
·Clinical Research·
Long-term
observation of vitrectomy without subretinal hemorrhage management for massive
vitreous hemorrhage secondary to polypoidal choroidal vasculopathy
Zhi-Xi Li1, Yi-Jun
Hu2, Alp Atik3, Lin Lu1,
Jie Hu1
1State Key Laboratory of
Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou
510060, Guangdong Province, China
2Aier School of Ophthalmology,
Central South University, Changsha 410000, Hunan Province, China
3Royal Victorian Eye and Ear
Hospital, Melbourne 3000, Australia
Co-first authors: Zhi-Xi Li and Yi-Jun Hu
Correspondence
to: Jie Hu.
State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen
University, Guangzhou 510060, Guangdong Province, China. hehujie@126.com
Received:
Abstract
AIM: To describe the long-term observation of vitrectomy without subretinal
hemorrhage (SRH) management for massive vitreous hemorrhage (VH) secondary to
polypoidal choroidal vasculopathy (PCV).
METHODS: This is a retrospective, consecutive case series. A
total of 86 eyes of 86 patients with >14d of massive VH associated with PCV
were included. All patients underwent vitrectomy without SRH management,
followed by intravitreal ranibizumab injections and/or photodynamic therapy
(PDT) as needed. The main outcome measures were best-corrected visual acuity
(BCVA), postoperative adverse events and the recurrence of VH.
RESULTS: The average follow-up period was 25.5±9.2mo (range
12-35mo). Mean BCVA at baseline (2.16±0.39 logMAR) had improved significantly,
both 3mo after surgery (1.42±0.66 logMAR, P<0.001) and by the last
visit (1.23±0.74 logMAR, P<0.001). The common postoperative complications
included macular subretinal fibrosis in 14 eyes (16.3%) and ciliary body
detachment in 4 eyes (4.7%). Nineteen eyes (22.1%) received following treatment
with ranibizumab injections without/with PDT, and 15 (17.4%) were resolved.
Four eyes (4.7%) had recurrent hemorrhage during the follow-up period. In
multiple regression analysis, thicker SRH (beta=0.33, P=0.025) in the
preoperative B-scan and the presence of foveal subretinal fibrosis (beta=0.28, P=0.018)
in the follow up were associated with poor postoperative BCVA.
CONCLUSION: Vitrectomy without SRH management for massive VH
secondary to PCV improved/stabilized visual function in the long-term
observation. Eyes presenting with thicker SRH preoperatively and forming foveal
subretinal fibrosis in the follow-up period tended to have worse BCVA.
KEYWORDS: polypoidal choroidal vasculopathy;
vitreous hemorrhage; vitrectomy; visual acuity
DOI:10.18240/ijo.2019.12.07
Citation: Li
ZX, Hu YJ, Atik A, Lu L, Hu J. Long-term observation of vitrectomy without subretinal
hemorrhage management for massive vitreous hemorrhage secondary to polypoidal
choroidal vasculopathy. Int J Ophthalmol
2019;12(12):1859-1864
INTRODUCTION
Polypoidal choroidal vasculopathy (PCV)
is a choroidal vascular abnormality characterized by a branching, polypoidal,
vascular network with choroidal lesions. PCV causes pigment epithelial and
neurosensory retinal detachment, a recurring problem associated with subretinal
leakage and hemorrhage[1-6]. In
those diagnosed with PCV, vitreous hemorrhage (VH) occurs in 19.9%; and among
patients at first PCV diagnosis, 4.5% present with VH[7].
The mechanism that allows a subretinal
hemorrhage (SRH) to cloud the vitreous has remained enigmatic[8-10]. If SRH is thick, the patient is at risk
for VH, which often results in a poor prognosis for central visual acuity[11-13].
Vitrectomy with or without
subretinal manipulation were the main surgical methods for PCV complicated by
VH. However, little information concerning associated surgery outcomes has been
reported and the attempt to define an ideal treatment has been inconclusive[14-15]. In fact, the VH is mainly
caused by the breakthrough of SRH and there is no consensus on the management
of chronic SRH. The long-term observation of relative surgical interventions in
such condition will help ophthalmologists develop treatment strategy. However,
the information is limited. Thus, the purpose of this study was to report the
long-term observation of a large sample of patients treated with vitrectomy
without SRH management for VH secondary to PCV.
