
·Clinical Research·
Chorioretinal
response to intravitreal aflibercept injection in acute central serous
chorioretinopathy
Byung Ju Jung, Kook Lee, Jin Hyung Park, Jae Hyung Lee
Department of Ophthalmology and
Visual Science, Seoul St. Mary’s Hospital, College of Medicine, the Catholic
University of Korea, Seoul 06591, Korea
Correspondence to: Jae Hyung Lee. Department of
Ophthalmology and Visual Science, Seoul St. Mary’s Hospital, College of
Medicine, the Catholic University of Korea, 505 Banpo-dong, Seocho-ku, Seoul
06591, Korea. voilaju@nate.com
Received:
Abstract
AIM: To evaluate chorioretinal responses to intravitreal aflibercept injection
(IAI) in patients with acute central serous chorioretinopathy (CSC).
METHODS: Seventy-one eyes from 71 patients with symptomatic
CSC for less than six months were included. Thirty-five eyes received a single
IAI and 36 eyes were observed without treatment. Best-corrected visual acuity
(BCVA), central subfield foveal thickness (CSFT), and subfoveal choroidal
thickness (SFCT) were assessed at baseline and at 1, 2, and 3mo.
RESULTS: The mean SFCT in the IAI group decreased at 1mo,
rebounded at 2mo and remained stable at 3mo compared to the baseline, while
significant change was not noted in the observation group. The mean CSFT
decreased significantly during the 3-month study period in both groups, and was
significantly lower in the IAI group at 1mo (P<0.001). A rebound of
CSFT between 1 and 2mo was noted in 14 eyes (40.0%) in the IAI group and in 1
eye (2.8%) in the observation group (P<0.001). The significant visual
improvement was achieved from 1mo in the IAI group, and from 2mo in the
observation group. The rate of complete absorption of subretinal fluid at 3mo
did not differ between the two groups. (45.7% vs 41.7%, P=0.813).
CONCLUSION: A single IAI for acute CSC induce a transient
decrease in SFCT and CSFT, which implies that IAI may have a pharmacological
effect on the underlying hyperpermeable choroid in acute CSC.
KEYWORDS: aflibercept; acute central serous
chorioretinopathy; anti-vascular endothelial growth factor; choroidal
hyperpermeability; choroidal thickness
DOI:10.18240/ijo.2019.12.08
Citation:
Jung BJ, Lee K, Park JH, Lee JH. Chorioretinal response to intravitreal
aflibercept injection in acute central serous chorioretinopathy. Int J
Ophthalmol 2019;12(12):1865-1871
INTRODUCTION
Central serous chorioretinopathy
(CSC) is characterized by serous neurosensory retinal detachment at the
posterior pole of the retina by the retinal pigment epithelium (RPE) leakage[1]. It was previously thought that the leakage from the
RPE after a breakdown of the outer blood-retinal barrier was primarily involved
in the pathophysiology of CSC. However, indocyanine-green angiography (ICGA)
findings in CSC, which are characterized as delayed choroidal infusion,
choroidal vascular hyperpermeability, and choroidal venous dilation suggest
that the choroid is primarily involved in the pathophysiology[2-3]. Studies using enhanced-depth imaging optical coherence
tomography (OCT) have shown that subfoveal choroidal thickness (SFCT) in eyes
with CSC was significantly larger than that of the unaffected eyes, or healthy
normal controls[4]. Additionally, topographic
studies have shown a high level of correlation between thickened choroid on OCT
and choroidal hyperfluorescence on ICGA[5-6].
Although CSC usually resolves
spontaneously within several months, some patients may progress chronic CSC,
which can cause photoreceptor degeneration and RPE atrophy, resulting in
irreversible anatomical and functional damage[7].
Photodynamic therapy (PDT) has been effective in resolving subretinal fluid in
recurrent or chronic CSC[8-11];
however, potential side effects of PDT hinder its extensive application for
acute CSC[12]. Several studies posit
that anti-vascular endothelial growth factor (VEGF) therapy may also lead to
the decrease of subretinal fluid in CSC by reducing choroidal vascular hyperpermeability,
based on its anti-permeability properties[13-14]. Although clinical results with bevacizumab and
ranibizumab were acceptable for the treatment of chronic CSC, they were not
promising compared to the anatomic resolution of low-fluence PDT treatment in
prospective comparative studies[15]. It seems
that these anti-VEGF drugs cannot fully address leakage from hyperpermeable
choroidal vessels to induce complete absorption of subretinal fluid, as PDT
does.
