
·Meta-Analysis·
Treating
with besifloxacin for acute bacterial conjunctivitis: a Meta-analysis
Jun-Jie Wang1, Xin-Yi Gao2,
Hong-Zhuo Li3, Shan-Shuang Du2
1Changzhi Medical College, Changzhi
046000, Shanxi Province, China
2Shaanxi Ophthalmic Medical Center,
Xi’an No.4 Hospital, Affiliated Guangren Hospital, School of Medicine, Xi’an
Jiaotong University, Xi’an 710004, Shaanxi Province, China
3Heping Hospital Affiliated to
Changzhi Medical College, Changzhi 046000, Shanxi Province, China
Co-first authors: Jun-Jie Wang and Xin-Yi Gao
Correspondence to: Hong-Zhuo Li. Heping Hospital
Affiliated to Changzhi Medical College, Changzhi 046000, Shanxi Province,
China. lihz0999@sina.com; Shan-Shuang Du. Shaanxi Ophthalmic Medical Center,
Xi’an No.4 Hospital, Affiliated Guangren Hospital, School of Medicine, Xi’an
Jiaotong University, Xi’an 710004, Shaanxi Province, China. sunsea2252@sina.com
Received:
Abstract
AIM: To evaluate the relative efficacy and safety of besifloxacin for
treatment of acute bacterial conjunctivitis.
METHODS: A comprehensive search in PubMed, EMBASE Web of
Science, Cochrane Central Database and CNKI was undertaken for randomized
controlled trials (RCTs) comparing besifloxacin with other treatments or
placebo. The primary outcome measures were clinical resolution, rates of
bacterial eradication, individual clinical outcomes, cure rates, and bacterial
eradication rates of different kinds of pathogens. Safety outcomes were the
number of adverse effects (AEs). The final search was performed on August 2018.
RESULTS: Six RCTs were included. Four studies compared the
efficacy and safety of besifloxacin with placebo, 1 study compared besifloxacin
with moxifloxacin, and 1 study compared besifloxacin with gatifloxacin. A total
of 2780 patients met the inclusion criteria. Besifloxacin presented higher
efficacy and safety than did placebo in clinical resolution, rates of bacterial
eradication, individual clinical outcomes, cure rates, bacterial eradication
rates of different kinds of pathogens and the number of AEs. There was no
significant difference between besifloxacin and moxifloxacin or gatifloxacin in
the comparison items mentioned above.
CONCLUSION: Besifloxacin is highly effective and safe for
treatment of acute bacterial conjunctivitis. Further comparative trials
regarding the effect of besifloxacin for treatment of acute bacterial
conjunctivitis will aid in treatment decisions.
KEYWORDS: besifloxacin; acute bacterial
conjunctivitis; Meta-analysis; randomized controlled trials
DOI:10.18240/ijo.2019.12.13
Citation:
Wang JJ, Gao XY, Li HZ, Du SS. Treating with besifloxacin for acute bacterial
conjunctivitis: a Meta-analysis. Int J Ophthalmol 2019;12(12):1898-1907
INTRODUCTION
Acute conjunctivitis, which is
characterized by a self-limited course of inflammation of the conjunctiva with
persistent mucopurulent discharge, erythema and discomfort, is a contagious
infection of the ocular surface that affects individuals ranging from neonates
to the elderly. As one of the most common eye disorders, acute conjunctivitis
can easily spread from one person to another, especially in situations in which
individuals are in close personal contact, such as schools, daycare centers,
and chronic health care facilities[1-2].
The pathogens of acute conjunctivitis can be viral or fungal in nature;
however, approximately 78% of cases in children and half of cases in adults are
caused by bacteria. The most common causative bacterial species are Haemophilus
influenza (H. influenza), Streptococcus pneumoniae (S.
pneumoniae), Staphylococcus aureus (S. aureus), and Staphylococcus
epidermidis (S. epidermidis)[3-4].
In fact, most acute conjunctivitis cases are caused by several bacterial
species simultaneously, therefore, treatment often relies on clinical
experience and is usually initiated with a broad-spectrum ophthalmic
antibacterial treatment. Although acute bacterial conjunctivitis is a
self-limited disease and can resolve spontaneously due to the host’s immune
factors in 1-2wk[5], topical ophthalmic
antibiotics are warranted as they hasten clinical resolution and
microbiological remission, decreasing the risk of relapse and the development
of complications such as keratitis, orbital cellulitis, and panophthalmitis[1,6]. Classical antibacterials options
include tobramycin, trimethoprim, ciprofloxacin, gatifloxacin and moxifloxacin[7]. However, the widespread use of broad-spectrum
antibiotics has resulted in the emergence of resistance to those typical
antibiotics[8-9]. Therefore,
developing new antibiotics with high efficacy and safety against some resistant
bacteria is necessary.
