
·Review Article·
Combined
corneal CXL and photorefractive keratectomy for treatment of keratoconus: a
review
Mansour M. Al-Mohaimeed
Department
of Ophthalmology, College of Medicine, Qassim University, Qassim, Buraidah
51452, Kingdom of Saudi Arabia
Correspondence
to: Mansour M.
Al-Mohaimeed. Department of Ophthalmology, College of Medicine, Qassim
University, Qassim, PO Box 6655, Buraidah 51452, Kingdom of Saudi Arabia.
drmohaimeed@qumed.edu.sa
Received:
Abstract
Keratoconus and iatrogenic
keratectasia are the corneal ectatic disorders occurring due to biomechanical
weakening of the cornea resulting in distorted images, myopia, and irregular
astigmatism. Corneal collagen cross-linking (CXL) is performed to arrest
keratoconus successfully. The main aim of this review is to discuss the safety
and efficacy of the adjuvant therapies, such as the combination of CXL and
photorefractive keratectomy (PRK) for the treatment of corneal ectatic
disorders. A comprehensive literature search was performed using PubMed,
MEDLINE, and Scopus using keywords ‘collagen’ ‘keratoconus’, ‘keratectasia’,
‘collagen cross-linking’, and ‘photorefractive keratectomy’. Search results
were restricted to clinical studies published in English. Corneal CXL
effectively arrests the progression of keratoconus by enhancing corneal
rigidity. However, functional vision is not improved by cross-linking.
Combining CXL to refractive surgeries such as topography-guided PRK or
transepithelial PRK is found to be a safe and effective method in providing
corneal stability as well as significantly improving functional visual acuity
with few minor complications. This combined technique also prevents regression
of keratoconus and reduce the risk of keratectasia. CXL combined with PRK is a
promising therapeutic approach in ophthalmology that can be successfully used
to treat progressive keratoconus and other corneal ectatic disorders and to
enhance visual acuity.
KEYWORDS: corneal collagen cross-linking;
photorefractive keratectomy; keratoconus
DOI:10.18240/ijo.2019.12.16
Citation: Al-Mohaimeed
MM. Combined corneal CXL and photorefractive keratectomy for treatment of
keratoconus: a review. Int J Ophthalmol 2019;12(12):1929-1938
INTRODUCTION
Keratoconus is a bilateral,
non-inflammatory, progressive ectatic disease characterized by apical bulging
of the cornea, thinning of the central cornea, and distortion of the cornea,
which affects mostly adolescent people[1-2].
With the advancement of the disease, ocular aberrations increase, and image
quality and visual acuity are diminished. In severe cases, axial corneal
scarring and irregular astigmatism were also noticed. The key objective of the
treatment of keratoconus involves halting the progression of ectasia, improving
the refractive errors, and bringing back the normal shape of the cornea[3]. Progressive keratectasia resulting from corneal
disease or a sequela of laser in situ keratomileusis (LASIK) surgery has
no appropriate treatment at present. Available treatment of keratoconus mostly
involves interventions done for tectonic, optical, or refractive purposes.
Treatment of keratoconus depends on the extent of disease progression and
disease severity[3]. Conventional approaches to
treating mild to moderate keratoconus involve eyeglasses and rigid gas
permeable contact lenses[3]. Nonetheless, some
patients are unable to tolerate contact lens and spectacle correction is
insufficient in some cases. Moreover, in advanced stages of keratoconus with
excessive corneal thinning/steepening and corneal scarring[4],
the traditional treatment approaches are not quite effective. Furthermore, none
of these therapeutic approaches are able to treat the principal causes of
ectasia and do not guarantee the absolute cessation of keratoconus progression[5]. One promising treatment approach gaining popularity
from the late decades of the twentieth century is the corneal collagen
cross-linking (CXL), which aimed to treat the underlying pathology of
keratoconic eyes and effectively stiffens the cornea by restoring its tensile
strength[4] and subsequently slow down or arrest
the advancement of keratoconus, or even reverses keratoconus in rare cases[5-7]. Additionally, a combination of CXL
with photorefractive keratectomy (PRK), a standard laser-assisted refractive
surgery is expected to have greater efficacy in the management of keratoconus.