Aflibercept has been reported to
have higher VEGF-binding affinity, and induce greater decreases in SFCT in eyes
with neovascular age-related macular degeneration (AMD) than ranibizumab[16-17]. Additionally, evidence
suggests that aflibercept induced a further reduction in choroidal and retinal
thickness in AMD patients who responded insufficiently to either ranibizumab or
bevacizumab[18-19]. These
results imply that aflibercept may have more influence on choroidal vasculature
than previous anti-VEGF drugs. Based on these results, aflibercept’s superior
potency in reducing leakage from hyperpermeable choroidal vessels could be
applied to the treatment of CSC. Indeed, Pitcher et al[20] reported that repeated aflibercept injections
resulted in a significant decrease in SFCT in chronic CSC. However, there are
currently no published studies evaluating the changes of subretinal fluid and
choroidal thickness after aflibercept injection in acute CSC. On the basis of
laboratory and clinical data, significant biological activity of aflibercept
(2.0 mg) is estimated to persist for 4 to 8wk after a single intravitreal
administration. Our hypothesis is that, if aflibercept can has pharmacologic
effect on the underlying choroid and lead to the reduction of subretinal fluid
in acute CSC, fluctuation of subretinal fluid may be observed as the effects of
aflibercept diminishes after a single injection. Indeed, the fluctuating
pattern of retinal thickness was seen noted when injection frequency was spaced
from 4 to 8wk in the VIEW studies. In the current pilot study, we aimed to
investigate the short-term response to intravitreal aflibercept injection in
patients with acute CSC by focusing on chorioretinal changes.
SUBJECTS AND METHODS
Ethical Approval We conducted a retrospective review
of the medical records of patients diagnosed with acute unilateral CSC between
January 2015 and August 2017 at the Seoul St. Mary’s Hospital of the Catholic
University of Korea in South Korea. This nonrandomized, retrospective,
comparative, interventional case series was approved by the Institutional
Review Board of the Catholic Medical Center, and was conducted according to the
tenets of the Declaration of Helsinki. Informed consent was obtained from all
patients before IAI and included mentions about other possible treatment options
such as observation, focal photocoagulation, PDT, and mineralocorticoid
blockers.
Inclusion criteria were as follows:
1) visual symptoms for less than six months; 2) subretinal fluid involving the
fovea, with or without RPE detachment, on spectral-domain (SD) OCT; 3) presence
of active focal leakage on fluorescein angiography (FA). Patients with a
history of previous treatment, including intravitreal anti-VEGF injection,
laser photocoagulation, or PDT, were excluded. Patients who had evidence of chronic
CSC (diffuse RPE change including atrophic dependent tracks and diffuse
hyperfluorescent leakage on FA), concomitant choroidal neovascularization,
polypoidal choroidal vasculopathy (PCV), or any other maculopathy causing
subretinal fluid accumulation, were also excluded. Patients divided into 2
groups: a single intravitreal aflibercept injection (IAI group) and observation
group.
Each patient was scheduled for a
monthly follow-up examination until 3mo after baseline visit. All patients
underwent complete ocular examinations at the baseline and at each subsequent
visit, which included the Snellen best-corrected visual acuity (BCVA) test,
dilated fundus examination with slit-lamp biomicroscopy, and OCT. Spectralis
OCT (Heidelberg Engineering, Heidelberg, Germany) was used to evaluate the
presence of fluid and central subfield foveal thickness (CSFT). CSFT was
defined as the mean retinal thickness in the
The main outcome measures include
changes of CSFT, SFCT and BCVA, from baseline and across visits. The proportion
of eyes achieving complete resolution of subretinal fluid at each follow-up
visits was assessed. Also, we evaluated the proportion of eyes that showed
significant changes in CSFT between each visit. For statistical analysis,
Snellen visual acuity was converted to the logarithm of the minimal angle of
resolution (logMAR). A repeated-measures analysis of variance (rmANOVA) with
Bonferroni’s correction for multiple comparisons was used to assess the time course
of changes in the CSFT, SFCT and BCVA over time. The unpaired t-test and
Chi-square test were used to compare continuous and categorical variables
between the two groups, respectively. SPSS for Windows ver. 20 (SPSS, Chicago,
IL, USA) was used for statistical analyses. The data are expressed as
mean±standard deviation; a P value less than 0.05 was considered
significant.