Besifloxacin is an
advanced-generation fluoroquinolone and represents the first
chlorofluoroquinolone developed specifically for ophthalmic use. Unlike older
fluoroquinolones that selectively target either DNA gyrase or topoisomerase IV,
besifloxacin has balanced activity against both of those enzymes[10-12]. In vitro studies have
demonstrated that its antibacterial capacity exceeds that of most other
fluoroquinolones and nonfluoroquinolones, especially against multidrug
resistant Staphylococci[13-14].
Several in vivo studies have also drawn optimistic conclusions regarding
the antibacterial potency of besifloxacin[15-16]. At present, besifloxacin ophthalmic suspension 0.6%,
a long-acting topical formulation using DuraSite technology (InSite Vision,
Alameda, California) that helps retain therapeutic doses of a drug on the
surface of the eye, has been approved in the United States, Canada, and various
countries in Latin America, Europe, and Asia for the treatment of acute
bacterial conjunctivitis[17]. However, some data
among in vivo studies are contradictory, for example, Karpecki et al[15] found that besifloxacin can eradicate S.
pneumoniae more efficiently than placebo, while Silverstein et al[18] argued that the eradication rate of S. pneumoniae
is not better than vehicle. Therefore, summarizing the data of published
studies and drawing a general conclusion to guide the clinical application of
besifloxacin are necessary. This review demonstrates the efficacy and safety of
besifloxacin for treatment of acute bacterial conjunctivitis via
Meta-analysis of randomized placebo-controlled trials. We also compare the
effect of besifloxacin with other antibiotics if necessary.
MATERIALS AND METHODS
Search Strategy Two trained investigators performed
an electronic literature search of major online databases, including PubMed,
Embase, Web of Science, Cochrane Central Database and CNKI (all relevant
studies were published in English or Chinese with the date range from inception
to August 31, 2018). Key terms for searching the title and abstract included
“besifloxacin”, “synaphymenitis”, “epipephysitis” and “conjunctivitis”.
Eligibility Criteria Articles were included if they met
the following criteria: 1) target population: individuals with acute bacterial
conjunctivitis; 2) intervention: besifloxacin and placebo or other antibiotics
as controls; 3) outcome: evaluated the clinical resolution, rates of bacterial
eradication and adverse effects (AEs); 4) type of studies: prospective,
randomized controlled trials (RCTs); and 5) full text published in English or
Chinese.
Study Identification Two investigators independently
identified articles using the eligibility criteria listed above. After reading
the title and the abstract, if the investigators considered the articles
potentially eligible, they would subsequently read the full text. If there was
any disagreement between the investigators, they discussed the issue with a
third investigator until they reached an agreement.
Risk of Bias and Assessment of Study
Quality The methodological quality of each
eligible study was independently determined by two investigators by using the
Cochrane Risk of Bias tool, provided in the Cochrane Handbook of Systematic
Reviews of Interventions (Version
Data Extraction The two investigators analyzed the
full text of all eligible articles and then extracted the following
information: study characteristics, publication years, number of participants
allocated to each group, the mean age of each group, number of males and
females in each group, the method of intervention and the assessment time. If
the two investigators disagreed with each other, they would ask for an opinion
from a third investigator until they finally came to a consensus.
Statistical Analysis The two investigators found and
recorded parameters for following outcomes: clinical resolution, rates of
bacterial eradication, individual clinical outcomes, cure rates, bacterial
eradication rates of different kinds of pathogens, and the number of AEs.
Statistical analyses were carried
out using RevMan 5.3 software. For all comparisons, odds ratios (ORs) and 95%
confidence intervals (CIs) were calculated as summary statistics for
dichotomous variables. The mean difference (MD) and 95%CI were calculated as
summary statistics for continuous variables. P<0.05 was regarded as
statistically significant. Statistical heterogeneity was quantified with the
use of Chi-square (χ2) and I2 tests. Pooled
summary statistics were calculated using a fixed-effect model ifsignificant
heterogeneity was not detected. If heterogeneity existed after determining by a
statistically significant P<0.05 and I2>50%, a
random effect model was applied to unsolved heterogeneity. Otherwise, a fixed
effect model was used. We also performed subgroup analysis and sensitivity
analysis to identify the source of heterogeneity.
RESULTS
Literature Search By using our search strategy, we
identified 29 citations from online databases, the same articles had been taken
away. Of those 29 studies, 15 articles were from PubMed, 3 from EMBASE, 5 from
Web of Science, 1 from the Cochrane Central Database and 5 from CNKI. Figure 1
describes the flow of candidate and eligible articles. After reading the titles
and abstracts of the 29 articles, we found that 12 studies were not randomized
controlled trials, the major focus of 3 articles was the effect of Besifloxacin
on conjunctivitis, and the full text of 1 article could not be acquired. After
reading the full text of the remaining 13 articles, the compared items of 5
articles did not meet our requirements, and the data of 2 studies were not
presented in the form of mean values and standard deviations. Ultimately, 6 articles
were considered eligible for our study.