The main purpose of the combined treatment of keratoconus with PRK/CXL involves
strengthening the cornea and halting the disease progression by CXL and to
improve the quality of vision via PRK[4].
The current paper intends to review
recent literature on the application of corneal CXL in combination with PRK for
treating keratoconus and other corneal ectatic disorders.
Basic Principles of CXL Collagen is a triple helical
structural protein present abundantly in the extracellular matrix in all
animals. Intermolecular cross-links between collagen monomers aid in
strengthening the collagen structure. CXL is a natural phenomenon occurring
within the corneas and crystalline lens either enzymatically or
non-enzymatically. The enzymatic cross-linking occurs via lysyl oxidase
enzyme[8]. Non-enzymatic cross-linking occurs via
glycation, where bond formation occurs between sugar and the amino group of a
protein; this mechanism commonly occurs with age, or in an individual with
diabetes mellitus, thereby strengthens the cornea in elderly people and lowers
the occurrence of keratoconus in diabetes mellitus patients. CXL can also be
induced by oxidation using ultraviolet (UV) irradiation to generate reactive
oxygen species (ROS) that polymerize the collagen monomers into cross-linked
polymers. The effect of CXL reduces with low oxygen tension indicating the
importance of oxygen and ROS in collagen polymerization[4].
History of CXL The most common application of CXL
is to fix tissue and strengthening the heart valve. CXL emerged from researches
conducted to detect biological glues to make cornea strong. The scientists
intended to obtain corneal cross-linking in non-diabetic corneas analogous to
natural cross-linking by glycosylation in diabetic patients[7].
Finally, in 2003, Wollensak et al[9]
introduced the CXL technique using 370 nm UVA irradiation and photomediator riboflavin
to cross-link stromal collagen fibrils for treating keratoconus[7]. This technique is widely followed at present. Food
& Drug Administration (FDA) in the USA also approved the use of CXL in 2016
for treating progressive keratoconus and the post-LASIK ectasia based on the
results of three 12-month clinical trials[4].
Use of Riboflavin in Corneal CXL
with UVA Corneal CXL is a minimally invasive
method of cross-linking corneal collagen in order to enhance the biomechanical
stability of the cornea, which is weakened due to progressive keratoconus or
post-operative keratectasia[10]. In this method,
riboflavin or vitamin B2 (a photosensitizing substance) and UVA are used to form
additional intra and inter-fibrillar covalent bonds via photosensitized
oxidation[11]. Riboflavin treated corneas have
three absorption peaks- 270, 365, and 370 nm. The peak between 365 and 370 nm
is normally used in the CXL procedure as this does not damage the retina[12]. Riboflavin is excited into a triplet state by UVA
light of wavelength 370 nm[12] and produces ROS
to activate natural lysyl oxidase pathway[5]. The
increased cross-links between and within collagen fibers stabilize the stromal
collagen fibers, thereby improving the collagen structure and corneal rigidity[10] and resist it from deformation[13].
The use of 0.1% riboflavin in CXL technique has been found to enhance corneal
UVA absorption by 95% compared to 30% when UVA was used alone. Moreover,
riboflavin reduces keratocyte cytotoxicity caused by UVA[8,10]. Furthermore, riboflavin is anticipated to serve as a
protective layer of the cornea, which may even reach up to 400 µm following
30min application, and protect the internal structures such as the retina,
crystalline lens, and the endothelium from the harmful effects of UVA[11].
