
·Clinical Research·
Dynamic
profile of ocular refraction in pediatric cataract patients after lens
surgeries
Zhen-Zhen Liu, Er-Ping Long, Duo-Ru Lin, Lei Ye, Yi-Fan
Xiang, Wang-Ting Li, Xiao-Hang Wu, Xu-Tu Zhao, Xiao-Ping
Liu, Lan-Qin Zhao, Xiu-Cheng Huang, Tong-Yong Yu, Hui
Chen, Jing-Jing Chen, Ming-Xing Wu, Hao-Tian
Lin, Wei-Rong Chen, Yi-Zhi Liu
State Key Laboratory of
Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou
510000, Guangdong Province, China
Co-first authors: Zhen-Zhen Liu, Er-Ping Long and
Duo-Ru Lin
Correspondence to: Hao-Tian Lin. 7# Jinsui Road, State
Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen
University, Guangzhou 510000, Guangdong Province, China. haot.lin@hotmail.com
Received:
Abstract
AIM: To study the change in ocular refraction in patients with pediatric
cataracts (PCs) after lens extraction.
METHODS: A total of 1258 patients who were undergoing cataract
extraction with/without intraocular lens (IOL) implantation were recruited
during preoperative examinations between Jan 2010 and Oct 2013. Patient ages
ranged from 1.5mo to 14y. Follow-ups were conducted at 1wk, 1, and 3mo
postoperatively and every 3mo in the first year, then 6mo thereafter. Ocular
refraction [evaluated as spherical equivalent (SE)] and yearly myopic shift
(YMS) were recorded and statistically analyzed among patients with age at
surgery, baseline ocular refraction, gender, postoperative time and laterality
(bilateral vs unilateral).
RESULTS: By Dec 31st 2015, 1172 participants had
been followed for more than 2y. The median follow-up period was 3y. The
critical factors affecting the ocular refraction of PC patients were baseline
ocular refraction, postoperative time for both aphakic and pseudophakic eyes.
YMS grew most rapidly in young childhood and early adolescence.
CONCLUSION: After lens surgeries, ocular refraction in PC
patients shows an individual difference of change. Further concerns should be
raising to monitor the rapid myopic shift at early adolescence of these
patients.
KEYWORDS: pediatric cataract; refraction;
intraocular lens; myopic shift
DOI:10.18240/ijo.2019.12.04
Citation: Liu
ZZ, Long EP, Lin DR, Ye L, Xiang YF, Li WT, Wu XH, Zhao XT, Liu XP, Zhao LQ,
Huang XC, Yu TY, Chen H, Chen JJ, Wu MX, Lin HT, Chen WR, Liu YZ. Dynamic
profile of ocular refraction in pediatric cataract patients after lens
surgeries. Int J Ophthalmol 2019;12(12):1839-1847
INTRODUCTION
Intraocular lens (IOL) implantation
is currently a commonly used means of optically rehabilitating children
undergoing cataract surgery. However, intriguing challenges remain in deciding
the best IOL power to be implanted in a specific child. The major problem is
that the variability of etiopathogenesis and treatment strategies for pediatric
cataract (PC) increases the variability of ocular refraction among these
patients. The postoperative/long-term refractive outcomes are not satisfactory
among PC patients despite great efforts made by many investigators.
Anticipating a myopic shift of
pseudophakic eye as a young child grows, several authors have recommended that
an appropriate hyperopic range be established for children in the immediate
postoperative period[1-2]. For
example, the rate of refractive growth (RRG), which uses a semi-logarithmic
model, is a formula designed to calculate the expected myopic shift in children[3-6]. However, some
controversy still exists among physicians about the postoperative refractive
goal. Some suggest that children should be made emmetropic after surgery so
that the amblyopia treatment will be more effective or easily performed[7].
It is not our purpose to resolve
this controversy. Instead, we seek to provide longitudinal refractive data from
PC patients after lens removal to demonstrate the actual refractive change in a
large cohort. In this study, we investigated the changing refractive status
among 1258 PC patients (from 1.5mo to 14y at enrollment, average 5.5±4.9y). We
aim to provide useful data from both aphakic and pseudophakic children to
improve the determination strategy of IOL power in PC patients.
