
DOI:10.18240/ijo.2019.12.03
Citation: Cao Q,
Deji QZ, Liu YJ, Ye W, Zhaba WD, Jiang Q, Yan F. The role of mechanical stretch
and TGF-β
INTRODUCTION
Proliferative vitreoretinopathy (PVR)
is most commonly found in long-term non-treated rhegmatogenous retinal
detachment (RRD) and the retinal detachment after surgical treatment. It has
long been credited with the main cause of failure of retinal detachment surgery
and significant vision loss[1].
Epiretinal membranes, a main
feature of PVR, is constantly contracting over the process of PVR, and leads to
retinal detachment and, eventually, the loss of vision[1].
During the formation of epiretinal membranes, human retinal pigment epithelial
(RPE) cells occurs epithelial mesenchymal transition (EMT) plays a decisive
role[2], and cell pathways and various cytokines are involved
in this EMT process, including the phosphatidylinositol 3-kinase-serine
threonine protein kinase (PI3K/Akt) pathway[3].
Transforming growth factor-beta2
(TGF-β2) was found abnormal high expressed in epiretinal membranes of PVR
patients[4-5] and is gotten labeled as an
induction factor of EMT, also in RPE cells. Additionally, a growing body of
studies have reported that microRNAs (miRNAs) have a strong capacity for gene
regulation in physiological processes and in some pathophysiological processes[6-9]. This small non-coding endogenous RNA
participates in the expression regulation of approximately 30%-50% of genes
encoding proteins by interacts with the
Published data have demonstrated
that mechanical stress plays an increasingly critical role in some
physiological processes, including cell proliferation, differentiation, apoptosis,
gene expression, organization growth, and some pathological processes[12]. These processes are similar to the PVR processes which are
caused by RPE cells abnormal proliferation. So, we can establish a more
accurate and more fit model of PVR in vitro to provide important methods for
further study of PVR. However, we still don’t have enough information about
this mechanism of the activation of retinal cells induced by excessive
mechanical stress from a pathogenetic point of view[13].
So, starting with the association
between PVR progression and PI3K-Akt signaling pathway and miRNA-29b, our study
expounds the mechanism of PVR and provide a well-established theoretical
foundation for further study of the prevention and treatment of PVR. We applied
mechanical stretching on human RPE cells and induced RPE cells EMT process
through the PI3K/Akt signaling pathway. In addition, we confirmed that TGF-β2
also can induce RPE cells EMT and inhibit the expression of miRNA-29b and this
inhibitory effect is more pronounced with increasing concentration and time in
vitro. The purpose of the present study was to investigate how human RPE cells
respond and adapt to mechanical stress.
MATERIALS AND METHODS
Cell Culture Treatment Fetal bovine serum (10%; Gibco,
California, USA) and 1% penicillin/streptomycin (Gibco, California, USA) were
added to the DMEM/F12 (Gibco, California, USA) to culture ARPE-19 cell line at
a humidity of 5% CO2 at
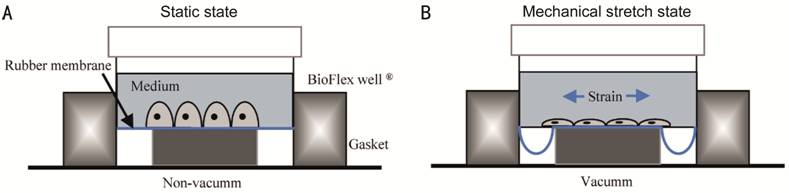
Stretch by the Device of
Mechanical Stress RPE cells were
inoculated on BioFex 6-well plates and the RPE cell densities were 1×106 cells
per well. RPE cells received 0, 1, 2, 3, or 4 mild stretch injuries delivered
3h apart after 24h of culture. The device of mechanical stress parameters were
set to sine wave, frequency 1 Hz, stretch strength 20% (Figure 1). The cells in
BioFex 6-well culture plate were collected and analyzed by Western blot and
confocal cell immunofluorescence.