RESULTS
Among the 84 eligible patients (84
eyes), 13 patients were excluded due to missed follow-ups or missing data.
Thus, we evaluated the clinical data from 71 patients, with 35 patients in the
IAI group and 36 patients in the observation group. The mean age of the
subjects was 49.7±9.6y (range: 31 to 66y), and the mean duration of symptoms
before study entry was 2.7±1.9mo (range: one to six months). On baseline FA,
all 71 eyes showed active focal fluorescein leakage. Choroidal
hyperpermeability was seen on ICGA in 27 eyes (77.1%) in the IAI group and 25
eyes (69.4%) in the observation group. Table 1 summarizes the baseline
characteristics of the patients in the two groups. There were no significant
differences in age, sex, duration of symptoms, number of previous episodes,
logMAR BCVA, CSFT, or SFCT between the two groups.
Table 1 Baseline demographic and
clinical characteristics of the both study population
|
Parameters |
Aflibercept group (n=35) |
Observation group (n=36) |
P |
|
Age (y) |
51.3±9.5 |
48.1±9.4 |
|
|
Gender (M/F) |
29/6 |
24/12 |
0.173b |
|
Duration of symptoms (mo) |
3.0±1.9 |
2.5±1.8 |
|
|
No. of previous episodes |
0.26±0.51 |
0.22±0.49 |
|
|
BCVA (logMAR) |
0.30±0.20 |
0.24±0.19 |
|
|
Central subfield foveal thickness
(µm) |
445±121 |
450±106 |
|
|
Subfoveal choroidal thickness (µm) |
444±100 |
426±95 |
|
|
Subfoveal choroidal thickness of
unaffected eye (µm) |
362±92 |
369±82 |
|
|
Choroidal hyperpermeability on
ICGA, n (%) |
27 (77.1) |
25 (69.4) |
0.594b |
BCVA: Best-corrected visual acuity;
logMAR: Logarithm of the minimal angle of resolution; ICGA: Indocyanine-green
angiography. Data are mean±standard deviation unless otherwise noted. aUnpaired
t-test. bChi-square test.
Table 2 summarizes the anatomical
and visual outcomes of the two groups. The mean CSFT changed significantly
during the 3-month study period in both groups (P<0.001, P<0.001,
rmANOVA). The mean CSFT in the IAI group decreased from 445±121 μm at baseline
to 276±62 μm at 1mo, 289±78 μm at 2mo, and 293±82 μm at 3mo. The mean CSFT in
the observation group also decreased from 449±106 μm at baseline to 358±70 μm
at 1mo, 318±92 μm at 2mo, and 303±92 μm at 3mo. CFST at each follow-up were all
significantly smaller than that at baseline in both groups (P<
Table 2 Anatomic and functional
outcomes after aflibercept injection or observation for the treatment of acute
CSC
|
Parameters |
Aflibercept group (n=35) |
Observation group (n=36) |
P |
|
Central subfield foveal thickness (µm) |
|
|
|
|
Baseline |
445±121 |
449±106 |
|
|
1mo |
276±62 (<0.001)d |
358±70 (<0.001)d |
< |
|
2mo |
289±78 (<0.001)d |
318±92 (<0.001)d |
|
|
3mo |
293±82 (<0.001)d |
303±92 (<0.001)d |
|
|
Subfoveal choroidal thickness (μm) |
|
|
|
|
Baseline |
444±100 |
426±95 |
|
|
1mo |
426±105 (<0.001)d |
425±93 (1.000)d |
|
|
2mo |
440±101 (0.918)d |
424±99 (0.632)d |
|
|
3mo |
437±101 (0.190)d |
423±90 (0.093)d |
|
|
Mean BCVA (logMAR) |
|
|
|
|
Baseline |
0.300 |
0.241 |
|
|
1mo |
0.222 (0.017)d |
0.191 (0.120)d |
|
|
2mo |
0.211 (0.016)d |
0.163 (0.015)d |
|
|
3mo |
0.188 (0.006)d |
0.152 (0.004)d |
|
|
No. of eyes rebound of central subfield foveal
thickness compared to the previous visit (%) |
|
|
|
|
1mo |
0 (0) |
3 (8.3) |
|
|
2mo |
14 (40.0) |
1 (2.8) |
<0.001b |
|
3mo |
4 (11.4) |
2 (5.6) |
|
|
No. of eyes with complete fluid absorption at 3mo (%) |
16 (45.7) |
15 (41.7) |
0.813b |
BCVA: Best-corrected visual acuity;
logMAR: Logarithm of the minimal angle of resolution; CSC: Central serous
chorioretinopathy. aUnpaired t-test; bChi-square
test; cFisher’s exact test; dPaired t-test
(compared with baseline).