SUBJECTS AND METHODS
Ethical Approval This study was approved by the
Ethical Review Committee of Zhongshan Ophthalmic Center, and the tenets of the
Declaration of Helsinki were followed throughout this study. Written consent
was obtained from patients’ guardian.
Enrollment Criteria Patients with PC who were undergoing
cataract extraction with/without IOL implantation were recruited prospectively
during preoperative screening at the Zhongshan Ophthalmic Center (ZOC),
Guangdong, China, from January 2010 to October 2013 (clinical trial identifier:
NCT02761850).
A patient was considered eligible
upon meeting the following inclusion criteria: 1) patients diagnosed with
congenital cataract or developmental cataract before surgery; congenital cataract
or developmental cataract were defined according to the 11th
Revision of the International Classification of Diseases (ICD-11) Beta Draft;
2) age 0-18y; and 3) informed, written consent provided by at least one
guardian. Patients with recorded refraction measurements and more than two
years of follow-up were included in the final analysis.
The exclusion criteria included the
presence of any of the following 1) the presence of any other ocular
comorbidities in the cataractous eye and/or the fellow eye, including and not
restricted to history of corneal disorders, glaucoma, lens luxation, persistent
hyperplastic primary vitreous and nanophthalmos, or systemic comorbidities
(including but not restricted to Down syndrome, congenital rubella syndrome and
juvenile idiopathic arthritis); and 2) cataracts secondary to other primary
diseases, such as complicated cataracts (due to ophthalmic inflammation or
degenerative changes), traumatic cataracts and metabolic cataracts.
Primary Data Collection Demographic information was obtained
from each eligible patient, including gender, age, etiology, and laterality.
The primary data collected also included the date of the surgery, the patient’s
age at the time of the surgery and the surgical strategy. All eyes of the subjects
underwent a thorough ophthalmic evaluation, including slit-lamp biomicroscopy,
fundus photography and B-scan ultrasonography.
Surgery Arrangement and Intraocular
Lens Calculation All of the surgeries were performed
by one of two experienced cataract surgeons (Liu YZ or Chen WR), and the
surgical strategy was implemented according to the patient’s age for all of the
surgeries. Surgical cataract extraction with/without IOL implantation [lens
irrigation/aspiration with posterior continuous curvilinear capsulorhexis and
anterior vitrectomy (I/A+PCCC+A-Vit) for patients younger than 2y; or lens
irrigation/aspiration with IOL implantation, posterior continuous curvilinear
capsulorhexis and anterior vitrectomy (I/A+IOL+PCCC+A-Vit) for patients of
2-3y; lens irrigation/aspiration with IOL implantation (I/A+IOL+PCCC) for
patients older than 3y] was performed in the included eye (refer to Table 1 for
more details). The IOL power was calculated using the SRK-II formula[8]. The Acrysof SN60AT and MA
Table 1 Arrangement of surgical strategies
|
Surgical indications |
Age of patients (y) |
Surgical strategies |
|
Dense lens opacity in the visual
axis, diameter > |
<2 |
I/A+PCCC+A-Vit |
|
2 to 3 |
I/A+IOL+PCCC+A-Vit |
|
|
>3 |
I/A+IOL+PCCC |
I/A: Lens aspiration; PCCC:
Posterior continuous curvilinear capsulorhexis; A-Vit: Anterior vitrectomy;
IOL: Intraocular lens.
Table 2 Postoperative refraction
correction
|
Laterality |
Treatmenta |
Postoperative refraction correction |
|
Bilateral |
Aphakia |
Glasses |
|
Unilateral |
Aphakia |
RGP lenses |
|
Bilateral & unilateral |
Pseudophakia |
Bifocal glasses |
RGP lenses: Rigid gas-permeable
contact lenses. a<2y: +3.0 D overcorrection; ≥2y: +2.5 D
overcorrection.
Follow-up of Participants and
Refraction Measurement The protocol called for follow-up
visits at 1wk, 1, and 3mo postoperatively (cataract removal and/or IOL
implantation), then every 3mo in the first year, then every 6mo thereafter.