Figure
Treatment with Transforming
Growth Factor-Beta2 When the
inoculated RPE cells in 6-well plates were around 60% confluent, serum was
reduced to 0 for 12h and recombinant human TGF-β2 (0, 1, 5, 10 ng/mL)
(Peprotech, Rocky Hill, USA) was added for 48h. During this period, observe the
changes in cell morphology with a phase contrast microscopy (Olympus, Tokyo,
Japan) and finally, cells in 6-well plates were collected and analyzed for
fibronectin (FN) and N-cadherin expression by Western blot analysis and
quantitative real-time polymerase chain reaction (qRT-PCR). For miRNA-29b
expression analysis, we used different concentrations (0, 1, 5, 10 ng/mL) of
TGF-β2 to stimulate RPE cells for 24h, and also used a constant concentration
(5 ng/mL) of TGF-β2 to stimulate RPE cells at 0, 3, 6, 12, 24, 48h, qRT-PCR
allowed the identification of miRNA-29b expression.
Western Blotting Analysis Firstly, RPE cells should be washed and
lysed with 400 µL radioimmunoprecipitation buffer [containing 50 mmol/L Tris
(pH 7.4), 1% Triton X‑100, 150 mmol/L NaCl, 1% sodium deoxycholate, 0.1% SDS, 5
mmol/L sodium orthovanadate, 1 mmol/L phenylmethanesulfonyl fluoride, 5 mmol/L
EDTA (Beyotime Institute of Biotechnology, Shanghai, China)] and 4 µL protease
inhibitors (Jiangsu KeyGen Bio Tech Corp, Ltd, Nanjing, China) at
Real-time Quantitative Polymerase
Chain Reaction After the total RNA
were isolated with TRIzol reagent (Invitrogen, California, USA), measured
concentration and purity via spectrophotometry, giving an RNAA260/280 ratio of
1.8-2.0 (GE, USA). Reverse transcription using a Prime Script RT Master Mix kit
(TaKaRa, Kusatsu, Japan), and the fluorescence of each cycle was quantified
with a 7300 RT-PCR system (Applied Biosystems, California, USA) using the SYBR1
Premix Ex TaqTM kit (TaKaRa, Kusatsu, Japan). As shown in Table 1, the specific
primers were used in this experiment. The time, temperature and cycle index of
the reaction were set according to manufacturer’s instructions. Using the 2-△△Ct method to
analyze the relative mRNA and miRNA expression level. GAPDH and U6 primers
served as the internal controls.
Table 1 Specific primers of quantitative
polymerase chain reaction
|
Genes |
Sequences
( |
|
FN |
F: |
|
|
R: |
|
N-Cadherin |
F: |
|
|
R: |
|
GAPDH |
F: |
|
|
R: |
F: Forward; R: Reverse; FN:
Fibronectin; N-Cadherin: Nerve calcium adhesion protein; GAPDH: Glyceraldehyde
3-phosphate dehydrogenase.
Confocal Cell
Immunofluorescence After the cells
were stretched with mechanical stress for 9h, they were fixed in 4%
paraformaldehyde, rinsed 3 times with PBS, kept in 0.5% Triton X-100 (Sigma,
Burlington, USA) and 5% goat serum. All the above operations are performed at
room temperature. And slides were kept in primary antibodies (p-PI3K, 1:50,
Affinity, Cincinnati, USA; p-Akt, 1:50, Bio world, Blooming, USA) overnight at
Statistical Analysis Results involved in this study were
independently completed at least three times, and the means± standard deviation
(SD) was used to present the quantitative data. Analyze data with SPSS 17.0
software. One-way analysis of variance (ANOVA) method was used to analyze the
relationship between different groups. As long as P<0.05, the data could be
regarded as a statistically significant results.
RESULTS
ARPE-19 Cells Treated With
Mechanical Stress to Assess the Impact of EMT After exposure to mechanical stretch for
9h, changes in expression levels of the mesenchymal marker were measured.
Western blot and confocal cell immunofluorescence showed that expression of two
mesenchymal proteins, α-SMA and Vimentin, were significantly enhanced in RPE
cells following stretch by the device of mechanical stress (Figure 2). Thus,
these data suggested that stretch can induce EMT in RPE cells.