Techniques of Corneal CXL Wollensak and Spoerl first developed
a photochemical CXL procedure at the University of Dresden, commonly referred
to as the Dresden protocol[9]. Till date many
protocols have been recommended for corneal cross-linking; however, the basis
of all these is the Dresden protocol established by Wollensak et al[9]. The entire procedure is conducted under sterile
condition. Corneal CXL begins with the removal of corneal epithelium since the
epithelial tight junctions block riboflavin absorption to some extent.
De-epithelization results in uniform riboflavin diffusion in the corneal stroma[6]. Under topical anesthesia, abrasion of the central 7
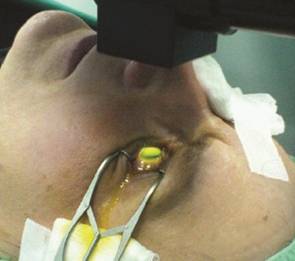
Figure 1 Treatment in progress with
the cornea soaked with riboflavin and irradiated by the UV lamp (represented
from Jankov et al[11]).
Clinical Study Results with Standard
CXL Procedure Wollensak[14]
performed the first clinical study of corneal CXL in 2003. This 3-year study
detected that following CXL treatment in patients with advanced keratoconus,
the progression of keratoconus was stopped in all patients along with
improvement in best corrected visual acuity (BCVA). Since then, a multitude of
clinical studies including prospective as well as retrospective studies has
been performed to explore the effectiveness of the standard CXL procedure. The
main parameters evaluated at the follow-up treatment are the maximal
keratometry (Kmax) value, BCVA, uncorrected distance visual acuity
(UDVA) and the follow-up period usually ranged between 1 and 6y[15]. Raiskup and colleagues in a retrospective study
determined the long-term efficacy of CXL in the stabilization of keratoconus
with a significant reduction of Kmax and Kmin values and
also improvement in BCVA. Another study with the largest follow-up time (48mo),
although detected initial deterioration (first 6mo), later found a substantial
improvement in next 42mo[4]. The results of the
majority of the clinical studies revealed that standard CXL has stabilized
corneal keratometry and improved BCVA, UDVA, visual acuity, and topographical
indexes in keratoconic eyes without altering corneal volume and anterior
chamber volume and depth[16]. Some studies
reported about improvement of visual acuity but no change in keratometry
values, whereas few other studies stated minor reduction in UDVA and BCVA
readings after 4-5y of CXL treatment. In majority of the cases, diminution of
irregular astigmatism was responsible for better visual acuity[16].
There are not much randomized
controlled trials to clarify the results of these studies. Nevertheless, the
findings of the first randomized clinical trial on the use of CXL in treating
progressive keratoconus conducted by Witting-Silva et al[17] with a follow-up period of three years substantiated
the effectiveness of standard CXL protocol in stabilizing keratoconus
progression and is considered to be a notable landmark. Another prospective,
non randomized clinical study on CXL for treating progressive keratoconus
determined statistical improvement in visual acuity and statistically
significant reduction of Kmax and Kmin values in the
treated group versus untreated group with no major change in endothelial cell
count at 12-month follow-up[12]. The long-term
results (48-60mo follow-up) of an open, prospective, nonrandomized, Phase II
clinical trial conducted by Caporossi et al[18]
also determined stability or improvement in 92% cases with a mean reduction of
average keratometry readings and substantial improvement in visual acuity,
BCVA, and UCVA following standard CXL, whereas the untreated fellow eyes showed
65% progression of keratoconus within 2y.
Treatment Failure Treatment failure is defined as the
continual progression of keratoconus with an enhancement of Kmax
reading of 1.0 D over the preoperative value. Treatment failure has been found
to occur in 8.1%-33.3% cases; one study by Poli et al[19]
stated about 11% failure rate during a follow-up period of 6y.