Each follow-up visit required a complete eye examination which last about 3h
including slit-lamp photography, tonometry, anterior segment analysis, and
refractive error inspection. Autorefraction was performed with an un-dilated
pupil. Objective retinoscopy was performed after dilating the pupil to evaluate
refractive status. Compound tropicamide was used to dilate the pupil before
examination. All refractions were performed by an experienced optometrist. Each
patient might have more than one refractive result per year. The result that
was taken about a year from his or her previous follow-up was included for
analysis.
Definitions and Data Recording
Ocular refraction The
refraction data from each follow-up visit were transformed and recorded as the
spherical equivalent [SE; algebraic sum in diopters (D), sphere +1/2 cylinder].
Yearly myopic shift
The yearly myopic shift (YMS)
was calculated as the SE in the yearN+1 minus the SE in the yearN
in diopters.
Non-affected eye
The fellow eye without cataract
in a patient with monocular cataract was defined as “healthy”. In our study,
fellow eyes were screened, and any ocular or systemic comorbidities were
excluded (see the enrollment criteria). “Healthy” was only applied to the
above-mentioned facets.
Statistical Analyses All of the statistical analyses were
performed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA). The Shapiro-Wilk
test was used to evaluate the normality of distribution for all variables. For
variables fitting a normal distribution, data were recorded as the
mean±standard deviation (SD). Otherwise, for variables not fitting a normal
distribution, data were recorded as the medians and 25th-75th
interquartile range. A linear mixed model (LMM) was used to analyze differences
in SE with laterality, age, and gender in both aphakia and pseudophakia data
separately. In the LMM, the ocular refractive SE that were repeatedly measured
at different follow-ups were regard as independent variable, and postoperative
time was included as a predictor. In addition, laterality (bilateral vs
unilateral) and gender were included in LMM as fixed effects[10].
In this way, we were able to statistically control the influences of other
factors when we looked at the effects on the refraction status caused either by
age, laterality or gender. The “reference” in the LMM regression models
referred to the category that was set as the reference level of a specific
categorical variable. For example, gender is a categorical variable, and
“female” was set as the reference level for gender. The AR (1) in LMM, which is
a first-order autoregressive structure with heterogenous variances, is used to
control the effects of repeated measurements of an individual. A paired t-test
was used to evaluate the difference in SE between the affected eye and the
fellow eye in unilateral PC patients. A two-tailed P-value <0.05 was
considered statistically significant for all tests.
RESULTS
Baseline Characteristics A total of 1258 patients were
enrolled, and 1172 (93%) were followed for more than two years and included in
the statistical analysis (the pipeline of the procedures is shown in Figure 1).
The median follow-up was 3y (interquartile range 2.5-4.5y). The ratio of
bilateral cataract to unilateral cataract was 2.63:1 (829:343). Demographic
information of the cohort is mentioned in detail in Table 3. The average number
of records per individual is 4.49±1.43 records/child.
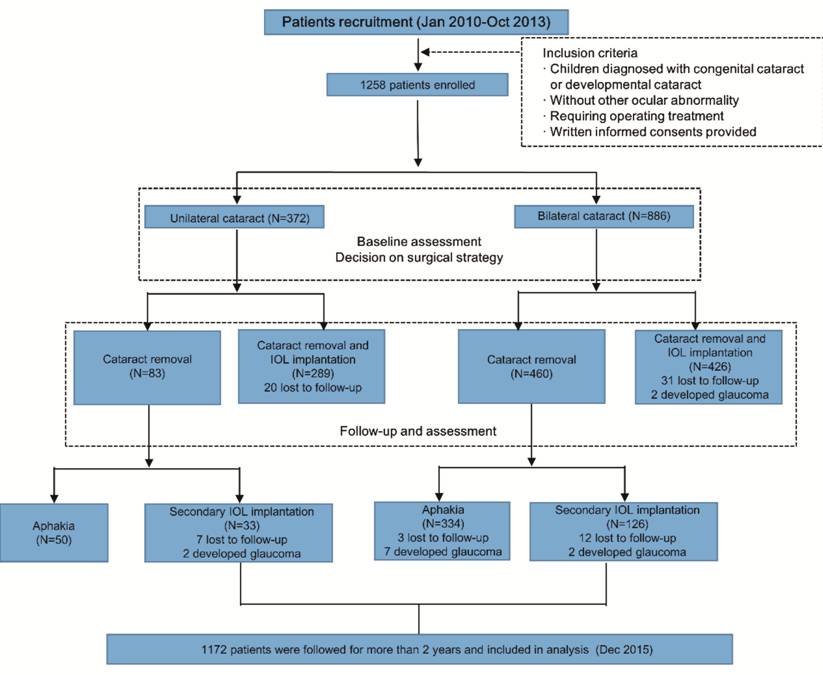
Figure 1 Pipeline detailing the
enrollment of subjects in the study.
