
·Review Article·
Corneal
alteration and pathogenesis in diabetes mellitus
Han Zhao1,2, Yan He1,2, Yue-Rong
Ren1,2, Bai-Hua Chen1,2
1Department of Ophthalmology, the
Second Xiangya Hospital, Central South University, Changsha 410011, Hunan
Province, China
2Hunan Clinical Research Center of
Ophthalmic Disease, Changsha 410011, Hunan Province, China
Correspondence to: Bai-Hua Chen. Department of
Ophthalmology, the Second Xiangya Hospital, Central South University, Changsha
410011, Hunan Province, China. chenbaihua2017@csu.edu.cn
Received:
Abstract
The incidence of diabetes
mellitus (DM) and its complications have increased considerably worldwide.
Diabetic keratopathy is the major complication of the cornea characterized by
delayed corneal wound healing, decreasing corneal epithelial sensitivity, and
recurrent corneal ulcers. There is accumulating evidence that diabetic
keratopathy is correlated with the hyperglycemic state. Different corneal
components may produce different alterations under hyperglycemia. In addition,
diabetic nerve alteration may become a novel biomarker of early-stage DM.
Abnormalities of the corneal nerve plexus have been associated with diabetic
inflammatory states. There is rapidly growing evidence based on investigations
of diabetic corneal nerves through in vivo confocal microscopy.
Understanding the molecular pathogenesis caused by hyperglycemia may assist in
the identification of novel biomarkers, as well as therapeutic targets for
early treatment. This review mainly summarizes recent findings on corneal
alteration and pathogenesis in DM.
KEYWORDS: diabetes mellitus; diabetic
keratopathy; diabetic neuropathy; in vivo confocal microscopy; advanced
glycation end products
DOI:10.18240/ijo.2019.12.17
Citation: Zhao
H, He Y, Ren YR, Chen BH. Corneal alteration and pathogenesis in diabetes
mellitus. Int J Ophthalmol 2019; 12(12):1939-1950
INTRODUCTION
With the rapid increase in the
prevalence of diabetes mellitus (DM), diabetic ocular complications [i.e.,
diabetic keratopathy (DK), diabetic cataract, dry eye, and diabetic retinopathy
(DR)] may lead to severe vision damage and blindness in adults worldwide[1]. In recent years, DK has gained increasing attention.
The main clinical manifestations include loss of corneal sensitivity, recurrent
erosions of the corneal epithelium, dry eye, and neurotrophic corneal
ulceration. The primary pathological manifestations include basement membrane
abnormality, lacrimal functional unit (LFU) dysfunction, corneal neuropathy,
and endothelial decompensation. In addition, diabetic neuropathy occurs even in
the pre-diabetic states, and worsens with the development of DM. Loss of nerve
innervation may result in the delay of corneal wound healing or neurotrophic
ulceration. Persistent hyperglycemia triggers the expression of various
cytokines, chemokines, and cell adhesion molecules (Figure 1). Over-expression
of cytokines, chemokines, and other pro-inflammatory proteins and pro-apoptotic
genes is a key contributor to developing DK[2].
This review summarizes the current findings and knowledge regarding the corneal
complications of DM (i.e., the morphology, pathophysiology, and cellular
mechanism).