Complications of Standard CXL Corneal CXL is a relatively safe and
effective revolutionary therapeutic approach to pause keratoconus progression
for at least five years and postoperative LASIK ectasia for a minimum of two
years with a low rate of complications[20]. The
complications of CXL can be either primary or direct arising from an incorrect
application of the technique or incorrect patient selection. The secondary or indirect
complications of CXL result from patient’s poor hygiene, therapeutic soft
contact lens, or other ocular surface diseases, such as bacterial keratitis
occurring due to epithelial defect or use of soft bandage contact lens
following surgery. The two most common direct complications of CXL include 1)
appearance of stromal haze due to back-scattered and reflected light; 2)
corneal edema due to endothelial damage[6].
Previous studies reported that CXL-associated corneal haze appearing as a
dust-like change in corneal stroma actually differs from other types of corneal
haze; this postoperative corneal haze usually increases within 1-3mo of surgery
and by 6mo, haze diminishes and the cornea appears to be clear[21].
Typically, corneal endothelial
damage occurs when safety limits about corneal thickness are not followed. CXL
results in corneal thinning, which starts at the initial phase of the procedure
and continues until 1-3mo’ post-treatment. Nonetheless, the optimal healing and
remodeling of the cornea occur in the first 6mo to 1-year period. In fact,
corneal thickness begins to recover from 3mo and attains baseline thickness (i.e.
corneal thickness before CXL procedure) within 1y. However, Kim et al[22] in their study reported statistically significant
reduction in the corneal thickness as compared to baseline value even 5y
post-CXL treatment. Corneal thinning following CXL probably occurs due to
corneal desiccation and dehydration owing to prolonged UVA exposure and this
actually results in endothelial damage[16]. The
endothelial damage can be prevented by keeping the corneal thickness over 400
µm prior to UV exposure[21].
Modifications of Conventional CXL
Technique Conventional CXL technique is
contraindicated for individuals with corneas thinner than 400 μm[5] in order to protect the cornea from endothelial
toxicity and cell death[23]. Hence, CXL using
standard protocol is proposed for keratoconic eyes with corneal thickness at
least 400 μm following de-epithelization. Progression has been reported in
about 25%-30% of keratoconus cases[23]. In order
to overcome the possible complications arising from the use of standard CXL
technique in keratoconus patients who are not good candidates for traditional
CXL (eyes with corneal thickness less than 400 μm) or to obtain quicker
results, several modifications have been made in the conventional Dresden
protocol[24]. The common modifications include:
1) use of hypoosmolar riboflavin to swell thin corneas artificially; 2)
accelerated CXL, altering irradiation dosage to reduce treatment duration; and
3) transepithelial CXL (TE-CXL), keeping epithelium intact and using various
compounds to enhance riboflavin penetration[25].
CXL with hypoosmolar riboflavin Original Dresden protocol mentions
the use of 0.1% riboflavin in 20% dextran solution. This riboflavin
concentration can treat only anterior 300 μm of the stroma and is ineffective
when corneal pachymetry is <400 μm after de-epithelization. A permanent
stromal scar was noticed in keratoconic eyes with thinner corneas and steeper
keratometric values following CXL using isomolar riboflavin[23].
In contrast to isotonic riboflavin, hypoosmolar riboflavin has lower colloidal
pressure (402.7 mOsmol/L vs 310 mOsmol/L) that causes stromal swelling
to double its thickness where stromal bed is less than 400 μm and thus
facilitates CXL technique[23,26].
In a study, Wollensak et al[9] used
hypoosmolar riboflavin alone in every 2min for 30min in kertaoconic eyes with
thin corneas (<400 μm) and observed stability in vision and keratometry with
no stromal scars at 12mo’ follow-up. Hafezi et al[10]
performed CXL in progressive keratoconus patients (cornea <400 μm) using
hypoosmolar riboflavin and detected halting of keratoconus progression in all
patients along with stable keratometry at 6-month follow-up. Stojanovic et
al[27] noticed that use of hypoosmolar riboflavin
with standard irradiation of 3 mW/cm2
for 30min arrested keratoconus
progression; however, the efficacy was lower than traditional CXL with isotonic
riboflavin. The possible explanation is that in hydrated corneas (using
hypoosmolar robiflavin) concentration of collagen fibrils is diminished, hence
fewer collagen fibrils are available for CXL[23].