Table 3 Demographic characteristics
of the patients included in final analysis
y, mean±SD
|
Treatment |
Total |
Male |
Female |
|||
|
n |
Age |
n |
Age |
n |
Age |
|
|
Bilateral |
|
|
|
|
|
|
|
Aphakia |
324 |
0.92±0.38 |
212 |
0.92±0.55 |
112 |
0.91±0.19 |
|
Primary IOL Implantation |
393 |
6.06±2.99 |
246 |
5.94±3.97 |
147 |
6.26±2.52 |
|
Secondary IOL Implantation |
112 |
2.89±0.82 |
76 |
2.85±0.62 |
36 |
2.98±0.36 |
|
Unilateral |
|
|
|
|
|
|
|
Aphakia |
50 |
0.74±0.39 |
28 |
0.75±0.39 |
22 |
0.71±0.37 |
|
Primary IOL Implantation |
249 |
4.80±3.37 |
146 |
5.01±3.70 |
103 |
4.49±2.85 |
|
Secondary IOL Implantation |
24 |
2.35±0.54 |
16 |
2.35±0.56 |
8 |
2.38±0.46 |
For aphakia and primary IOL
implantation, “Age” was referred to the age at enrollment. For secondary IOL
implantation, “Age” was referred to the age when receiving IOL implantation.
Ocular Refraction in Bilateral and
Unilateral Aphakia There was no statistically
significant difference in the age at surgery and the immediate postoperative
refraction between unilateral aphakia and bilateral aphakia (Table 4). SE
refractive error decreased with age in both bilateral and unilateral aphakic
eyes (Figure 2). We found that SE refractive error became myopic by 0.89 D
yearly in aphakia after lens removal (P<0.0001). Males tended to have
more myopic than females (P<0.0001). Factors that significantly
affect the ocular refraction were gender, postoperative time and baseline
ocular refraction (Table 5).
Table 4 Comparison of ocular
refraction and age at baseline in aphakia (n=510)
|
Variable |
Unilateral |
Bilateral |
Statistics value |
P |
|
Age at surgery (y) |
0.92 (0.83, 1.15) |
0.92 (0.67, 1.58) |
17299 |
0.271 |
|
Baseline SE (D) |
16.48±2.78 |
16.77±2.99 |
-0.86 |
0.411 |
Using two independent sample t
test for SE with t statistics, Wilcoxon rank sum test for age with W
statistics. SE: Spherical equivalent.
Table 5 Multivariate analyses of
factors independently associated with ocular refraction in aphakia using LMM
|
Variable |
Estimate |
95%CI |
SE |
t-value |
P-value |
|
Age at surgery |
-0.11 |
-0.57 to 0.34 |
0.23 |
-0.49 |
0.624 |
|
Gender |
|
|
|
|
|
|
Male |
-0.75 |
0.29 to 1.21 |
0.23 |
3.20 |
< |
|
Female |
Reference |
|
|
|
|
|
Laterality |
|
|
|
|
|
|
Unilateral |
0.23 |
-0.55 to 1.03 |
0.40 |
0.60 |
0.552 |
|
Bilateral |
Reference |
|
|
|
|
|
Postoperative time |
-0.89 |
-1.03 to -0.74 |
0.07 |
-12.13 |
< |
|
Baseline ocular refraction |
0.69 |
0.57 to 0.80 |
0.06 |
11.67 |
< |
LMM: Linear mixed model; CI:
Confidence interval; SE: Standard error. “Reference” referred to the category
that was set as the reference level of a specific categorical variable. aStatistically
significant.