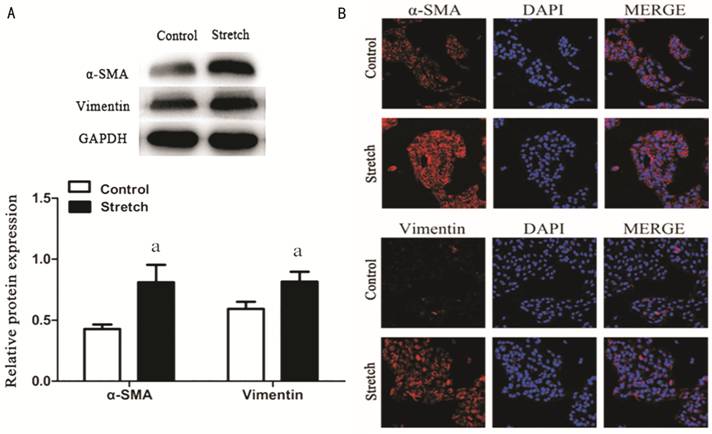
Figure 2 Stretch induced RPE
cells EMT RPE cells were stretched
by the device of mechanical stress for 9h before detection. A: Western blotting revealed an increase
in α-SMA and Vimentin proteins levels. GAPDH was selected for internal
reference (aP<0.05); B: α-SMA and Vimentin expression by confocal cell
immunofluorescence. Performed all experiments in triplicate.
Increased Protein Expression of
p-AKT and p-PI3K in RPE Cell To
confirm that stretching RPE cells can activate the PI3K/Akt pathway in ways
that lead to EMT (RPE cells were stretched for 0, 1, 3, 6, 9h), we next
detected the phosphorylation level of Akt and PI3K by Western blotting and
confocal immunofluorescence. Results from quantitative immunoblotting analysis
indicates that phosphorylation of PI3K and Akt increased depending on time
(Figure

Figure 3 Mechanical stress
induces EMT and active PI3K/Akt signaling pathway in RPE cells RPE cells were stretched by the device
of mechanical stress (0, 1, 3, 6, 9h).
A: Protein abundance of p-Akt and p-PI3K in mechanical stress stretched
RPE cell was quantified by Western blotting and increased with increasing time
(aP<0.05). B: Investigate the p-Akt and p-PI3K expression by confocal cell
immunofluorescence. Three independent experiments were performed.
Increased Proteins Expression of
FN and N-Cadherin in TGF-β2 Induced RPE Cells After stimulating RPE cells for 48h with
various concentrations of TGF-β2 (0, 1, 5, 10 ng/mL), the shape of cells was
changed and presented with this classical spindle-shaped appearance which was
more obvious with increase of concentration of TGF-β2 (Figure
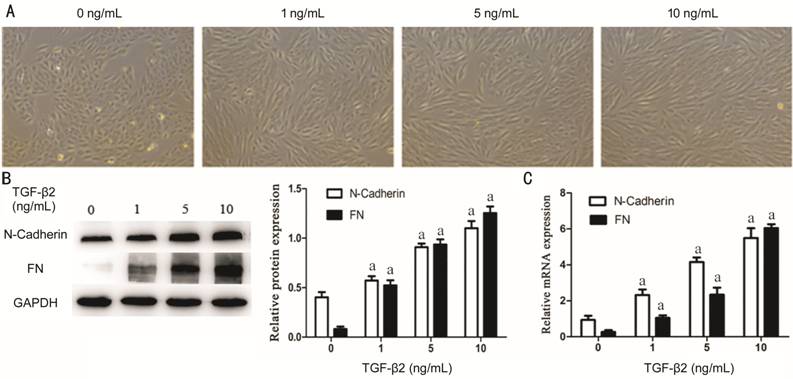
Figure 4 TGF-β2 induce EMT in RPE
cells RPE cells were exposed to
three different concentrations (1, 5, 10 ng/mL) of TGF-β2 for 48h and 0 ng/mL
TGF-β2 is a blank control group. A:
The cell morphological appearance was analyzed by a phase-contrast microscope
at 100× magnification; B: Western blotting showed TGF-β2 could induce
N-Cadherin and FN proteins expression with obvious dose-dependence. GAPDH was
selected for internal reference (aP<0.05). C: qRT-PCR analysis showed
N-Cadherin and FN mRNA expression increases with the TGF-β2 concentration.