Accelerated versus conventional CXL
in treating keratoconus The duration of standard CXL is
about 1h and exposure of the cornea to UVA for this time period may cause
damage to corneas thinner than 400 μm. To quicken the treatment process,
“accelerated CXL” is performed. This technique utilizes high energy up to 30
mW/cm2 for a shorter duration of time such as 3-10min, still keeping
the total radiant exposure to be 5.4 J/cm2[2].
Several studies were conducted to compare the efficacy of accelerated CXL with
that of conventional CXL by using different irradiation intensity and it was
observed that Accelerated protocols have acceptable efficacy[4].
However, a recent study comparing accelerated vs conventional CXL in
keratoconic eyes was unable to detect any significant difference in visual
acuity, keratometry reading, and endothelial cell count at 1-year follow-up
among these two techniques[26].
Transepithelial CXL vs
conventional epithelium-off CXL Wollensak et al[9] performed CXL by excision of corneal epithelium to
facilitate penetration of riboflavin since riboflavin being hydrophilic unable
to penetrate properly through the lipophilic epithelial membrane. However,
removal of epithelium is a painful method, requires more healing time, has a
higher probability of developing infections, and leads to corneal melting[4]. To minimize these problems, currently, a modified CXL
technique known as TE-CXL, where corneal epithelium remains intact is being
performed[4]. The entry of riboflavin through
corneal epithelium is aided by the addition of certain chemicals such as
tetracaine, benzalkonium chloride, and trometamol, which loosen the epithelial
tight junctions[4,11].
Stojanovic et al[27] did a comparative
study with and without epithelial removal to treat progressive keratoconus and
concluded that both methods were equally safe and effective in stabilization of
keratoconus. While different studies revealed that visual acuity appears to be
similar following TE-CXL and epithelium-off CXL, the efficacy of TE-CXL in
terms of topographic indices is less than CXL with de-epithelization[4,11]. In one study limited CXL effect
was observed in eyes with intact epithelium; the possible reasons may be
insufficient riboflavin concentration in the stroma and lesser oxygen diffusion
into the stroma. It is anticipated that rise in biomechanical rigidity
following TE-CXL and standard epithelium-off CXL is about 64% and 320%
respectively[23] suggesting that the effect of
TE-CXL is more superficial than conventional CXL[26].
Despite this, TE-CXL has several
advantages over regular epithelium-off CXL, including less time-consuming, no
operation room required, quicker visual recovery, applicable for patients with
corneal thickness less than 400 μm, safer technique since intact cornea acts as
a barrier to prevent the entry of pathogen, reducing the occurrence of
infectious keratitis[4]. In addition, stromal
haze, postoperative pain, burning sensation, healing reaction, and other
complications are less in TE-CXL[23,27].
Iantophoresis-assisted CXL Riboflavin is a crucial component of
CXL since by virtue of its photosensitizing power, it forms the CXL and
provides tensile strength to cornea. Thus, proper penetration of riboflavin to
the stroma is vital. Iantophoresis is a non-invasive unique technique to
facilitate riboflavin infiltration using small electric current. Riboflavin,
being negatively charged is a good candidate for iontophoresis. Following only
5min of 1 mA current flow, an adequate level of riboflavin penetrates into the
corneal stroma, thus epithelial integrity is maintained[15,23]. Initial clinical study results exhibited that
iontophoresis-assisted CXL can stop keratoconus advancement without
considerable complications; even so, further long-term follow-up studies are
needed to determine its efficacy in keratoconus management[15].