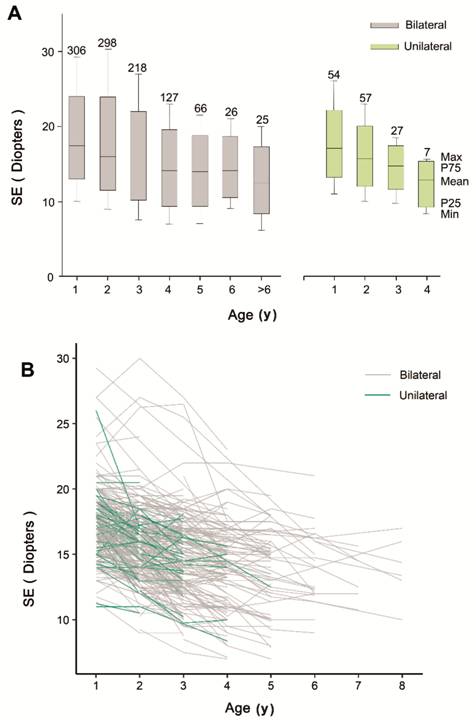
Figure 2 Ocular refraction in bilateral
and unilateral aphakia with age A: The boxplot shows the SE in
bilateral (grey) and unilateral (green) aphakia in each age group; B: The
spaghetti plot shows the changing trend for SE of each bilateral (grey) and
unilateral (green) patient in aphakia. SE decreases with age in bilateral and
unilateral aphakic eyes.
Ocular Refraction in Bilateral and
Unilateral Pseudophakia There was significant difference in
the age at surgery and the immediate postoperative refraction between
unilateral pseudophakia and bilateral pseudophakia (Table 6). We further
stratified the data of the immediate postoperative refraction according to the
age of IOL implantation. There was no difference between unilateral
pseudophakia and bilateral pseudophakia in most of the age range after
stratification. We found significant difference in the patients younger than 2y
and those older than 10y. However, it could not be concluded that the immediate
postoperative refraction was different between unilateral pseudophakia and
bilateral pseudophakia in these age ranges. The number of patients in some age
group were too small, and we had to combine them for statistical analysis. The
difference was likely due to the uneven distribution in age for these two age
ranges.
Table 6 Comparison of ocular
refraction and age at baseline in pseudophakia (n=798)
|
Variable |
Unilateral |
Bilateral |
Statistics value |
P |
|
Age at surgery |
4.00 (2.90, 5.80) |
4.90 (3.70, 6.80) |
55088 |
< |
|
Baseline SE |
1.01±2.35 |
0.23±3.25 |
3.87 |
< |
Using two independent sample t
test for SE with t statistics, Wilcoxon rank sum test for age with W
statistics. SE: Spherical equivalent. aStatistically significant.
SE refractive error decreased with
age in both bilateral and unilateral pseudophakic eyes (Figure
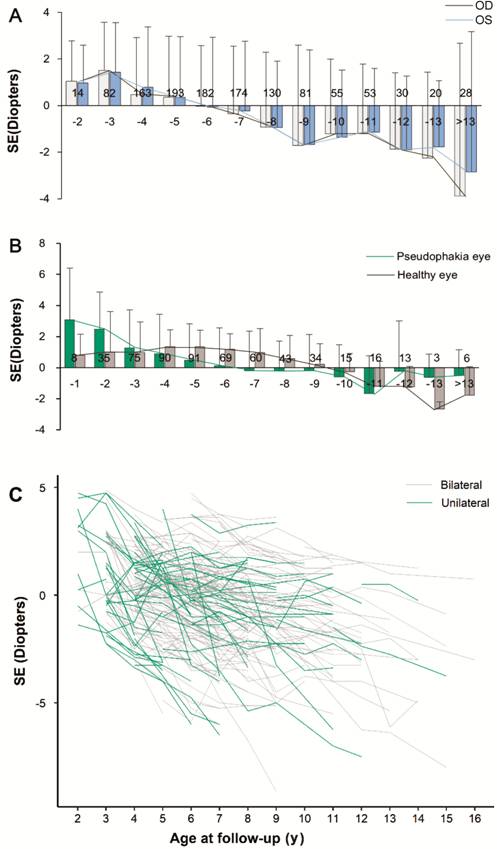
Figure 3 Ocular refraction in
bilateral and unilateral pseudophakia with age The bar and line chart show the
changing trend (mean) of SE in bilateral (A) and unilateral (B) pseudophakia.