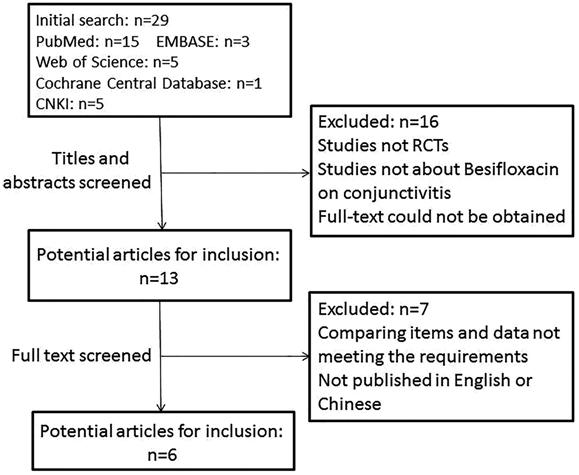
Figure 1 Flow diagram of studies
included in this Meta-analysis.
Study Characteristics The main characteristics of the 6
eligible studies are summarized in Table 1. Four studies compared the efficacy
and safety of Besifloxacin with placebo, 1 studies compared Besifloxacin with
Moxifloxacin, and 1 study compared Besifloxacin with Gatifloxacin. The earliest
study was published in 2009, while the latest study was accepted in 2017. The
number of participants varied from 16 to 482. A total of 2780 patients met the
inclusion criteria. The mean age of each group ranged from 15.2 days to 38.3
years old (the subjects of one study were neonates). The assessment time can be
regarded as identical, the first visit day was the 4th day after
intervention, while the second visit time was the 8th day after
treatment. The patterns of intervention varied slightly among those studies.
Table 1 Characteristics of
randomized controlled trials included in this Meta-analysis
|
Author(s), year |
Groups |
No. of patients |
Mean age,
y |
Male/female |
Intervention methods |
|
Karpecki et al[15], 2009 |
Besifloxacin |
137 |
33.3 |
51/86 |
Besifloxacin (0.6%) or vehicle 3 times daily for 5d |
|
Contrast |
132 |
35.1 |
56/76 |
||
|
Tepedino et al[19], 2009 |
Besifloxacin |
475 |
27.3 |
173/302 |
Besifloxacin (0.6%) or vehicle 3 times daily for 5d |
|
Contrast |
482 |
27.3 |
182/300 |
||
|
DeLeon et al[20], 2012 |
Besifloxacin |
231 |
29.4 |
89/142 |
Besifloxacin (0.6%) or vehicle twice daily for 3d |
|
Contrast |
243 |
26.4 |
110/133 |
||
|
Malhotra et al[21], 2013 |
Besifloxacin |
344 |
29.6 |
140/204 |
Besifloxacin (0.6%) or vehicle 3 times daily for 7d |
|
Contrast |
170 |
30.5 |
75/95 |
||
|
McDonald et al[22], 2009 |
Besifloxacin |
252 |
31.6 |
109/143 |
Besifloxacin (0.6%) or moxifloxacin (0.5%) 3 times
daily for 5d |
|
Contrast |
281 |
38.3 |
139/142 |
||
|
Sanfilippo et al[16], 2017 |
Besifloxacin |
16 |
15.8d |
4/12 |
Besifloxacin (0.6%) or gatifloxacin (0.3%) 3 times
daily for 7d |
|
Contrast |
17 |
15.2d |
10/7 |
Study Quality Figure 2 shows the study quality
assessment of the included studies. Two studies did not provide detailed information
about randomization. Three studies did not mention allocation concealment. All
included studies had a low risk of bias in terms of selective reporting and
blinding method. One studies had a high risk of attrition bias.
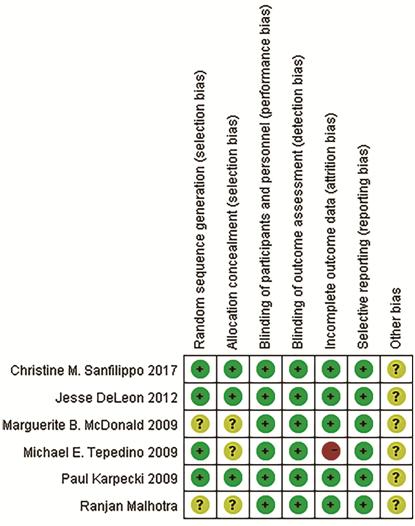
Figure 2 Risk of bias of included
studies.
According to a guideline of the
Cochrane library[23], assessment for publication
bias is not reliable for fewer than 10 pooled studies. Therefore, we did not
evaluate the existence of publication bias by the Egger’s test for funnel plot
asymmetry.
Results of Forest Plots
Clinical resolution Significantly more patients
receiving besifloxacin than placebo had clinical resolution of the baseline
infection at days 4 and 8 after intervention, and there was no significant
difference between besifloxacin and moxifloxacin or gatifloxacin (Figures 3 and
4).