PHOTOREFRACTIVE KERATECTOMY IN COMBINATION WITH CXL TO TREAT KERATOCONUS AND
POST-LASIK KERATECTASIA
Corneal CXL is a promising technique
for management of keratoconus as it provides tensile strength and stability to
the cornea by inducing cross-links at the corneal stroma and thus arrests
keratoconus. For prophylactic use, virtually any patient can be treated with
cross-linking to reduce the chance of future development of ectasia, especially
patients with thinner than normal corneas, irregular corneal astigmatism,
asymmetry on corneal topography, against-the-rule astigmatism or steeper than
normal corneas. Majority of the studies indicated more than 90% success rate in
stabilizing the advancement of keratoconus following CXL technique[28]. However, CXL alone is unable to improve functional
vision[29] and yields a better result for the
patients suffering from early-to-moderate keratoconus compared to end-stage
keratoconus[6]. The limitation of CXL can be
resolved by combining CXL with PRK. Although previous studies revealed the
effectiveness of PRK in treating stable or early keratoconus, its application
in combination with CXL proved to be superior[30].
Presently, a novel technique referred to as ‘CXL Plus’, which combines two
surgical procedures is performed to treat a corneal ectatic disorder such as
keratoconus. In CXL-Plus, the fundamental method is CXL, which is combined with
other refractive procedures such as topography-guided PRK, or transepithelial
topography-guided PRK, or intracorneal ring segments (ICRS), or phakic
intraocular lens implantation (PIOL), or multiple techniques combined with CXL,
either sequentially or simultaneously[29,31].
CXL-Plus is advantageous over typical CXL because it enhances CXL result, by
improving corneal stability as well as by providing functional visual acuity[29]. Topography-guided PRK combined with CXL was the
first CXL Plus method using excimer laser ablation and is considered to be an
effective treatment of choice for keratoconus and keratectasia[29]. Several modifications of the technique have been
suggested, involving the timing of the two procedures (simultaneous or sequential),
highest advised ablation depth, and the use of mitomycin[28].
Sequential vs Simultaneous
Topography-Guided PRK in Combination with CXL in Treating Keratoconus and
Post-Surgical Corneal Ectasia Previous studies have reported
considerable improvement in the functional vision of keratoconic eyes treated
with a two-step procedure of corneal CXL and sequential topography-guided PRK,
performed one year after CXL[29,32].
Nonetheless, there are some limitations of this sequential CXL and PRK
technique, such as 1) the ablation rate of cross-linked corneas may vary from
that of the normal corneas, which may yield arbitrary results; 2) the
probability of post-PRK haze formation is higher; and 3) most importantly, the
removal of corneal tissue stiffened by CXL during PRK diminishes the benefits
of CXL[1,29]. Later
Kanellopoulos[1] introduced an alternative
approach involving simultaneous topography-guided PRK followed by CXL at the
same day referred to as CXL-Plus to produce more regular corneal shape and
improve the quality of vision further and is believed to amplify the outcome of
CXL alone in keratoconus patient[21]. This
procedure commonly referred to as the Athens protocol is widely used nowadays[1]. The Athens protocol initiated by Kanellopoulos[1] involved excimer laser ablation of about 50 µm of the
anterior corneal epithelium to rectify irregularities of corneal surface and
simultaneous epithelium-off CXL with riboflavin and UVA to arrest keratoconus
progression[1,31].
The main advantages of simultaneous
PRK and CXL over sequential topography-guided PRK after CXL in keratoconus
treatment are: the cross-linked portion of the cornea remains unaffected by
laser ablation and the probable stromal scarring occurring due to PRK alone is
minimized[1,21]. Combined CXL
and topography-guided PRK simultaneously in patients with moderate keratectasia
and sufficient corneal thickness (about 400 µm) resulted in rigid corneal
collagen along with significant enhancement in UCVA, corrected visual acuity
(CVA), reduced spherical error, and keratometry readings leading to
considerable improvement of vision[1,5,29]. Multiple studies revealed the safety and efficacy of
simultaneous topography-guided PRK and CXL for the treatment of patients with
keratoconus and post-LASIK corneal ectasia (Table 1).