With the current strategy for IOL calculation, SE in bilateral pseudophakia
becomes myopic with age and reached -0.49 to +0.49 D at approximately 6 years
of age (A). A similar profile of SE is observed in the affected eyes in
unilateral PCs; the SE in the non-affected eyes of unilateral PCs reaches
emmetropia at approximately 8-10 years of age (B). C: The spaghetti plot shows
the changing trend for SE of each bilateral (grey) and unilateral (green)
pseudophakic patient, indicating that SE decreases with age.
Table 7 Multivariate analyses of factors
independently associated with ocular refraction in pseudophakia using LMM
|
Variable |
Estimate |
95%CI |
SE |
t-value |
P-value |
|
Age at surgery |
0.49 |
-0.21 to 0.30 |
0.13 |
0.38 |
0.704 |
|
Gender |
|
|
|
|
|
|
Male |
-0.09 |
-0.44 to 0.26 |
0.18 |
-0.53 |
0.598 |
|
Female |
Reference |
|
|
|
|
|
Laterality |
|
|
|
|
|
|
Unilateral |
-0.47 |
-0.85 to -0.09 |
0.19 |
-2.49 |
|
|
Bilateral |
Reference |
|
|
|
|
|
Postoperative time |
-0.43 |
-0.52 to -0.34 |
0.05 |
-9.40 |
< |
|
Baseline ocular refraction |
0.72 |
0.63 to 0.81 |
0.04 |
16.11 |
< |
LMM: Linear mixed model; CI:
Confidence interval; SE: Standard error. “Reference” referred to the category
that was set as the reference level of a specific categorical variable. aStatistically
significant.
To study differences in ocular
refraction between pseudophakia and healthy eyes, data from the non-affected
eyes of unilateral PC patients were introduced into a linear mixed model
analysis (Table 8). The ocular refraction became myopic for both pseudophakic
and phakic eyes. SE in pseudophakia was more myopic than that of healthy eyes (P=0.017).
The paired t-test showed that the differences in SE between the affected
eye and the fellow eye in unilateral PC was significant (P=0.0013).
Table 8 Multivariate analyses of
factors independently associated with ocular refraction in pseudophakia and
non-affected eyes using LMM
|
Variable |
Estimate |
95%CI |
SE |
t-value |
P-value |
|
Age at surgery |
-0.12 |
-0.33 to 0.08 |
0.10 |
|
0.254 |
|
Gender |
|
|
|
|
|
|
Male |
0.25 |
-0.05 to 0.56 |
0.15 |
1.61 |
0.109 |
|
Female |
Reference |
|
|
|
|
|
Diagnosis |
|
|
|
|
|
|
Bilateral |
-0.42 |
-0.76 to -0.08 |
0.17 |
-2.41 |
|
|
Non-affected |
Reference |
|
|
|
|
|
Postoperative time |
-0.33 |
-0.42 to -0.24 |
0.05 |
-7.18 |
< |
|
Baseline ocular refraction |
0.78 |
0.69 to 0.86 |
0.04 |
17.67 |
< |
LMM: Linear mixed model; CI:
Confidence interval; SE: Standard error. “Reference” referred to the category
that was set as the reference level of a specific categorical variable. aStatistically
significant.
Yearly Myopic Shift in Bilateral and
Unilateral Pseudophakia The extent of YMS had a double-peak
profile, one in young adulthood and another in early adolescence in pseudophakic
and healthy eyes (Figure 4). In most of the age groups, the eye affected by PC
showed a higher YMS, regardless of its laterality.