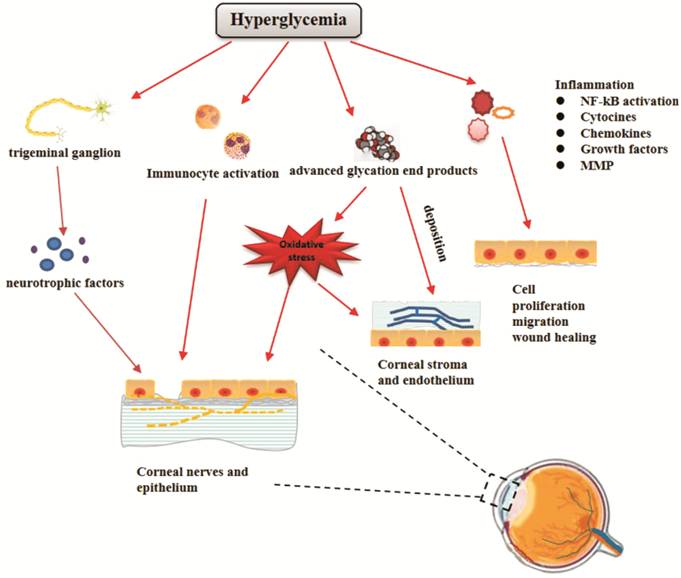
Figure 1 Schematic showing the
pathogenesis of diabetic keratopathy
Hyperglycemia
has distinct effects on different parts of the cornea, including advanced
glycation end products, oxidative stress, diabetic neuropathy, inflammatory
reaction, and immunocyte activation. These effects eventually lead to defective
wound healing in the corneal epithelium, abnormalities of sub-basal and stromal
nerves, and corneal stromal and endothelial dysfunction. NF-κB: Nuclear factor
kappa-light-chain-enhancer of activated B cells transcription factor; MMP:
Matrix metalloproteinase.
DIABETIC CORNEAL NEUROPATHY
Diabetic corneal neuropathy is a
potential visual impairment condition caused by damage to the trigeminal nerve
under chronic hyperglycemia, and results in reduction or loss of corneal
innervation. Diabetic corneal neuropathy is characterized by photophobia,
ocular irritation, or pain. The majority of corneal symptoms are the result of
damage to the small Aδ and C nerve fibers of the cornea[3].
The loss of corneal sensory innervation causes corneal epithelial breakdown,
delayed wound healing, and subsequently progresses to corneal ulceration,
melting, and perforation. However, those symptoms may not correlate with the
severity of corneal neuropathy. A number of patients with diabetic corneal
neuropathy often present without symptoms; this may be due to the decreased
innervation of the cornea (Figure 2).
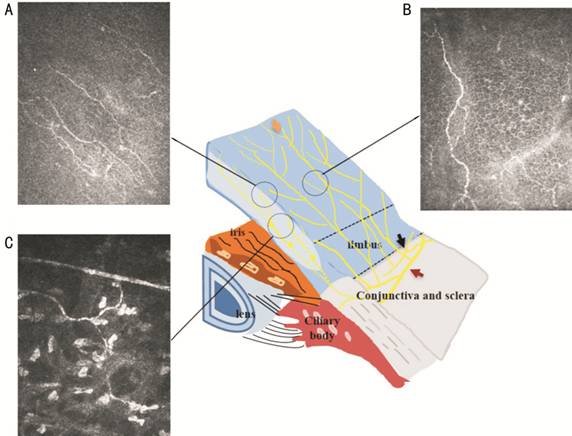
Figure 2 Transected view of the
entire corneal nerve alterations The epithelial innervation (yellow
arrow) is supplied by two nerve networks, namely the limbal superficial nerve
network and sub-conjunctiva nerve network (black arrows). Corneal stromal
nerves originate from the sclera and branch into the epithelium (red arrow).
Representative IVCM images are shown for A: Sub-basal nerves; B: Corneal
epithelial nerves; C: Corneal stromal nerves in patients with DM.
In vivo confocal microscopy (IVCM) has
revealed several significant findings in the epithelial nerve. The long nerve
fiber bundles in the corneal sub-basal nerve plexus had significantly decreased
in patients with DM and corneal sensitivity was negatively correlated with long
nerve fiber length[4]. In addition, the corneal
sub-basal nerves in diabetic patients showed pronounced thickening than those
observed in control subjects[5]. Some studies
showed that patients with DM had significantly decreased corneal sub-basal
nerve fiber length and branch density[6]. Changes
in nerve fibers correlated with the development of DR. Patients with
proliferative DR showed significantly thicker, tortuous, and lower density
nerve measurements than those without proliferative DR[7].
Kallinikos et al[8] reported that reduction
of corneal sub-basal nerve tortuosity may predict the severity of somatic
neuropathy in patients with DM. Recent IVCM studies conducted by Deák et al[9] showed a significant reduction in corneal nerve fiber
density in patients with DR.