SUBJECTS AND METHODS
Ethical Approval The study followed the guidelines of
the Declaration of Helsinki. Ethical approval was obtained from the
Institutional Review Board of Zhongshan Ophthalmic Center and written informed
consent was obtained from all subjects.
The medical records of patients with
PCV-related VH were reviewed retrospectively in Zhongshan Ophthalmic Center in
Guangzhou city of China. Analysis was performed on 86 consecutive patients (86
eyes) who were presented with PCV secondary to VH, between August 10, 2012 and
October 31, 2017. Patients were treated with a 3-port pars plana vitrectomy
(PPV), using a standard 23-gauge sutureless system. The inclusion criteria were
as follows: 1) Vision deterioration because of massive VH, defined as dense VH
with complete obscured fundus by slit lamp examination after full mydriasis; 2)
Diagnoses of PCV made either preoperatively or postoperatively, based on the
results of fundus examination, optical coherence tomography (OCT), fundus
fluorescein angiography (FFA) and indocyanine green angiography (ICGA). PCV was
defined as the presence of a branching vascular network with polypoidal or
aneurysmal structures at any visits as determined by ICGA, and/or the presence
of elevated orange-red lesions observed at fundus examination during the
operation, and/or multiple sero-sanguinous retinal pigment epithelium
detachments, and/or double-layer sign or thumb-like polyps on OCT[16-17]; 3) The absence of
other ocular diseases that affect visual acuity (i.e., age related
macular degeneration, retinal vein occlusion, diabetic retinopathy, choroidal
melanoma, retinal detachment and retinal vasculitis). Patients with VH
secondary to other eye diseases, any severe systemic diseases and any retinal
tears with ultrasound preoperatively have been excluded.
All patients underwent a
comprehensive ophthalmologic examination, including a test of best-corrected visual
acuity (BCVA), slit-lamp microscope examination, ultrasound biomicroscopy (UBM)
and B-scan ultrasonography. Preoperative data included BCVA, duration of
symptoms, and the characteristics of VH. Postoperative BCVA, fundus
photography, spectral-domain OCT (SD-OCT; Heidelberg, Germany), FFA and ICGA
were also obtained.
All patients underwent a 23-gauge
PPV, under local or general anesthesia, without subretinal manipulation. The
surgical procedure consisted of a core and peripheral vitrectomy. If a retinal
tear was observed during the operation, endophotocoagulation was used to create
chorioretinal adhesions and silicone oil used for an intraocular tamponade. For
those without retinal tears, vitrectomy was performed without external
indentation, SRH management, or intraocular tamponade.
Following treatment was done if
lesion activity was supposed to be present as follows: early-stage intense
saccular hyperfluorescence and late-stage leakage/staining of the polypoidal
lesions and accumulation of fluid in ICGA[18].
Intravitreal ranibizumab (0.5 mg/0.05 mL; Lucentis; Genentech, Inc) alone
or combined with photodynamic therapy (PDT) were applied to these eyes
postoperatively.
BCVA was measured by a standard
Snellen visual acuity chart and converted to a logarithm of minimal angle of
resolution (logMAR) scale for statistical analysis. Visual acuity was described
as improved or worsened if there was a change of more than two Snellen lines,
and stable if within two lines from baseline. According to previous methods[19-20], no light perception
was set at 2.9 logMAR, light perception at 2.6 logMAR, hand movements at 2.3
logMAR, and counting fingers (CF) at 1.85 logMAR.
Statistical Analysis The Mann-Whitney U test was used
for comparison of preoperative, 3mo after surgery, and final postoperative
BCVAs. Univariate and multiple regression analyses were performed to explore
the association between BCVA at final visit with age, gender, history of
diabetes mellitus, history of hypertension, duration of symptom, area of SRH,
and thickness of SRH and foveal subretinal fibrosis. A P value of 0.05
or less was considered statistically significant. All statistical analyses were
performed using SPSS for Windows version 17.0 (SPSS, Inc, Chicago, Illinois,
USA).