GAPDH was selected for internal reference (aP<0.05). Performed all
experiments in triplicate.
TGF-β2 Decreased the miRNA-29b
Expression in RPE Cells To examine
the miRNA-29b expression, the RPE cells stimulated for 24h with different
concentration (0, 1, 5, 10 ng/mL) of TGF-β2 and stimulated with 5 ng/mL
TGF-β2 for 0, 3, 6, 12, 24, 48h.
The result of qRT-PCR showed that, miRNA-29b expression was decreased with the
increase of TGF-β2 concentration, and reached the lowest in 5 ng/mL TGF-β2
induced group (Figure
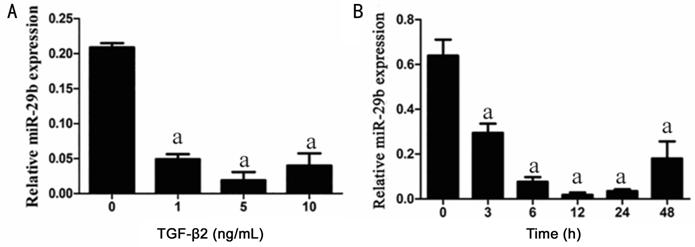
Figure 5 The miRNA-29b expression
was inhibited after induced with different concentrations and different time of
TGF-β
DISCUSSION
EMT is a biological process with
many regulation factors, which is accompanied with significant reduction of
mesenchymal markers and increase of epithelial markers. There are series
physiological and biological reactions in the course of EMT, and these
reactions can seriously affect cell motility, proliferation, apoptosis, and
protein expression. Pulmonary fibrosis, liver fibrosis, renal fibrosis and
breast cancer metastasis are closely related to the EMT process[14-17]. It has been shown that RPE cells that have undergone EMT
is the main cause of retinal traction and surgery failure and the initiation
factor of PVR[18]. However, the mechanisms involved during
PVR are remains unexplored.
Flex cell tissue mechanical
culture system can provide different stress, magnitude of the force and
simulate different stress time. Therefore, Flex cell device of mechanical
stress stimulates in vitro to induce RPE cells to EMT could be not only more
accurate but also produce persistent mechanical stretch similar to produce by
fibrous proliferative membrane. Therefore, mechanical stress could be used to
simulate the pathophysiological process of PVR[19]. In our
study, after exposure to mechanical stretch for 9h, changes in expression
levels of the mesenchymal marker were measured. So we demonstrated that
mechanical stress induce EMT in RPE cells and established a PVR model in vitro.
It has been reported a number of
factors are involved in cell growth, proliferation, as well as the migration
and the
Furthermore, we noticed that
after stimulating RPE cells for 48h with various concentrations of TGF-β2, the
RPE cells could undergo an EMT process. And more interestingly, according to
the reference of a great deal of documents, mechanical stress induces VEGF
expression and promotes angiogenesis in RPE cells, and regulation of VEGF mRNA
expression and protein secretion by TGF-β
miRNA, as a small non-coding
endogenous RNAs, can participated in gene expression regulation and importantly
affect many different mRNAs through miRNA-mRNA interactions (miRNA interacts
directly with
In conclusion, our result
suggests that Akt/PI3K signaling pathway was activated during EMT induced by
mechanical stress in RPE cells. Furthermore, the miRNA-29b down regulated by a
time-dose dependence in TGFβ2 -induced EMT. These findings suggest that
mechanical stress and TGF-β2 can induce RPE cells EMT and further studies could
focus on inhibitors of the PI3K/Akt pathway and overexpression of miRNA-29b to
prevent or treat PVR. Knowledge of these mechanisms could further understand
the pathogenesis of PVR, and indicate new prevention strategies and therapeutic
targets.
ACKNOWLEDGEMENTS
We are grateful to Key Laboratory
for Oral Disease Research of Nanjing Medical University kindly to provide the
experimental facilities and equipment for this study.
Foundations: Supported by the
National Natural Science Foundation of China (No.81600754).
Conflicts of Interest: Cao Q,
None; Deji QZ, None; Liu YJ, None; Ye W, None; Zhaba WD, None; Jiang Q, None; Yan
F, None.
REFERENCES