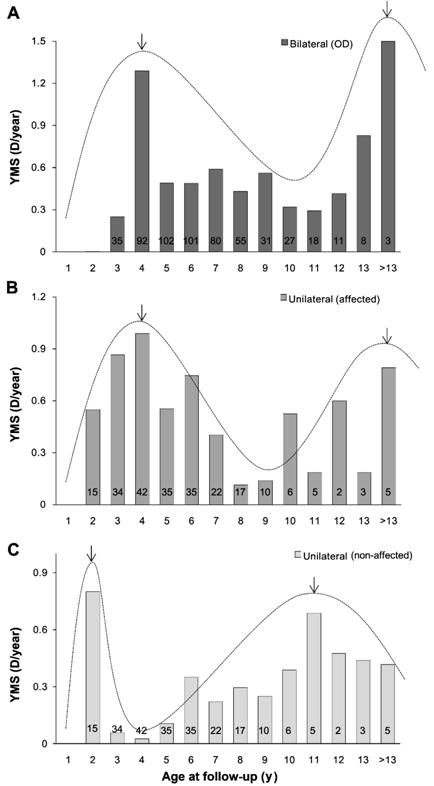
Figure 4 YMS in bilateral and
unilateral pseudophakia and healthy eyes with age The bar chart shows the YMS in
bilateral (A) and unilateral (B) pseudophakia, as well as in healthy eyes in
patients with unilateral congenital cataract (C). Black arrows and the peak of
the curve represent the peak of YMS. YMS is most fast in young childhood and
early adolescence in pseudophakia and healthy eyes. In most age groups, the eye
affected by PC shows a higher YMS, regardless of laterality.
DISCUSSION
PC causes defocus and/or form
deprivation during the critical period of ocular development; a PC patient who
has an opaque lens removed requires optical correction of the resulting
extensive hyperopia. Deciding on IOL power is a key step for optical
rehabilitation for PC patients, and yet, is a long-lasting controversial issue.
It is extremely difficult to predict when the refraction will stabilize for an
individual patient. The postoperative refractive shift may vary from 0.52 to
36.3 diopters[9,11-13].
Furthermore, there is insufficient source data to fully characterize the
dynamic refraction profile of PC patients to guide treatment strategy.
In the current study, we
prospectively recruited 1172 PC patients at one medical center, grouping and
analyzing the changing refractive pattern of the subjects by laterality. In
this longitudinal study with a large cohort, we observed some interesting
results.
We used a linear mixture model to
observe the effects of single factor on refractive changes while controlling
other factors. The average refractive power became myopic by 0.89 D every year
for aphakic eyes, and 0.43 D for pseudophakic eyes.
We found that among PC patients, the
refractive pattern differed with gender in aphakic eyes. SE refractive error in
males was more myopic than that in females. This is consistent with our
previous study, in which we found that before cataract removal, the axial
length of male PC patients was longer than that of females[14].
Furthermore, our result showed a difference between SE in unilateral of
bilateral pseudophakia.
Data from our cohort demonstrated
that the YMS grew the most rapidly in both young childhood (<3y) and early
adolescence (>12y). Some studies have demonstrated that refraction changes
rapidly in PC patients until the age of 1.5 to 3y and then stabilizes at the
age of 8 to 10y, while other studies have found further myopic shift into early
adolescence[1,9,15-16]. Currently the hyperopic range established
for children at the time of IOL implantation is predicted according to the
expected myopic shift in patients before 8 to 10y[1-2,9,17-18].
However, our data showed that in both cataractous and healthy eyes there
is another rapid changing period of refraction during early adolescence. These
results are consistent with the classical RRG serial studies, which
demonstrated that the refraction development did not follow a simple linear
pattern[3-6]. The
double peak profile of the YMS illustrated that the ocular refraction became
myopic fastest both during young childhood and early adolescence. That is why
the refraction development could not be calculated with a linear equation. The
rapid growth during early adolescence observed in our cohort may be a result of
ethnic differences given the high rate of myopia seen in Asia[19-21]. In populations with
a large risk of developing high myopia, strategies for IOL power determination
may need to reflect these differences. The possible solutions for this
phenomenon are to leave a larger hyperopic range after IOL implantation and/or
to suppress the significant myopia shift through early post-operative
overcorrection. This is the main focus of our further study in refractive
development and IOL power determination of congenital cataract patients.