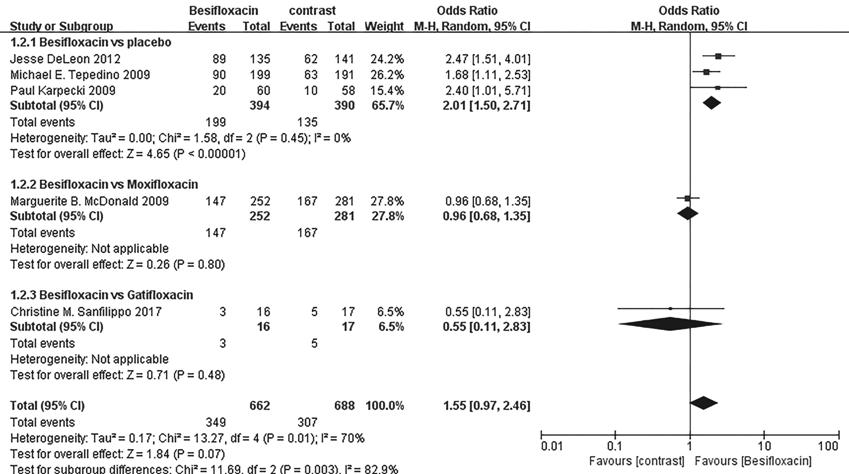
Figure 3 Estimated odds ratio for
changes in clinical resolution at day 4.
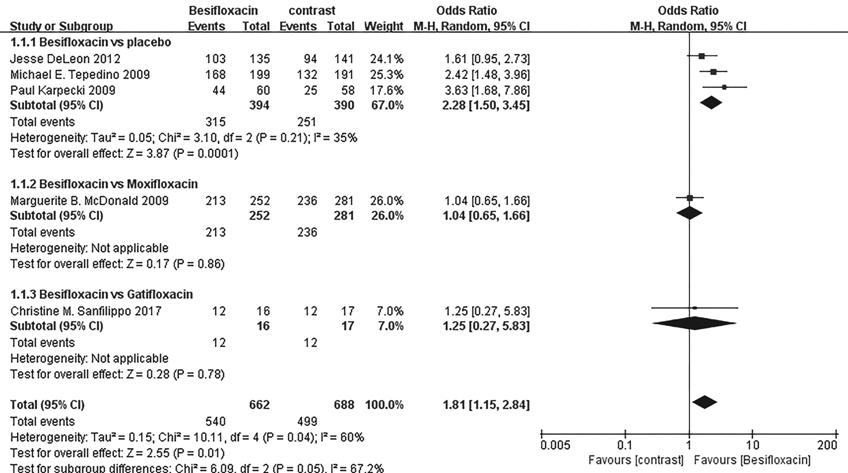
Figure 4 Estimated odds ratio for
changes in clinical resolution at day 8.
Rates of bacterial eradication The rates of bacterial eradication
were also significantly greater in those using besifloxacin than using vehicle
on days 4 and 8, and no significant difference was observed between
besifloxacin and moxifloxacin or gatifloxacin (Figures 5 and 6).
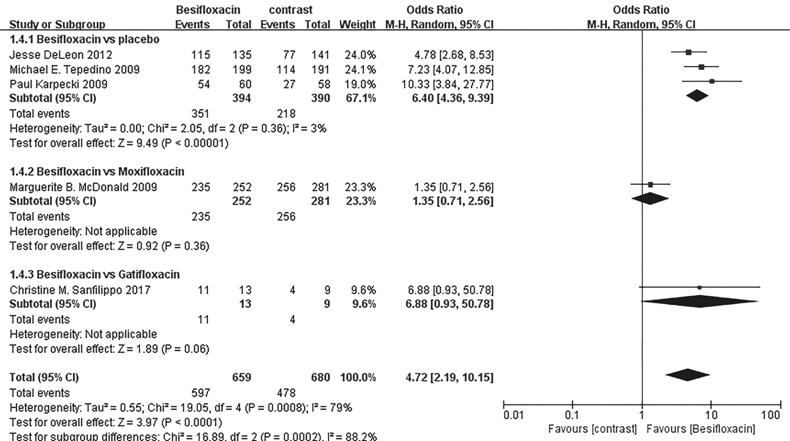
Figure 5 Estimated odds ratio for
changes in bacterial eradication rates at day 4.