RESULTS
Eighty-six consecutively treated
eyes of 86 patients were included in this study. The baseline demographic data
of subjects and ocular characteristics are shown in Table 1. Nine eyes (10.5%)
had received previous treatments for PCV, including intravitreal ranibizumab in
7 eyes (8.2%) and PDT in 2 eyes (2.3%). The other 77 eyes (89.5%) had VH as
their initial presenting feature, which had an undefined diagnosis before
undergoing 23-gauge PPV and were diagnosed postoperatively (Figure 1). The
average duration from the onset of visual symptoms to the first visit was
72.2±53.8d (range 20-270d). B-scan showed VH in all eyes, extensive SRH in 54
eyes (62.8%) with mean thickness of 4.61±
Table 1 Demographics and clinical
characteristics of patients with massive VH secondary to PCV treated by
23-gauge PPV
|
Parameters |
Values |
|
Age (y) |
|
|
Mean (SD) |
59.9 (8.1) |
|
Range |
39-87 |
|
Gender, n (%) |
|
|
Male |
56 (65.1) |
|
Female |
30 (34.9) |
|
Hypertension, n (%) |
|
|
No |
54 (62.8) |
|
Yes |
32 (37.2) |
|
Diabetes mellitus, n (%) |
|
|
No |
79 (91.9) |
|
Yes |
7 (8.1) |
|
PCV lesion, n (%) |
|
|
Monocular |
77 (89.5) |
|
Binocular |
9 (10.5) |
|
Duration of VH (d) |
|
|
Mean (SD) |
72.2 (53.8) |
|
Range |
20-270 |
|
Follow-up time (mo) |
|
|
Mean (SD) |
25.5 (9.2) |
|
Range |
12-35 |
SD: Standard deviation.
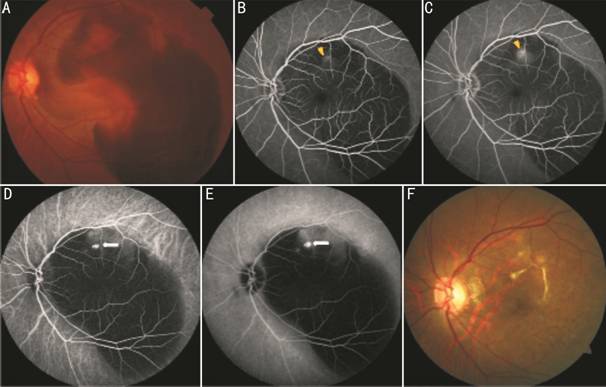
Figure 1 Submacular hemorrhage due
to PCV after vitrectomy A: Fundus photography shows
the dense SRH with CF/
Eighty-four eyes (97.7%) without a
retinal tear received 23-gauge vitrectomy; and 2 (2.3%) underwent vitrectomy
combined with endophotocoagulation and silicone oil tamponade, when a retinal
tear was observed during the operation. The yellowish thick organized SRH
companied by reddish-brown SRH on the border was observed in 79 eyes (91.9%)
and macular subretinal fibrosis was found in 7 eyes (8.1%) during the surgery.
The average size of the SRH was 28.9 disc areas (range 8.2 to 86.7 disc areas),
based on fundus photography one week after surgery. The submacular hemorrhage
was absorbed in 3.7±1.6mo (range 1-7mo) and SRH was almost automatically
absorbed clearly in 6.9±3.4mo (range 4-11mo).
The mean follow-up time was
25.5±9.2mo (range 12-35mo). During the follow-up period, 15 eyes (17.4%)
received phacoemulsification and intraocular lens implantation at a follow-up
visit. At the final visit, subretinal fibrosis in the posterior pole area were
found in 14 eyes (16.3%). Among these eyes, 6 (7.0%) involved the fovea.