In most of the age groups, the YMS
of PC eyes was relatively higher than that of healthy eyes, which could be
partially explained by the fixed refractive power of the IOL that could not
grow and compensate for the refractive change in the cornea as natural lens.
Though a hyperopic range was established at the time of IOL implantation,
eventually the pseudophakic eyes were more myopic than healthy contralateral
eyes in our cohort.
In summary, our results support to
set up target refraction basing on laterality of cataract involvement, age at
IOL implantation and baseline ocular refraction. For those kindergarten
patients, treatment of amblyopia is of priority, and the second peak of myopia
shift is less important. For relative older patients who have better compliance
with spectacle wearing and other treatments, we would like them to be 1 to 2 D
myopic as adults so that they can have good uncorrected near acuity and
reasonably clear uncorrected distance vision. We suggest postponing the age of
IOL implantation to solve the dilemma of visual function development and more
extensive myopic shift in our patients. This suggestion is consistent with a
recent published study of 256 children with congenital or infantile cataract[22]. The prerequisite was proper refraction correction
under ophthalmologists’ instructions. Our recommendations for target
refractions based on the results of our cohort are presented in Table 9.
Table 9
Recommended strategies and target refractions
|
Conditions (y) |
Strategies and target refractions |
|
Bilateral |
|
|
<2 |
IOL implantation not recommended |
|
2-5 |
Spectacles correction recommended;
IOL implantation: refer to Enyedi et al[9] |
|
5-8 |
+4, +3, +2, +1 D |
|
Unilateral |
|
|
<2 |
IOL implantation not recommended |
|
2-5 |
RGP correction recommended; IOL
implantation: refer to Enyedi et al[9] |
|
5-8 |
2-3 D hyperopia than the
non-affected eye |
|
Baseline spherical equivalent of
ocular refraction: more hyperopic than (28-3×age) D |
The reserved hyperopia should be 1
D more than that of the above-mentioned target refractions. |
The results and interpretation of
the current study must be understood within the context of its strengths and
limitations. In our cohort, some binocular patients underwent a secondary IOL
implantation at an older age compared to monocular patients. The late surgical
time brings possible influences on the refractive outcome of unilateral and
bilateral patients. Although patients were referred to the hospital from all
parts of China, all the subjects were treated and followed at one medical
center. The results may not be representative for other cohorts. Although we
used the linear mixed model to adjust for confounding factors, the use of
non-affected eyes of patients with unilateral PC as a “healthy” control possesses
potential influences on the results[23].
Despite these limitations, the
results of our study confirm critical factors, such as baseline ocular
refraction and post-operative time, contributing to the refractive outcome in
PC patients. What is more, further concerns should be raising to monitor the
rapid myopic shift at early adolescence of these patients.
ACKNOWLEDGEMENTS
Authors’ contributions: Conception and design: Liu ZZ, Long
EP, Lin HT; Collection and assembly of data: Liu ZZ, Long EP, Lin DR, Ye L,
Xiang YF, Li WT, Wu XH, Zhao XT, Liu XP, Zhao LQ, Huang XC, Yu TY, Chen H, Chen
JJ; Data analysis and interpretation: Huang XC, Yu TY, Chen H, Chen JJ, Wu MX,
Lin HT, Chen WR, Liu YZ; Manuscript Writing: Liu ZZ, Long EP, Lin DR; Final
approval of manuscript: all authors.
Foundations: Supported by National Natural
Science Foundation of China (No.81873675; No.81770967); National Key R&D Program of China (No.2018YFC0116500;
No.2017YFC1104600); Fundamental Research Funds for the Central Universities
(No.16ykjc28).
Conflicts of Interest: Liu ZZ, None; Long EP,
None; Lin DR, None; Ye L, None; Xiang YF, None; Li WT, None;
Wu XH, None; Zhao XT, None; Liu XP, None; Zhao LQ,
None; Huang XC, None; Yu TY, None; Chen H, None; Chen
JJ, None; Wu MX, None; Lin HT, None; Chen WR, None; Liu
YZ, None.
REFERENCES