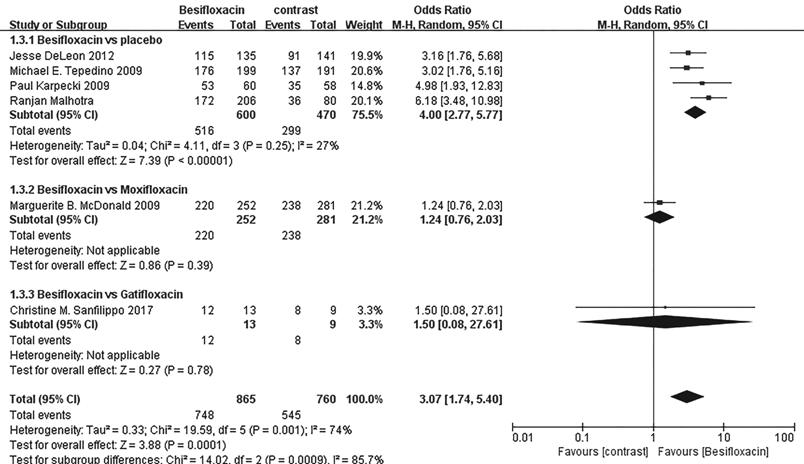
Figure 6 Estimated odds ratio for
changes in bacterial eradication rates at day 8.
Individual clinical outcomes The percentage of patients treated
with Besifloxacin who had resolution of ocular discharge was significantly
greater at days 4 and 8 compared with that of treated with placebo, and
significantly greater percentages of patients treated with Besifloxacin had
normal bulbar conjunctival injection than did those treated with vehicle at
days 4 and 8 (Figure 7). In addition, Besifloxacin cured more patients at day 4
and 8 than did placebo (Figures 8 and 9). No significant difference was
observed in cure rates between Besifloxacin and Moxifloxacin (Figures 8 and 9).

Figure 7 Estimated odds ratio for
changes in ocular discharge and bulbar conjunctival injection A: Resolution of ocular discharge at
day 4; B: Resolution of ocular discharge at day 8; C: Normal bulbar
conjunctival injection at day 4; D: Normal bulbar conjunctival injection at day
8.
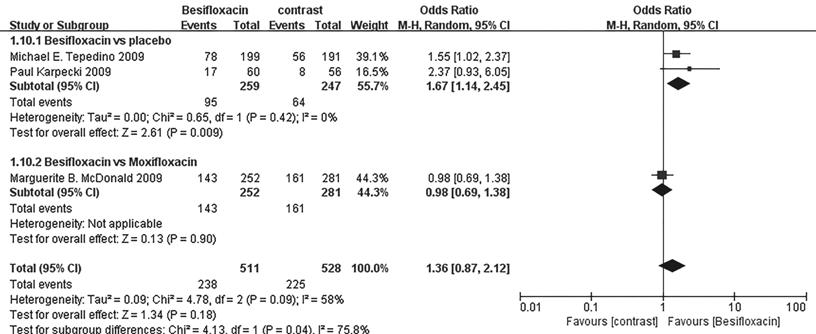
Figure 8 Estimated odds ratio for
changes in cure rates at day 4.
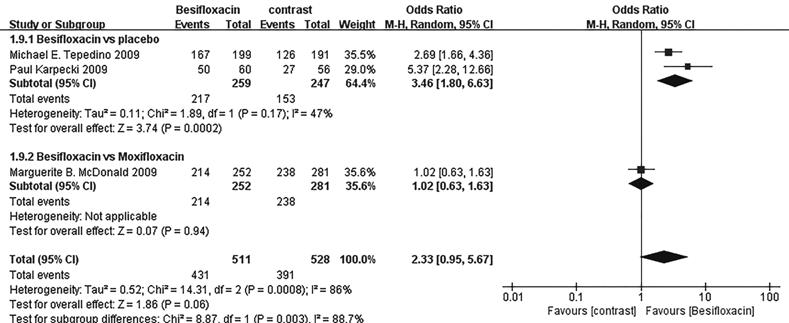
Figure 9 Estimated odds ratio for
changes in cure rates at day 8.
Pathogens Besifloxacin-treated subjects had a
higher rate of bacterial eradication and clinical resolution in Gram-positive
bacteria at days 4 and 8, and Gram-negative bacteria at day 8 than did
placebo-treated subjects. Bacterial eradication rates were significantly better
in Besifloxacin-treated eyes than in placebo-treated eyes for infections caused
by H. influenza, S. pneumoniae, S. aureus and S.
epidermidis at days 4 and 8. There was no overall significant difference in
those comparison items between Besifloxacin and Moxifloxacin or Gatifloxacin
(Table 2).