In the IAI group, intraocular
complications including endophthalmitis, cataract formation, intraocular
pressure changes, and rhegmatogenous retinal detachment were not notified. All
eyes showed a significant decrease of CSFT at 1mo compared to baseline.
However, an increase of CSFT more than 7% was noted in 14 of 35 eyes (40.0%)
between 1 and 2mo. In 4 eyes (11.4%), CSFT increased significantly between 2
and 3mo. In the observation group, an increase of CSFT more than 7% compared to
the previous visit was noted in 3 eyes (8.3%) at 1mo, 1 eye (2.8%) at 2mo, and
2 eyes (5.6%) at 3mo. The proportion of eyes that showed a rebound of CSFT was
significantly higher in the IAI group at 2mo (P<0.001).
The mean SFCT in the IAI group
changed significantly during the 3-month study period. Compared with the
baseline value of 444±100 μm, the mean SFCT in the IAI group decreased to
426±105 μm at 1mo (P<0.001), rebounded to 440±101 μm at 2mo (P=0.918)
and remained stable at 3mo (437±101 μm, P=0.190). The SFCT in the
observation group was 426±95 μm at baseline, 425±93 μm at 1mo, 424±99 μm at
2mo, and 423±90 μm at 3mo, and the change was not significant (P=0.254,
rmANOVA). The representative cases are shown in Figure 1.
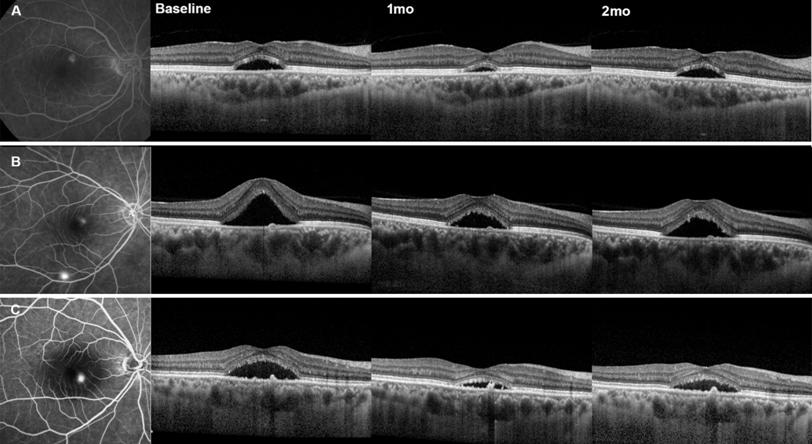
Figure 1 Representative three cases with
acute CSC treated with intravitreal aflibercept injection After single intravitreal
aflibercept injection, all eyes showed a transient decrease in SFCT and CSFT at
1mo, and showed rebounding tendency at 2mo.