Ciliary body detachment developed in 4 eyes (4.7%) at one week after the
operation but fortunately, this resolved itself within 4 to 12wk without
specific treatment. There were no other complications including tractional
retinal/choroidal detachment, glaucoma, or endophthalmitis.
Nineteen eyes (22.1%) needed
postoperative management of active PCV. Intravitreal ranibizumab was injected
into 15 eyes (17.4%), 4 (4.7%) received combined treatment with PDT. The
average number of intravitreal ranibizumab injections was 1.81±0.93 (range 1 to
3 injections). Four eyes (4.7%) had recurrent VH in the follow-up period. One
of them received 3 ranibizumab injections and the other underwent the combined
treatment (intravitreal ranibizumab and PDT).
At baseline, 78 eyes (90.7%) had
visual acuity ranging from CF to no light perception. Mean preoperative BCVA
was 2.16±0.39 (logMAR; range, 1.2-2.9); while postoperatively, visual acuity
improved to 1.42±0.66 (P<0.001; range 0.1-2.9) 3mo after vitrectomy
and 1.23±0.74 (P<0.001; range 0.2-3.2) at the final visit. BCVA
improved in 54 (62.8%) of the 86 eyes, remained unchanged in 28 eyes (32.5%),
and aggravated in 4 eyes (4.7%) at the finial visit caused by recurrent VH.
In univariate regression analysis,
thicker SRH in the B-scan preoperatively (P<0.001) and subretinal
fibrosis involving fovea (P<0.001) at the final visit were predictors
for worse BCVA at the final visit, which was consistent with previous studies[11-13]. At no time-point did visual
outcomes appear to correlate with age (P=0.165), gender (P=0.536),
history of diabetic mellitus (P=0.219), history of hypertension (P=0.382),
hemorrhage duration (P=0.750) or area of SRH (P=0.849).
In multiple regression analysis,
increased thickness of SRH (beta=0.33, P=0.025) in the B-scan before
surgery and the presence of foveal subretinal fibrosis (beta=0.28, P=0.018)
predicted the worse postoperative BCVA.
DISCUSSION
PCV is considered a posterior uveal
bleeding syndrome, and often results in a large, thick VH. Various
vitrectomy-based methods are used to manage severe hemorrhagic complications
caused by PCV but many complications have been reported with vitrectomy
combined with retinotomy. Abdel-Meguid et al[21]
reported postoperative complications among 39 eyes with SRH that
underwent PPV with retinotomy. In their study, 10 eyes (25.6%) had
proliferative vitreoretinopathy leading to postoperative retinal detachment; and
11 eyes (28.2%) had postoperative hypotony (intraocular pressure less than
For SRH related to PCV, reports on
vitrectomy with subretinal tissue plasminogen activator (tPA) injection are
conflicting. In a sample of 15 eyes, Kimura et al[23]
reported encouraging results of complication-free surgeries. However,
compared to our subjects, their study had patients with smaller/thinner SRHs
(mean 5.7±4.9 disc diameters) with shorter symptom duration (mean 9.5±4.5d) and
with less follow-up time (mean 9.4±3.1mo). Another large, retrospective review
of submacular hemorrhage treated with vitrectomy combined with subretinal tPA
injection found partial or no hemorrhage displacement in 18 eyes (18%),
rhegmatogenous retinal detachment in 4 eyes (4%), VH in 2 eyes (2%), and
recurrent SRH in 6 eyes (6%)[24]. The differing
results among these studies were primarily the result of variations in the
amount of time elapsed between onset and treatment, the area and thickness of
SRH, and whether the fovea were involved.
In our study, the mean interval from
symptom onset to the first visit were quite long (72.2±53.8d) and the SRH was
larger, thicker and organized. Thicker SRH is associated with increased iron,
hemosiderin and fibrin deposition is toxic to photoreceptors, large clot retraction
could sheer and damage photoreceptors and a large physical separation of the
photoreceptors from the RPE in this stage usually causes atrophy and disciform
scar formation. The SRH in our series also encircled the optic nerve and
adhered to the underlying retinal pigment epithelium or subretinal surface.