Table 2 Clinical resolution and
bacterial eradication rates of different species
|
Parameters |
Treatments |
No. of patients |
OR |
95%CI |
P |
χ2 |
I2 |
Effect model |
|
|
Rates of bacterial eradication |
|||||||||
|
4d |
|
|
|
|
|
|
|
|
|
|
Gram-positive |
Besifloxacin vs placebo |
755 |
7.33 |
4.18-12.85 |
<0.00001 |
0.11 |
50% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
368 |
1.26 |
0.63-2.55 |
0.51 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
31 |
9.33 |
1.50-58.20 |
0.02 |
- |
- |
Random effect |
|
|
Gram-negative |
Besifloxacin vs placebo |
438 |
4.29 |
2.52-7.28 |
<0.00001 |
0.92 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
214 |
1.26 |
0.34-4.59 |
0.73 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
10 |
5.57 |
0.18-176.26 |
0.33 |
- |
- |
Random effect |
|
|
H. influenzae |
Besifloxacin vs placebo |
281 |
4.01 |
2.19-7.32 |
<0.00001 |
0.90 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
169 |
1.16 |
0.30-4.48 |
0.83 |
- |
- |
Random effect |
|
|
S. pneumoniae |
Besifloxacin vs placebo |
215 |
2.21 |
1.14-4.29 |
0.02 |
0.60 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
122 |
1.02 |
0.37-2.85 |
0.97 |
- |
- |
Random effect |
|
|
S. aureus |
Besifloxacin vs placebo |
141 |
9.11 |
3.50-23.72 |
<0.00001 |
0.39 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
113 |
1.55 |
0.55-4.40 |
0.41 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
5 |
7.00 |
0.17-291.34 |
0.31 |
- |
- |
Random effect |
|
|
S. epidermidis |
Besifloxacin vs placebo |
109 |
4.58 |
1.74-12.10 |
0.002 |
0.36 |
3% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
63 |
4.8 |
0.55-42.23 |
0.16 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
7 |
7.00 |
0.22-218.95 |
0.27 |
- |
- |
Random effect |
|
|
8d |
|
|
|
|
|
|
|
|
|
|
Gram-positive |
Besifloxacin vs placebo |
753 |
4.23 |
2.90-6.17 |
<0.00001 |
0.75 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
368 |
1.04 |
0.58-1.87 |
0.88 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
31 |
1.42 |
0.08-24.95 |
0.81 |
- |
- |
Random effect |
|
|
Gram-negative |
Besifloxacin vs placebo |
437 |
2.14 |
1.29-3.57 |
0.003 |
0.61 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
214 |
1.9 |
0.82-4.41 |
0.14 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
10 |
2.13 |
1.41-3.21 |
- |
- |
- |
Random effect |
|
|
H. influenzae |
Besifloxacin vs placebo |
369 |
2.36 |
1.31-4.23 |
0.004 |
0.34 |
11% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
169 |
1.17 |
0.44-3.13 |
0.75 |
- |
- |
Random effect |
|
|
S. pneumoniae |
Besifloxacin vs placebo |
215 |
2.21 |
1.14,4.29 |
0.02 |
0.6 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
122 |
1.02 |
0.37-2.85 |
0.97 |
- |
- |
Random effect |
|
|
S. aureus |
Besifloxacin vs placebo |
210 |
9.53 |
4.47-20.32 |
<0.00001 |
0.74 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
115 |
1.15 |
0.45-2.96 |
0.78 |
- |
- |
Random effect |
|
|
S. epidermidis |
Besifloxacin vs placebo |
225 |
9.86 |
3.80-25.59 |
<0.0001 |
0.25 |
27% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
70 |
2.02 |
0.56-7.21 |
0.28 |
- |
- |
Random effect |
|
|
|
Besifloxacin vs gatifloxacin |
7 |
1.67 |
0.05-58.28 |
0.78 |
- |
- |
Random effect |
|
|
Clinical resolution |
|
|
|
|
|
|
|
||
|
4d |
|
|
|
|
|
|
|
|
|
|
Gram-positive |
Besifloxacin vs placebo |
532 |
2.12 |
1.47-3.06 |
<0.0001 |
0.82 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
368 |
0.91 |
0.60-1.37 |
0.65 |
- |
- |
Random effect |
|
|
Gram-negative |
Besifloxacin vs placebo |
328 |
1.63 |
0.98-2.69 |
0.06 |
0.31 |
15% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
214 |
1.2 |
0.68-2.14 |
0.52 |
- |
- |
Random effect |
|
|
8d |
|
|
|
|
|
|
|
|
|
|
Gram-positive |
Besifloxacin vs placebo |
532 |
1.97 |
1.34-2.90 |
0.0005 |
0.4 |
0 |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
368 |
1.14 |
0.67-1.94 |
0.64 |
- |
- |
Random effect |
|
|
Gram-negative |
Besifloxacin vs placebo |
331 |
2.47 |
1.19-5.13 |
0.02 |
0.18 |
41% |
Random effect |
|
|
|
Besifloxacin vs moxifloxacin |
214 |
0.81 |
0.35-1.89 |
0.63 |
- |
- |
Random effect |
|
Safety Figure 10 demonstrates there were
more people in the placebo group suffering from AEs than those of Besifloxacin
group, and no significant difference in AEs risk between Besifloxacin and
Moxifloxacin was found.