Most studies are focused on the
diabetic changes in corneal sub-basal nerves, with limited research focusing on
the corneal stromal nerves. Patel and McGhee[10]
found that the mean stromal nerve thickness and the proportion of curved
stromal nerves were significantly higher in patients with DM. Moreover, they
confirmed that patients with proliferative retinopathy had thicker stromal
nerves than patients with background retinopathy. Nevertheless, the stromal
nerve density can not calculated, because it has course obliquely in the
corneal stroma and cannot be imaged through confocal microscopy. According to
corneal immunofluorescence staining, stromal nerve fiber loops are one of the
striking changes observed in corneal stromal nerves. Under hyperglycemia, the
basement membrane may resist the stromal nerves entering the epithelium,
leading to the occurrence of nerve fiber loops. Moreover, the alteration of the
extracellular matrix in the diabetic corneal stroma may also result in the
formation of nerve fiber loops[11].
Pathogenesis of Diabetic Corneal
Neuropathy Multiple mechanisms, such as
hyperglycemia-mediated inflammation, oxidative stress, and signal pathways, may
play an important role in diabetic neuropathy. Advanced glycation end-products
(AGEs) are reactive metabolites produced by the non-enzymatic glycosylation of
sugar molecules, which are caused by hyperglycemia in DM[12].
Recent studies have demonstrated that the accumulation of AGEs may result in
retinal diabetic neuropathy[13-14].
AGEs and their receptors (RAGE) cause the formation of oxygen radicals and the
release of pro-inflammatory cytokines[15].
Some studies have confirmed that
poly (ADP-ribose) polymerase plays an important role in corneal neuropathy,
which may trigger the mechanism of oxidative stress both in the diabetic rat
and mouse model[16]. Chronic hyperglycemia can
lead to the generation of reactive oxygen species (ROS), which results in
mitochondrial damage[17]. Yagihashi et al[18] showed that mitochondrial damage in nerve fibers may
lead to demyelination and conduction dysfunction. In that study, immune
mechanisms were suggested to play a prominent role in the progression of
diabetic corneal neuropathy. The presence of immunocytes in the cornea can be
observed via confocal microscopy. Studies have reported that the
proportion of dendritic cells and Langerhans cells (LCs) was significantly
increased in diabetic patients compared with control subjects. Furthermore, LC
density was significantly increased in diabetic patients, and was significantly
correlated with the severity of neuropathy[19].
The corneal nerve plexus plays an
essential role in maintaining epithelial homeostasis and promoting wound
healing through secretion of neuropeptides, growth factors, and cytokines.
Chronic hyperglycemia may impair corneal nerve secretion of neuropeptides[20]. Notably, the ciliary neurotrophic factor (CNTF) may
promote epithelial wound healing and nerve regeneration[21].
Interestingly, the proportion of dendritic cells is decreased in the diabetic
cornea, which is the primary source of CNTF. As a systemic metabolic disease,
DM may disrupt both the immune and neuroendocrine systems[22]. Recently, in the diabetic mice model, treatment with
pigment epithelium-derived factor, docosahexaenoic acid, and ω-3 fatty acid was
shown to promote epithelial wound healing and nerve regeneration[23].
CORNEAL EPITHELIUM ABNORMALITY
The corneal epithelium consists of
cell layers and the basement membrane. The epithelium is an important barrier
to the cornea, which can resist attacks from pathogens. However, diabetic
patients are vulnerable to corneal epithelium dysfunctions, such as superficial
puncture keratitis and epithelium erosion. Corneal epithelium abnormality
is one of the most common and long-term complications of DM.
Corneal Epithelial Basal Cells Corneal epithelial basal cells
(CEBCs) are derived from the corneal stem cells at the limbus, and play an
important role in forming the basement membrane. Under physiological
conditions, CEBCs are presented as alternately dark and bright dense cluster
polygonal cells, with a high reflective cell border and low reflective
cytoplasm using IVCM. In DM, abnormal hyper-signals were detected at the
interface between the epithelium and the anterior stroma. These abnormalities
may reflect the accumulation of AGEs[24]. Qu et
al[25] showed an increase in LCs and decrease
in CEBCs in patients with type 2 DM.
