
·Brief
Report·
Reactive
uveitis, retinal vasculitis and scleritis as ocular end-stage of Acanthamoeba
keratitis: a histological study
Lei Shi1,2, Tobias Hager1, Fabian
Norbert Fries1, Loay Daas1, Leonard Holbach3,
Carmen Hofmann-Rummelt3, Elena Zemova1, Berthold Seitz1,
Nóra Szentmáry1,4
1Department of Ophthalmology,
Saarland University Medical Center, Homburg/Saar 66424, Germany
2Department of Ophthalmology, The
First Affiliated Hospital of USTC, Division of Life Sciences and Medicine,
University of Science and Technology of China, Hefei 230001, Anhui Province,
P.R. China
3Department of Ophthalmology,
Friedrich-Alexander University Erlangen-Nürnberg, Erlangen 91052, Germany
4Department of Ophthalmology,
Semmelweis University, Budapest 1093, Hungary
Correspondence to: Lei Shi. Department of
Ophthalmology, Saarland University Medical Center, Homburg/Saar, Kirrberger
Str. 100. 66424, Germany. shileidr@outlook.com
Received:
Abstract
We analysed histologically two Acanthamoeba
keratitis (AK) eyes with anterior and posterior segment inflammation and
blindness. Two enucleated eyes of 2 patients (age 45 and 51y) with AK (PCR of
epithelial abrasion positive) were analysed. Histological analysis was
performed using hematoxylin-eosin, periodic acid-Schiff and Gömöri-methenamine
silver staining. We could not observe Acanthamoeba trophozoites or cysts
neither in the cornea nor in other ocular tissues. Meanwhile, we found uveitis,
retinal vasculitis and scleritis in these eyes, due to the long-standing,
recalcitrant AK. So in this stage of AK, systemic immune suppression may be
necessary for a longer time period.
KEYWORDS: Acanthamoeba keratitis; enucleation; uveitis;
retinal vasculitis; scleritis
DOI:10.18240/ijo.2019.12.20
Citation: Shi
L, Hager T, Fries FN, Daas L, Holbach L, Hofmann-Rummelt C, Zemova E, Setiz B,
Szentmáry N. Reactive uveitis, retinal vasculitis and scleritis as ocular
end-stage of Acanthamoeba keratitis: a histological study. Int J
Ophthalmol 2019;12(12):1966-1971
INTRODUCTION
Acanthamoeba keratitis (AK) is a progressive,
sight-threatening disease, occurring mostly in contact lens wearers. Its annual
incidence was 17.53 to 21.14 per one million contact lens wearers in the UK[1]. In Germany, with about 80 million inhabitants, about
150 new cases have been reported in a 10-year-period[2].
Studies showed that 68%-92.3% of AK patients are contact lens wearers[1,3-4]. Expression of
mannosilated glycoproteins on corneal epithelial cell surface is upregulated in
contact lens wearers[3]. This plays an important
role in AK pathogenesis. The Acanthamoeba trophozoite binds to these
proteins though its mannose-binding site in order to release the so-called
mannose-induced protease 133 (MIP-133) and Acanthamoeba plasminogen
activator (aPA). MIP-133 and aPA give rise to lysis of epithelial, stromal
cells and stromal matrix, leading to corneal erosions and ulceration[4]. Presence of bacteria or fungi also supports Acanthamoeba
growth, often resulting in co-infection[5].
Although contact lens wear is considered as a risk of AK development, most
interestingly, not each contact lens wearer tends to develop AK, implying that
the individual immune response may play a crucial role. In many aspects, the
immunology of AK needs further research to better understand its pathogenesis
and to find potential intervention points to prohibit its development and
optimize the human immune response[6-11].