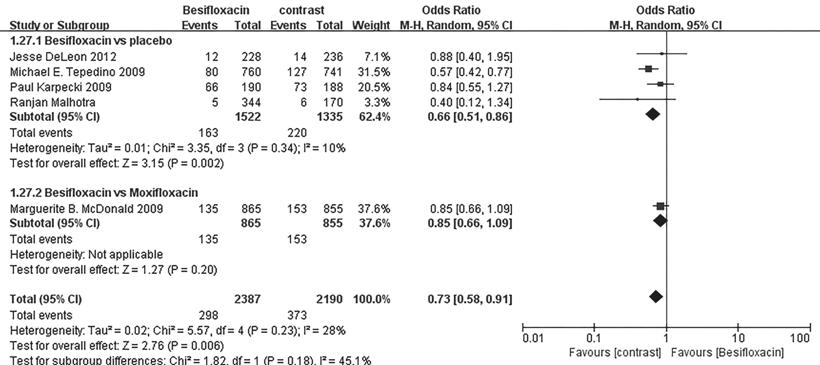
Figure 10 Estimated odds ratio for
the risk of AEs.
Sensitivity Analysis To find the source of heterogeneity,
we performed sensitivity analysis. Studies by Jesse DeLeon et al. was omitted
to achieve lower heterogeneity in the comparison of the bacterial eradication
rates of Gram-positive bacteria at day 4 (Figure 11).
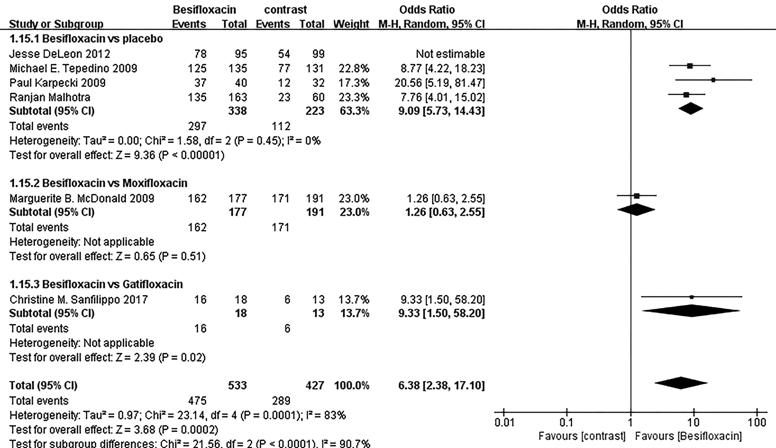
Figure 11 Sensitivity analysis for
the bacterial eradication rates of Gram-positive bacteria at day 4.
DISCUSSION
To our knowledge, this Meta-analysis
is the first to summarize the efficacy and safety of besifloxacin for treatment
of acute bacterial conjunctivitis. We have extensively searched electronic
databases, including PubMed, EMBASE, Web of Science, Cochrane Central Database
and CNKI, and 6 RCTs were ultimately included in our Meta-analysis. The forest
plot results suggest that compared with placebo, besifloxacin can significantly
promote clinical resolution, eradication of bacteria, improved clinical signs,
symptoms and cure rates. Moreover, there is no significant difference in the
occurrence of AEs. Compared with moxifloxacin and gatifloxacin, besifloxacin
showed no significant difference in efficacy and safety.
Clinical resolution is defined as
the absence of conjunctival discharge and bulbar conjunctival injection on the
assessment days, and the rates of bacterial eradication indicate the absence of
all bacterial species on assessment days that were present at or above the
threshold before intervention. These two parameters are the most important
clinical outcomes used to evaluate the efficacy of treatments for
conjunctivitis. We recorded the number of participants who achieved clinical
resolution and bacterial eradication. Pooled analyses indicate that besifloxacin
is highly effective in enhancing clinical resolution and rates of bacterial
eradication, and its efficacy is as high as that of moxifloxacin and
gatifloxacin. Nonetheless, we noticed a declining trend in bacterial
eradication rates in 3 studies from day 4 to day 8[15,19,22], which suggests bacterial
resistance to besifloxacin. Since all 3 studies were conducted in 2009, we
inferred that the reason may lie in the development of pharmaceutical and study
design. Bacterial resistance is of great importance for antibiotics, and its
results can change with time. Recent data should be updated to assess the effect
of besifloxacin on bacterial eradication rates.
Individual clinical outcomes include
ocular conjunctival discharge grading (0=absent, 1=mild, 2=moderate, and
3=severe), bulbar conjunctival injection grading (0=normal, 1=mild, 2=moderate,
3=severe) and cure rates on assessment days. We compared the number of patients
who were cured and graded 0. The results of forest plots suggest that
besifloxacin can significantly improve individual signs and symptoms and cure
acute bacterial conjunctivitis. The cure rates of besifloxacin can be
considered the same as those of moxifloxacin. There was relatively high
heterogeneity in the comparison of cure rates between besifloxacin versus
placebo, and we hypothesized that two reasons may account for this
heterogeneity. The sample size and percentage of male participants were
obviously different between the two studies. Tepedino et al[19] used data from a modified intent-to-treat population
instead of all patients or culture-confirmed patients who completed the study.