There is a significant reduction of
central corneal thickness (CCT) in severely diabetic rat models, indicating
disruption of the normal homeostasis of the corneal epithelium[4]. However, this reduction was observed only in severe
diabetic neuropathy[26]. Chang et al[27] revealed that changes in corneal epithelial
parameters, including reduction of CEBC density, increased variability in cell
size, and wider intracellular space were observed in patients with DM. In
addition, they reported that reduction in the CEBC density was significantly
correlated with nerve branch density and nerve fiber density. Other studies
using high-frequency ultrasound revealed changes occurring in the corneal
epithelium during hyperglycemia, which can be useful for the early detection of
damage to the corneal epithelium[28].
Alteration of innervation may be a
major cause of CEBC decrease in patients with DM. As mentioned earlier in this
review, corneal nerve fibers release multiple neuropeptides to maintain corneal
epithelial homeostasis. Accumulating evidence suggests that neurotrophic
factors, as pivotal regulatory molecules, play an important role in DK[29]. Nerve growth factor and CNTF may also reverse
corneal pathologic alteration and accelerate corneal epithelial wound healing
by attenuating apoptosis and inflammation in the diabetic cornea[30-31]. Similarly, fibronectin-derived
peptide (PHSRN) eye drop significantly facilitated the healing of corneal
epithelial wounds in diabetic rats[32]. Other
studies have shown that nerve growth factor promoted human corneal epithelial
wound healing by stimulating phosphorylation of the Akt pathway. This finding
suggests that the PI3K-Akt pathway is involved in corneal epithelial wound
healing[33]. Akhtar et al[34] reported that Substance P–a neuropeptide mainly secreted by
sensory nerve fibers–promoted diabetic corneal epithelial wound healing. This
effect was exerted through the substance P-neurokinin 1 receptor signal pathway
by recovering the activation of Akt, epidermal growth factor receptor (EGFR),
and silent mating type information regulation 2 homolog 1 (SIRT1), ameliorating
the mitochondrial function, and increasing the ROS scavenging capacity. In
addition, a number of miRNAs showed a close relationship with the corneal wound
healing process. For example, miR-204-5p mediated regulation of SIRT1
contributes to the delay of epithelial cell cycle traversal in DK[35]. Furthermore, overexpression of SIRT1 strongly
promoted wound healing in Ins2 mice[36]. The miR
Corneal Epithelial Basement
Membrane Delayed epithelial wound healing and
abnormal epithelial adhesion is attributed to alteration in the basement
membrane by DM. Using transmission electron microscopy, Taylor and Kimsey[39] reported that the thickness of the corneal basement
membrane was greater in diabetic patients. However, Morishige et al[32] reported that the Z-scan may provide a
light-scattering index (LSI), a quantitative parameter of the light
reflectivity of tissues at the basement membrane. The LSI was significantly
increased in diabetic patients; this parameter is relatively reproducible and
correlated with the severity of diabetes. These results imply that measurement
of the LSI may be a marker for the early detection of DM[40].
Multiple mechanisms have been
proposed to play a role in pathological alteration of the basement membrane in
DM. Ljubimov et al[41] reported a
reduction in the CEBC layer occupied by hemidesmosomes in the diabetic cornea.
Diminished expression of the components of the basement membrane (e.g.,
nidogen-1/entactin, laminins, and binding partner integrin α3β1) was observed
in patients with DM[42]. These alterations may be
attributable to abnormal basement membrane metabolism. Accumulating evidence
has suggested that a number of matrix metalloproteinases (MMPs) play a pivotal
role in corneal wound healing. In particular, the expression of
MMP-10/stromelysin-2 is attributed to the proteolytic degradation of basement
membrane components in DM[43-44].
In addition, the expression of MMP-9 was enhanced in diabetic corneal
epithelium wound healing models. It may also damage the type IV collagen and
deteriorate its normal interaction with other proteins involved in cell
attachment[45]. It is widely established that
AGEs play an important role in diabetic epitheliopathy[46].