AK patients at the early stage of
the disease suffer from tearing and ocular pain. At this time-point, the
ophthalmologists observe a relative mild ophthalmological status, compared to
the pronounced discomfort of the patient. A pseudodendritiformic
epitheliopathy, “dirty epithelium”, typically spot-like multifocal stromal
infiltrates and radial perineuritis can be observed at this stage. Some days
later, a Wessely immune ring around the infected area is observed. In case of
bacterial or mycotic coinfection, a dense stromal infiltrate and hypopyon may
also be present. In later stages secondary glaucoma, iris atrophy, mature
cataract, scleritis and chorioretinitis may occur.
Until now, there is no standardized
treatment of AK and there is no topical or systemic drug which could explicitly
eliminate Acanthamoeba cysts from the human cornea. Topically, diamidines,
biguanides and neomycin are most often used. In some cases, penetrating
keratoplasty (PKP), amniotic membrane transplantation and corneal collagen
crosslinking (CXL) treatment are applied as surgical therapy, but the removal
of the eye through enucleation may also be necessary[12].
The purpose of this study was to
histologically analyze two AK eyes with anterior and posterior segment
inflammation and blindness.
SUBJECTS AND METHODS
Ethical Approval This retrospective study was
performed in accordance with the Declaration of Helsinki Guidelines for Human
Research and the Health Insurance Portability and Accountability Act. The
research project was approved by the Ethics Committee of Saarland (Number 213/18).
Patient History We performed a retrospective record
review between January 2006 and December 2017, at the Department of
Ophthalmology of Saarland University Medical Center, Homburg/Saar searching for
patients with the diagnosis of AK [polymerase-chain reaction (PCR) positive]
and subsequent enucleation. During this time period, there were 30 PCR positive
AK patients and 2 of them underwent enucleation.
These two patients were both contact
lens wearers and their clinical history is described below. In these two eyes
of 2 female patients (aged 45 and 51y) PCR of epithelial abrasion confirmed the
clinical diagnosis of AK (time to diagnosis after first symptoms 2wk and 3mo).
These cases had been treated previously as herpetic or herpetic/bacterial keratitis
in another hospital, respectively. There was no evidence of previous or
subsequent systemic disease in any of the patients. Best corrected visual
acuity at the time of diagnosis was 0.2 and 0.05 and clinical signs of AK were
dirty epithelium and multifocal stromal infiltrates (Figure

Figure 1 Images
of the first case AK with “dirty
epithelium” and multifocal stromal infiltrates (A), after first PKP (B),
recurrence of AK and calcification of recipient along interface (arrows; C),
repeat PKP with amniotic membrane transplantation as patch (D) and with ocular
hypotony, retinal and choroidal detachment (E).

Figure 2 Images
of the second case AK with corneal ulcer, ring infiltrate,
intrastromal bleeding and posterior synechiae (A), after first PKP and amniotic
membrane transplantation as patch (B), with mature cataract (C) and with
“filamentous, spider-net-like” inflammatory reaction in the anterior chamber
(D).
Up-to date, there is no specific
treatment for the Acanthamoeba isolates, causing keratitis. However, in
Germany, mainly triple-topical therapy (polyhexamethilen-biguanide,
propamidin-isethionat and neomycin) is used. Both patients underwent
triple-topical therapy and with failed recovery (2 and 5mo after first AK
symptoms and with continuous triple-topical therapy), surgical therapies
followed. Before surgery, during continuous triple therapy there were
persisiting epithelial defects in both patients, with the size of about 4×
The first patient received CXL with
amniotic membrane transplantation as patch (2mo after first symptoms). All
amniotic membranes have been prepared in our eye bank and were used following
cold storage (
The second patient underwent CXL,
subsequent corneal cryotherapy with PKP and amniotic membrane transplantation
as patch (7.5/
Beside our “standard” systemic
immune modulatory treatment after PKPs
(250-150-150-125-125-100-100-80-80-64-64-32-32-16-
Following PKPs, best corrected
visual acuity was hand movement and 0.1. Triple-topical therapy was continued
5× daily with additional prednisolone-acetate eye drops 5× daily. However, the
epithelial defects further did not heal and the patients developed secondary
glaucoma 3 and 6mo after presentation of first AK symptoms, which was
successfully cured with conservative therapy. This was followed by central
artery retinal occlusion (CRAO) in the first patient 5mo and with central vein
occlusion (CRVO) in the second patient 6mo after first AK symptoms. CRAO and
CRVO were diagnosed through fundus examination. Fluorescein angiography could
not give us additional information through the deepithelialized and oedematous
transplanted corneas.