The mean logMAR BCVA changed
significantly during the 3-month study period in the IAI (P<0.001,
rmANOVA) and observation groups (P=0.001, rmANOVA). In the IAI group,
the visual improvement was statistically significant from 1mo (0.22±0.16,
Snellen equivalent of 20/33) compared with baseline values (0.30±0.20, Snellen
equivalent of 20/40) (P=0.017). In the observation group, the visual
improvement was statistically significant from 2mo (0.16±0.18, Snellen equivalent
of 20/29) compared with baseline values (0.24±0.21, Snellen equivalent of
20/35; P=0.015). At 3mo, complete absorption of subretinal fluid was
noted in 16 of 35 eyes (45.7%) in the IAI group, and in 15 of 36 eyes (41.7%)
in the observation group (P=0.813). The changes in SFCT, CSFT, and
logMAR BCVA are shown in Figure 2.
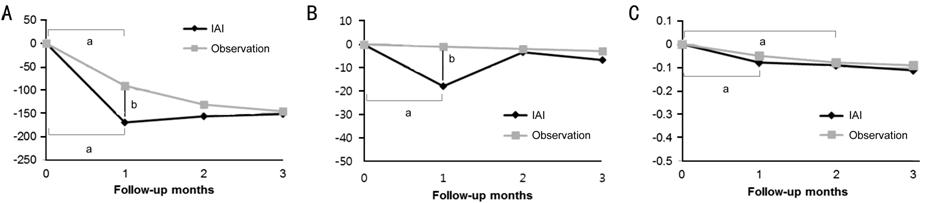
Figure 2 Graphs showing treatment
outcomes in the study A: Mean change in CSFT; B: Mean
change in SFCT; C: Mean change in BCVA. aP<0.05 compared
with baseline. bP<0.05 compared between the two groups.
DISCUSSION
CSC is commonly classified into
acute and chronic cases, but the underlying ICGA abnormality of both types is
choroidal vascular hyperpermeability. PDT directly targets choroidal
circulation and damages the choriocapillaris, leading to decreased choroidal
vascular hyperpermeability that is represented as a decrease in choroidal
thickness on OCT[21-22]. Zhao et
al[23] reported a significant decrease
in choroidal thickness in chronic CSC patients who received 50%-dose PDT. But
in patients who received 30%-dose PDT, the results were inferior in resolving
subretinal fluid. Furthermore, recent studies have demonstrated that SFCT
increased toward baseline values at recurrence after either PDT or bevacizumab
injection, and the extent of the SFCT reduction was associated with the rate of
recurrence in chronic CSC[22,24].
Therefore, choroidal thickness changes may be used as a relevant
parameter in the assessment of treatment effects, and monitoring exudative
activity of CSC. In the current study, a single IAI induced a significant
decrease in SFCT at 1mo then rebounded at 2mo. The mean CSFT also significantly
decreased at 1mo, and an increase of CSFT was noted at 2 and 3mo in about half
of the patients in the IAI group. The exudation from the choroid in eyes with acute
CSC seems to be transiently controlled after a single IAI. This implies that
choroidal hyperpermeability in CSC may potentially be VEGF mediated, and that
aflibercept indeed causes choroidal thinning.
A decrease in choroidal thickness,
and subsequent decrease in subretinal fluid, in CSC could be explained by
anti-VEGF drugs inducing choroidal vasoconstriction by decreasing levels of
nitric oxide, or by anti-VEGF drugs reducing choroidal fenestrations[25]. VEGF plays a crucial role in choriocapillaris
maintenance, and the choriocapillaris has been shown to be vulnerable to VEGF
inhibition. Experimental studies using monkeys demonstrated a reduction in the
number of fenestrations and choriocapillaris endothelium thickness after one
injection of ranibizumab and aflibercept, but showed a more pronounced
reduction after aflibercept treatment[25-26].
Blockade of VEGF-A, and/or simultaneous inhibition of multiple molecules in the
VEGF family, such as VEGF-B and placental growth factor (PlGF)[27], may explain the prominent effect of aflibercept on
the choroid. Additionally, this may be the reason as to why aflibercept appears
to be more effective in diseases associated with a thicker choroid and
choroidal vascular hyperpermeability, such as PCV and CSC[16,20].