Ishida et al[47] were the first to detect
elevated corneal autofluorescence in diabetic patients compared with healthy
individuals. The corneal autofluorescence was correlated with deposition of
AGEs in the diabetic cornea. Accumulation of AGEs has been detected at the site
of the corneal epithelium and the epithelial basement membrane in diabetic rats[48]. The AGEs are particularly distributed on the
basement membrane laminin[49]. Furthermore, Sato et
al[35] reported the corneal AGE
autofluorescence corresponding to the severity of DR. AGEs may induce apoptosis
in human corneal epithelial cells through activation of the c-Jun N-terminal
kinase and p38 mitogen-activated protein kinase pathways and generation of ROS[50].
CORNEAL STROMA ABNORMALITY
DM may also cause alterations in the
corneal stroma leading to corneal stroma disorder. DM may induce both
structural and functional alterations in the corneal stroma, and these
processes result in loss of corneal transparency and threaten the vision of the
patients[51]. Studies showed that CCT increases
in parallel with the severity of diabetic peripheral neuropathy due to an
increase in stromal thickness, suggesting that the increase in CCT is an
important clinical implication[52]. Using
transmission electron microscopy, it was shown that the organization of the
anterior stroma matrix was different in the diabetic cornea. In the center of
diabetic corneas, although the structure of collagen lamellae was similar to
that observed in the normal cornea, the basal epithelial lamina appeared
thicker than that reported in the normal cornea. In the peripheral cornea, an
abnormally tile-shaped collagen fibril appeared in the anterior epithelial
basal lamina[24]. According to a long-term
streptozotocin-induced diabetic monkey model, stroma changes affect the
transparency of the cornea. Abnormal collagen fibril bundles with different
thickness and variable spacing can be found in the corneal stroma, and AGE
immune reactivity may also be observed in the corneal stroma. Importantly, AGE
immune reactivity was detected throughout the corneal stroma, which may lead to collagen
crosslinking and contribute to the stromal abnormality[53].
Additionally, keratocyte cell density in the posterior stroma was higher in
young patients with type 1 DM, and the accumulation of ROS and several growth
factors induces the proliferation and activation of keratocytes[7,54]. However, Kalteniece et al[55] demonstrated that a reduction in keratocyte cell
density, which was associated with damage to the corneal sub-basal nerve
plexus. Furthermore, treatment with an EGFR inhibitor may reverse corneal
stroma abnormality by modulating the level of AGEs. In particular, it reverses
the abnormality of the collagen fibrils and proteoglycans. This study suggests
that the EGFR signal pathway contributes to the development of diabetes-induced
corneal stroma remodeling[34]. The MMPs and
tissue inhibitors of metalloproteinases (TIMPs) play a crucial role in the
synthesis and degradation of the extracellular matrix. DM destroys the delicate
balance between MMPs and TIMPs; in DR corneas, MMP-3 and MMP-10 were
upregulated, whereas TIMPs-4 was downregulated[43,56] (Figure 3).

Figure 3 Schematic showing changes
in the components of the stroma in diabetes mellitus Abnormally aggregated collagen
fibrils scattered in the corneal stroma. The accumulation of AGEs in the stroma
causes abnormal cross-linking between the collagen fibers. Moreover,
significantly higher keratocyte cell density was found in diabetes. The
abnormal accumulation of AGEs, ROS, MMP, and some growth factors may result in
the activation or proliferation of keratocytes.
Schwarz et al[57] observed an increased biophysical adhesion strength
of the endothelium-Descemet membrane complex in the diabetic cornea. The
increased adhesive interface between the Descemet membrane and the underlying
stroma may be associated with chronic hyperglycemia, and this study provided a
novel direction for further investigations. Moreover, using complete metabolism
and liposome analysis, Priyadarsini et al[58]
identified potential novel biomarkers in the corneal stroma (e.g.,
aminoadipic acid, pipecolic acid, and dihydroorotate). These potential
biomarkers are significantly up-regulated in diabetic corneas, indicating that
they may be involved in the corneal stroma response to a chronic hyperglycemic
insult. Such biomarkers may be indicative of diabetes-induced stromal damage,
allowing the prompt prediction of DM complications.