The first patient ended up with
ciliary body, choroid and retinal detachment 11mo after first keratitis
symptoms and, therefore, sclerotomy, pars plana vitrectomy with silicon oil
implantation was performed. The second patient, with subsequent CRVO, received
intravitreal bevacizumab 9 and 10mo after first AK symptoms.
Ocular hypotony became obvious in
both patients 11 and 9mo, respectively, after the first AK symptoms (Figures 1E
Three months after repeat PKP, both
patients had no light perception (7 and 12mo after the first symptoms of AK)
and subsequently, also due to pain, the inflamed blind eyes were enucleated
(13mo after the first symptoms of AK in both patients).
Histological Analysis Histological analysis of the enucleated
eyes was performed at the Department of Pathology of Saarland University,
Homburg/Saar, Germany and at the Department of Ophthalmology of the
Friedrich-Alexander University of Erlangen-Nürnberg, Erlangen, Germany.
After
formaline-fixation and paraffin wax-embedding of the patients’ enucleated eyes,
3 μm thickness sections were cut using a standard microtome and transferred
onto microscope slides (SuperFrost, Menzel-Gläser, Braunschweig, Germany). We
performed serial sections anteroposteriorly (parallel to the optical axis) and
cross-sections of the optic nerves. The slides were dried at
RESULTS
Images of the histological analysis
are shown at Figure 3. There was no central corneal epithelium on the analysed
globes. We could not observe Acanthamoeba trophozoites or cysts neither
in the cornea (Figure
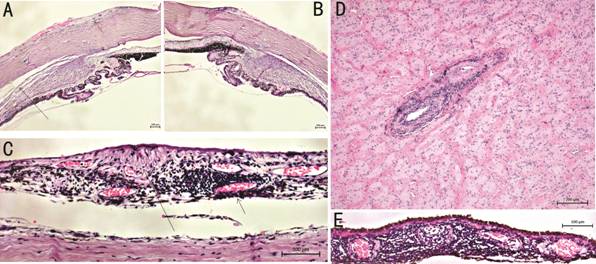
Figure 3 Histological images of both
cases In the first case (A and B,
haematoxylin-eosin), Acanthamoeba trophozoites or cysts were not
detectable in the corneal or other ocular tissues, but lymphocytic infiltration
of the episclera (arrow) and choroidal detachment (long arrow) were shown. In
the first case (C, haematoxylin-eosin), perivascular lymphocytic infiltration
(arrow) and retinal atrophy (long arrow) and perivascular lympocytic
infiltration around central retinal artery was detectable (D,
haematoxylin-eosin). In the second case, there was lymphocytic infiltration of
the choroid (E, haematoxylin-eosin).
There were anterior synechiae in the
chamber angle of both cases and lymphocytic infiltration around the central
retinal artery and vein, associated with fibrous metaplasia of the retinal
pigment epithelium (Figure
Additionally, we observed
perivascular inflammatory cell infiltration (mainly lymphocytes) in the episclera
and around ciliary nerves, analyzing the first case (Figure
Histopathologic studies of the
second case revealed a multifocal, non-granulomatous choroiditis with
lymphocytic infiltration (Figure 3E).
DISCUSSION
In 2007, Awwad et al[13] reported chronic chorioretinal inflammation with
perivascular lymphocytic infiltration and diffuse neuroretinal ischemia as a
new potentially blinding syndrome in 4 of 5 enucleated eyes after AK. In 4 of
these patients, there were Acanthamoeba cysts in the cornea.