These two reasons may have influenced our Meta-analysis. However, more studies
are necessary to verify our hypothesis. Considering that there were only two
studies in this comparison, further high quality studies are needed to lower the
heterogeneity and confirm our results.
Our Meta-analysis demonstrates that
the clinical resolution and bacterial eradication rates were significantly
higher on days 4 and 8 for Gram-positive and Gram-negative species in
Besifloxacin-treated patients than in placebo-treated patients, with the
exception of Gram-negative species at day 4. Further studies are needed to
confirm this phenomenon. One study also presented the results on day 11, which
were mostly consistent with those on days 4 and 8[21].
Compared with Moxifloxacin and Gatifloxacin, Besifloxacin presents no overall
significant difference in these comparison items, which suggests that the
efficacy of Besifloxacin is similar to that of Moxifloxacin and Gatifloxacin. S.
epidermidis, H. influenzae, S. aureus, S. treptococcus
mitis and S. pneumoniae were the most common species isolated from
eyes in eligible studies. We analyzed the eradication rates of four species,
and pooled analyses showed that treatment with Besifloxacin is associated with
high rates of bacterial eradication of each of these species, the rates is
approximate to that of moxifloxacin and gatifloxacin. Even though the clinical
resolution and bacterial eradication rates of Besifloxacin were simiar to those
of moxifloxacin and gatifloxacin, several included studies determined that 90%
of the minimal inhibitory concentration (MIC90) values for Besifloxacin against
these clinical species were lower (0.06-0.5 μg/mL) than those for moxifloxacin
and gatifloxacin[15,18], which
may reflect besifloxacin’s potency against isolates that were resistant to
other kinds of antibiotics. Reports have already suggested that there is
emerging resistance to the fourth-generation fluoroquinolones Moxifloxacin and
Gatifloxacin among ocular pathogens, therefore, it is necessary to develop new
antibiotics with improved activity against resistant strains[24-25]. Unlike other fluoroquinolones, besifloxacin is being
developed exclusively for ophthalmic use. Hence, selective pressure for
resistance stemming from systemic use of besifloxacin is not expected to be a
factor. This factor, along with its activity against drug-resistant strains and
balanced activity against topoisomerase IV and DNA gyrase, may be an important
property of besifloxacin in the fight against emerging antibacterial
resistance. Moreover, one study suggested that besifloxacin can eradicate
bacteria more rapidly than gatifloxacin[14].
However, the sample size in current trials remains somewhat small, further
studies are required to verify these properties of besifloxacin. Compared with
moxifloxacin and gatifloxacin, besifloxacin has an advantage for the treatment
of acute bacterial conjunctivitis in the long interval between its application
to eyes. This characteristic makes besifloxacin more convenient for patients.
Current studies lack direct comparisons between besifloxacin and other drugs for
the efficacy of acute bacterial conjunctivitis, thus, future studies are needed
to explore this subject, which is important for clinical decision making.
Common AEs caused by besifloxacin
include conjunctivitis, eye pain, blurred vision, and eyelid erythema, however,
their occurrence rates were relatively low (all were lower than 5%, and most
were lower than 1%). A pooled analysis of safety data from three included
clinical studies reported that blurred vision, eye irritation, and
conjunctivitis were significantly less frequent in patients treated with
besifloxacin than in patients treated with placebo or moxifloxacin[26]. Our Meta-analysis further demonstrates that AE
frequency was significantly less in patients treated with Besifloxacin than in
patients treated with placebo and there was no significant difference between
the Besifloxacin and Moxifloxacin groups in the AE frequency. Further studies
are needed to verify this hypothesis.
Even though the methodological
quality of nearly all included studies is relatively high, this Meta-analysis
has some limitations. First, the intervention methods in the included studies
varied, which may influence the results of forest plots. Second, the number of
included studies was somewhat small, and there were only 6 included articles.
One study compared besifloxacin with moxifloxacin, and 1 study compared
besifloxacin with gatifloxacin. The small sample size of the included studies
can influence the results of our analysis. In addition, we cannot test the
publication bias via funnel plots. Third, there was no recent research
that compares besifloxacin and placebo, which may interfere with the results of
our Meta-analysis. Fourth, because of our limitations, we could screen only
English and Chinese articles.
This Meta-analysis demonstrates the
high efficacy and safety of besifloxacin for treatment of acute bacterial
conjunctivitis. Besifloxacin can promote the recovery of acute bacterial
conjunctivitis and the eradication of bacteria with few AEs and high
convenience. However, compared with moxifloxacin and gatifloxacin, current
studies do not provide enough evidence for the efficacy of besifloxacin in
managing antibiotic-resistant species. Moreover, the small sample size may
influence the results of our analysis, and further comparative trials on the
efficacy and safety of besifloxacin compared with placebo and other drugs for
treatment of acute bacterial conjunctivitis are needed.
ACKNOWLEDGEMENTS
Conflicts of Interest: Wang JJ, None; Gao XY, None; Li HZ,
None; Du SS, None.
REFERENCES