CORNEAL ENDOTHELIUM ABNORMALITY
DM also exerts a profound effect on
the corneal endothelium. Changes in endothelial morphological parameters, such
as endothelial cell density (ECD), hexagonality, and CCT have been reported in
DM[48]. Liaboe et al[59]
showed that patients with DM had a markedly lower mean ECD. The coefficient of
variation of the cell area was higher in the diabetic cornea. Although the
lower percentage of hexagonal cells was not statistically significant, it may
reflect the abnormality of the corneal endothelial recovery process[60-63]. Functional disturbances may
lead to increased endothelial permeability and endothelial autofluorescence,
which subsequently result in the impairment of cornea dehydration and lead to
corneal swelling with increased CCT[64].
Moreover, the lower ECD was associated with a higher level of hemoglobin A
The endothelium contains many immune
and inflammatory factors, such as vascular endothelial growth factor, tumor
necrosis factor-α, interleukin (IL), and MMP. These factors may also insult the
corneal endothelium and lead to alterations in endothelial function and
morphology, as well as changes at the molecular level. Of note, the function of
the corneal endothelial barrier is impaired, and recovery of endothelial cells
becomes slower and weaker[68-69].
Hyperglycemia causes non-enzymatic
glycosylation of proteins and abnormal accumulation of sorbitol. Accumulation
of AGEs may cause a decrease in corneal endothelial cells with aging and
disturbing endothelial cell metabolism[49,70].
Other probable mechanisms of changes in the corneal endothelium include
mitochondrial dysfunction, which results in the accumulation of ROS and
mitochondrial injury[71-72].
In addition, glycation of membrane adenosine triphosphatase may play a role in
the disorders of oxygen metabolism[64].
The Descemet membrane is the
basement membrane of the corneal endothelium, which plays a vital role in
withstanding greater shear stresses from biological and mechanical pathogenic
factors[73]. Using confocal microscopy,
hyper-reflective and rod-shaped structures were detected in the peripheral
Descemet membrane of the diabetic cornea; these structures have been identified
as long-spacing collagen fibril. The abnormal secretion of long-spacing collagen
fibril may also occur due to the deposition of AGEs[24].
However, confocal microscopy provided poorly contrasted images of these
abnormalities and lacked specificity. At present, second harmonic generation
(SHG) microscopy is a new imaging technique for the detection of collagen-rich
tissues. SHG can overcome these disadvantages and SHG microscopy can show the
deposition in the Descemet membrane[74]. Using
electron microscopy and laser confocal microscopy, Akimoto et al[75] have also reported that the abnormal long-spacing
collagen fibril bundles were frequently observed in the Descemet membrane of
the diabetic rat model. Interestingly, several diabetic alterations in
collagen-rich tissue (e.g., age-like changes) and the diabetic rat model
showed an age-dependent increase in the density of long-spacing collagen.
Moreover, the formation of long-spacing collagen may be suppressed by
antidiabetic agents. Thus, long-spacing collagen may be a new biomarker for
measuring the effect of antidiabetic agents (Figure 4).
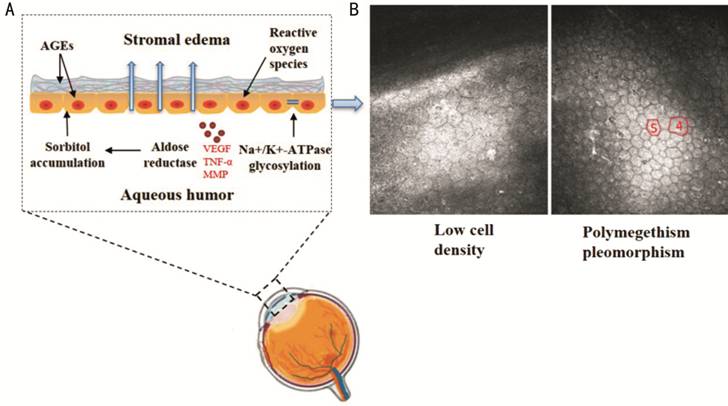
Figure 4 Schematic showing the
pathogenesis of corneal endothelium and Descemet membrane in diabetes mellitus
showing morphological and functional changes, including accumulation of AGEs,
glycation of membrane ATPase, overproduction of ROS, and accumulation of
sorbitol pathway products A: Functional disturbances
may lead to increased endothelial permeability, damage to cellular components,
and stromal edema; B: Representative confocal microscopy image of low ECD and
endothelial cells with polymegethism and pleomorphism in diabetic patients.