It is interesting that the effect of
a single IAI did not last up to 2mo in this study. Aflibercept is known to
maintain significant intravitreal VEGF-binding activity for 10-12wk after a
single injection, as predicted by a mathematical model[28],
which is further supported by major clinical trials[29-30]. The predicted biological activity of a therapeutic
macromolecule depends to a large degree on both its intraocular half-life and
its binding affinity. The binding affinity for VEGF of aflibercept is about 100
times higher than that of ranibizumab or bevacizumab[27].
The half-life of aflibercept (molecular weight: 115 kDa) in human eyes has not
yet been studied. Since the intraocular half-life of a macromolecule is
primarily determined by its molecular size, aflibercept may be estimated to
have a half-life between ranibizumab (molecular weight: 48 kDa) and bevacizumab
(molecular weight: 149 kDa)[31]. Therefore, the
main reason for the longer action of aflibercept could be explained by a large
increase in binding affinity, rather than similar elimination half-times. A
recent study demonstrated VEGF concentrations in aqueous humor were decreased
below the lower limit of quantification after intravitreal aflibercept
injections for about 10wk[32]. In contrast, in
patients with CSC, the aqueous humor level of VEGF was not significantly
increased compared to the healthy controls[33]. Therefore,
binding affinity to VEGF may not be the major decisive factor in determining
the biological activity of the anti-VEGF drug in CSC. We believe that the level
of VEGF in CSC may be insufficient for the molecules of aflibercept to bind and
demonstrate longer biologic activity, compared to that of ranibizumab or
bevacizumab.
The mean SFCT of the IAI group
returned to its baseline level at 2mo, and the mean CSFT at 2 and 3mo and the
rate of complete absorption of subretinal fluid at 3mo did not differ between
the IAI group and the observation group. The rates of our study are similar to
the results from previous comparative studies; the reported rate of complete
resolution of subretinal fluid ranged from 18.2% to 21.1% at 1mo, and from
27.3% to 44.0% at 3mo[34-36].
This may imply that aflibercept can suppress hyperpermeable choroidal vessels
only temporarily. This may lead to faster resolution of subretinal fluid and
higher rate of complete fluid resolution. Further comparative studies with
prospective designs are warranted to confirm this hypothesis.
There are some limitations to our
study, including a short follow-up period and the small number of patients. The
change in SFCT was measured manually, which carries the inherent possibility of
under- or overestimation. However, a similar rebound was noted at 2mo after a
single IAI in SFCT changes and automatically measured CSFT. We observed a
pharmacologic effect of aflibercept on the choroid by measuring choroidal
thickness on OCT, and angiographic improvements, such as a cessation of leakage
on FA or a decrease of choroidal hyperpermeability on ICGA was not assessed.
However, it was not feasible to routinely perform angiography after IAI, and
OCT seems to be a better modality for quantifying transient and subtle changes
in choroid. Finally, in this pilot study, the patients in the IAI group did not
receive 3 loading injections due to the economic burden, which might have been
more effective to assess the efficacy of aflibercept in acute CSC. However, the
purpose of this study was to investigate whether aflibercept can suppress the
exudation from the hyperpermeable choroid in acute CSC, and we did not aim to
evaluate the efficacy of aflibercept in the treatment of acute CSC. The rebound
of SFCT and CSFT after a single IAI observed in this study can clearly
demonstrate that aflibercept injection has pharmacologic effect temporarily on
the underlying choroid.
To our knowledge, our study is the
first to report anatomic and visual changes after aflibercept injection in
acute CSC. A transient decrease in choroidal thickness followed by a
significant decrease in subretinal fluid was observed after a single
aflibercept injection. Although anatomic and visual improvement was noted up to
three months, definitive conclusions cannot be made as to whether these
improvements would be significantly different from those of simple observation
in acute CSC. However, this pilot study demonstrates that hyperpermeable
choroidal vessels in acute CSC indeed respond to aflibercept. The results of
our study lend credence to the need for further comparative studies with
protocols involving repeated injections and longer follow-up periods to explore
the possible role of IAI in the treatment of acute CSC.
ACKNOWLEDGEMENTS
Conflicts of Interest: Jung BJ, None; Lee K,
None; Park JH, None; Lee JH, None.
REFERENCES