Such cases were frequently excluded from studies of SRH management with
subretinal tPA injection[23-25].
The blood does not liquefy with conventional doses of tPA in these cases
and the dose of tPA needed in this situation is far more than the recommended
maximum of 50 μg. In addition, a compulsory physical separation of the
photoreceptors from the RPE in this stage can cause atrophy and disciform scar
formation. Thus, it is too early to consider subretinal tPA injection as the
gold-standard treatment for organized SRH.
Previous studies have shown that,
during the chronic stage (>14d) of massive of SRH, BCVA was not better than
the CF even after surgery[26-27].
Nevertheless, some studies demonstrated more favorable visual outcomes of
vitrectomy without SRH management, but the validity was limited by their small
sizes and short follow up period[14,16].
We found that BCVA improved significantly, both at 3mo after surgery and
at the final visit, consistent with studies on vitrectomy for VH secondary to
PCV[14-16]. Narayanan
et al[16] investigated outcomes of PPV
without drainage of SRH in 27 eyes with PCV and their findings showed that
57.1% of eyes had improved BCVAs by two or more Snellen lines postoperatively.
Serious postoperative complications included retinal tear/detachment (n=5,
17.9%) and choroidal detachment (n=1, 3.6%). Other complications
included macular subretinal fibrosis, organized dehemoglobinized blood and recurrent
VH. These results are in accordance with our study. In our study, 90.7% of eyes
included had shown severe vision loss (CF to no light perception), whereas 54
(62.8%) of the included 86 eyes had improved visual acuity by two Snellen
lines. We did not have any incidence of choroidal detachment, iatrogenic
retinal tear or retinal detachment. The ciliary body detachment that occurred
in 4 eyes (4.7%), resolved itself without specific treatment within 3mo. We
speculated that the low retinal complication rate in our study was associated
with carefully removal of the vitreous cortex and prohibition of external
indentation to avoid iatrogenic retinal tears, especially in the uplift areas
with SRH.
There is limited data on recurrence
rate of VH after vitrectomy in PCV. Narayanan et al[16]
noted recurrence rate of VH was 10.7% in 28 eyes. However, in our study, only 4
out of 86 eyes (4.7%) had recurrent VH during the follow-up period. This high
recurrence rate in the earlier study was mostly because of relatively high rate
of retinal tear (17.8%), as organized hemorrhage underneath and around retinal
tear would decrease the effect of retinal photocoagulation and SRH would cloud
the media of vitreous cavity through the retinal tear even after vitrectomy. In
addition, the destruction of abnormal vessels during the course of hemorrhage
breakthrough from polypoidal structures was also an explanation for the low
recurrence rate of PCV-related VH in our study.
Our study was limited by its lack of
a comparison group and thus difficult to underline the difference of impacts of
this surgical procedure with those of other management. Despite this and its
retrospective nature, we believe it offers important implications for
treatment. A prospective, randomized controlled clinical study is needed to
fully clarify the impact of this surgical procedure.
In
conclusion, this study reports the long-term outcomes in a large series of
patients with massive VH secondary to PCV treated by PPV without SRH management
and demonstrated vitrectomy without SRH management for such patients had
favorable visual outcome and less complications even after a long follow-up
period. As treatment for patients with VH secondary to PCV and chronic stage of
SRH was not well documented, our study provides a feasible method for the
management of such patients. Certainly, in the future, a randomized controlled
study with the surgical procedures of PPV and SRH management would bring better
understanding for the management of such condition.
ACKNOWLEDGEMENTS
Authors’
contributions: Li ZX, Hu
YJ, Hu J and Lu L were involved in the concept, design and conduct of the
study. Li ZX, Hu YJ contributed to the acquisition, analysis and wrote the
paper. Atik A involved in the analysis of data. All authors revised and edited
the manuscript.
Foundations:
Supported by
the National Natural Science Foundation of China (No.81271009); the Science and
Technology Planning Project of Guangdong Province, China (No
Conflicts of
Interest: Li ZX, None; Hu
YJ, None; Atik A, None; Lu L, None; Hu J, None.
REFERENCES