CORNEAL LIMBAL STEM CELL ABNORMALITY
Corneal limbus is a narrow band of
tissue that encircles the cornea. Under physiological conditions, corneal
limbal epithelial stem cells (LESCs) give rise to progeny (transit amplifying
cells), which differentiate into mature corneal epithelium during their radial
migration towards the central cornea. The renewal of the corneal epithelium by
LESCs may explain the clinically observed delays in diabetic wound healing.
Using the IVCM, the limbus of the
cornea showed loss of the regular limbal epithelium, presence of
intraepithelial cystic changes, and a mosaic pattern of cells of differing
morphology. In addition, the more profound stroma of limbal palisades of Vogt
showed irregularly arranged fibrous strands with scattered islands of basal
limbal epithelial cells[76].
In DM, a reduction in the expression
of LESC markers and slower wound healing in cultured diabetic LESCs have been
observed, which may account for diabetic LESC dysfunction[77].
Overexpression of c-met, MMP-10, and cathepsin F gene in LESCs was shown to
normalize wound healing, and increase diabetes-altered staining for putative
markers of LESCs (i.e., ΔNp63α, ABCG2, keratins 15 and 17, and laminin
γ3 chain)[78-79]. Furthermore,
treatment with insulin-like growth factor-1 exerts a preventive effect, which
can protect against corneal damage in diabetes[80].
A study performed by Kulkarni et al[81] identified
miR-10b as one of the most abundant miRNAs in corneal limbal, which may control
corneal epithelial homeostasis and stem cell functions. Such miRNAs may be a
new tool for the treatment of DK.
TEAR FILM ABNORMALITY
The tear film is the primary interface
between the ocular surface and the external environment, and plays pivotal
roles in maintaining the morphological and functional integrity of the cornea.
In addition, the lacrimal glands, lacrimal drainage system, and interconnecting
innervation work together as the LFU.
DM is also associated with film
abnormality and LFU insufficiency, which can deteriorate corneal components.
Owing to the lack of tearing or abnormal tear dynamics, the diabetic patients
are more prone to suffer from dry eye syndrome (DES)[82].
DES is very common in diabetic patients, especially in those with DR. DES is a
potential visual impairment syndrome and can lead to superficial punctuate
keratopathy, secondary bacterial infection, and even perforation. The decrease
in lacrimal gland secretory function is the cardinal problem in DES[83].
Many mechanisms contribute to the
onset and progression of the tear film abnormality in diabetic patients.
Notably, chronic inflammation and peripheral neuropathy in diabetes play a
vital role in DES. Chronic hyperglycemia is the main causative mechanism
underlying the pathogenesis of tear film abnormality[84].
In addition, there was a significant elevation of inflammation or
pre-inflammation markers in the tears and conjunctiva of diabetic patients,
such as IL-1α, IL-1β, IL-6, and tumor necrosis factor-α[85].
As previously stated, MMP is an important mediator of inflammation in diabetes
and contributes to tissue impairment. It was reported that elevated MMP-9 was
significantly correlated with ocular surface inflammation[86].
In addition, the level of substance P was significantly lower in the
tears of diabetic patients[20]. A recent study
showed that the increasing level of metallic elements in the tears of patients
with DM may be an indicator of ocular damage[87].
In addition, oxidative stress in the diabetic rat model leads to pathological
alteration of the lacrimal gland acinar cells. An experimental study
demonstrated that overexpression of SIRT
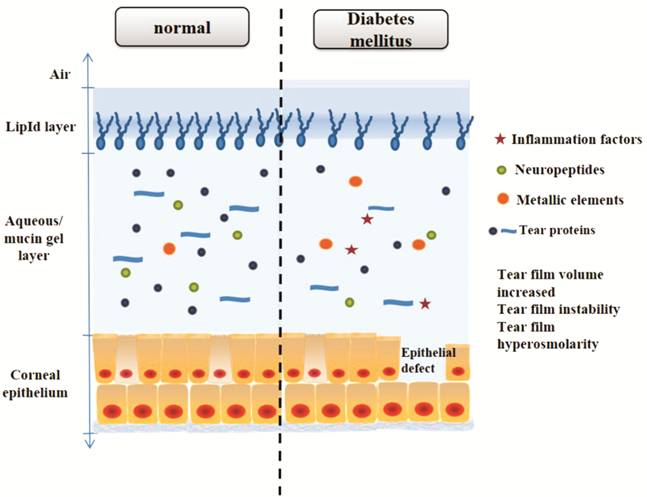
Figure 5 Schematic showing changes
in components of the tear film in diabetes mellitus As a result, the levels of tear
proteins and neuropeptides (secreted by trigeminal sensory nerves on the cornea)
in diabetics are often significantly lower than those reported in healthy
individuals, whereas the levels of some inflammation factors are higher. The
osmolarity of diabetic tears also increases. Regarding the tear fluid itself,
the higher glucose concentration in tears alters the capability for corneal
epithelial wound healing.
Lacrimal nerve fibers play a pivotal
role in the maintenance of tear production and integrity of the LFU. Diabetic
neuropathy may compromise the innervation of the LFU. Moreover, impairment of
the LFU sensory nerve may also inhibit tear secretion associated with the
reduced threshold of corneal sensitivity[92].
Interestingly, using confocal microscopy, the number of corneal sub-basal
nerves was significantly correlated with Schirmer test values[93]. Such a phenomenon may indirectly reveal alterations
in the corneal innervations in DES patients with diabetes. Furthermore,
exposure to high levels of glucose is deleterious for human meibomian gland
epithelial cells, and may help explain the importance of hyperglycemia for LFU
in patients with DM (Figure 6)[94].
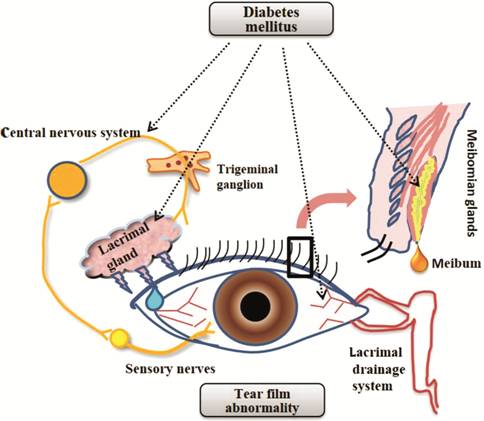
Figure 6 Schematic depiction of the
key components of the LFU The LFU consists of the lacrimal
gland, conjunctival goblet cells, meibomian gland, as well as sensory and motor
nerves. DM exerts distinct effects on different parts of the LFU, resulting in
LFU dysfunction. Diabetic neuropathy may damage both corneal afferent fibers
and efferent nerves. The concomitant inflammatory response with DM may also
affect the meibomian gland, lacrimal gland, and conjunctival goblet cells.
FUTURE PERSPECTIVES
The prevalence of DM has increased
in recent years as a metabolic disease that can influence all structures of the
eye. The clinical manifestations of DK are variable and mainly concern epithelial
lesions, neuropathy, and tear film abnormalities. The molecular mechanisms
responsible for DK remain to be elucidated. As summarized in this review,
numerous underlying pathophysiologic mechanisms participated in changes to the
cornea. Several novel and accurate methods have been developed to investigate
alterations in the diabetic cornea. There is increasing research regarding the
use of IVCM in corneal morphological alterations in diabetic patients.
Therefore, such parameters may be noninvasive biomarkers for diabetic
peripheral neuropathy. An improved understanding of both alterations and
pathogenesis of the DK would be important for the optimal management of DM.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural
Science Foundation of China (No.81371054; No.81600714).
Conflicts of Interest: Zhao H, None; He Y, None; Ren YR,
None; Chen BH, None.
REFERENCES