Nevertheless, the posterior segment of the eye failed to demonstrate Acanthamoeba
cysts or trophozoites. Burke et al[14] had
reported similar results in one patient in 1992.
Most interestingly, we observed
episcleritis, non-granulomatous uveitis with choroidal and central retinal
artery/vein lymphocytic infiltration (vasculitis) and neuroretinal
degeneration, without presence of Acanthamoeba trophozoites or cysts in
the cornea or other ocular tissues, in two enucleated eyes of two patients.
Extracorneal invasion of Acanthamoeba
had only been described in 8 patients between 1975 and 2013, in the literature.
In three of these cases, scleral invasion and in 5 others Acanthamoeba
sclerokeratitis have been described. Iovieno et al[15]
reported 18.5%
occurrence of sclerokeratitis in their case series with presence of degraded
necrotic cysts in scleral nodule biopsy of these patients. They considered
sclerokeratitis as a T-cell-mediated immune response, which requires systemic
immunosuppression[10,16]. Acanthamoeba
antigens elicit an immune response that leads to generation of T cell clones.
These T cell clones then cross-react with antigens expressed in the normal eye,
which may lead to the generation of additional T cell clones by a process
called “epitope spreading”[17].
We hypothesize that lymphocytes are
more efficient than neutrophils and macrophages to chemoattract Acanthamoeba.
But on the other hand, it can induce an immune response, which may also destroy
other structures of the eye.
Lee et al[18]
has reported, that corneal antigen presenting cells can reside in the central
cornea, migrate to the cervical lymph nodes and activate T-cells. These T-cells
then trigger an inflammatory reaction in the vascularized ocular tissues, such
as uvea and retina. Interestingly, Johns et al[19]
reported on chorioretinitis without vitritis in the contralateral eye of an
immunocompetent AK patient, which might have been a regional immune-related
inflammation, induced by local tissue infection through Acanthamoeba.
There is another hypothesis that Acanthamoeba
may induce a state of autoimmunity through molecular mimicry via corneal
antigen presenting cells or a type III immune reaction, which may target
vascular receptors leading to vasculitis and thrombosis.
In our cases, there was CRAO in one
patient and CRVO in the second patient, before enucleation. Histopathological
examination found lymphocytic infiltration of these vessels. This may indicate
a possible local immune-mediated vasculitis with secondary thrombosis and
occlusion. We hypothesize that the peripheral vasculitis might be rather
related to reactive inflammation than to the Acanthamoeba itself. This
could have happened similarly in three patients reported by Awwad et al[13] and Burke et al[14].
In our two patients, conservative and surgical treatment even could have been
successful. However, the immune reaction to the Acanthamoeba seemed to
generate an ocular inflammatory disease leading to blindness.
Interestingly, necrotizing
vasculitis, leukocytoclastic vasculitis, thrombosis of small vessels and
thrombo-occlusive vasculitis have also been described in systemic Acanthamoeba-related
diseases, such as cutaneous Acanthamoeba infections and Acanthamoeba
encephalitis.
There are only 4 case reports on Acanthamoeba
in the posterior part of the eye. Jones et al[20]
described a case in a 7-year-old boy with meningoencephalitis, with
trophozoites in the ciliary body. Heffler et al[21]
reported on Acanthamoeba cysts in the aqueous humor and in the vitreous
in a patient with acquired immune deficiency syndrome. In both patients,
choroiditis and retinal vasculitis were present. Moshari et al[22] found Acanthamoeba cysts and trophozoites in
the human retina, without chronic choroidal and retinal perivascular
inflammation. Mammo et al[23] report a
recurrent Acanthamoeba infection presenting initially as keratitis,
followed by retinitis and histopathology confirmed endophthalmitis.
Interestingly, Clarke et al[24] showed that the clearance of the anterior chamber
happens within 15d following injection of Acanthamoeba trophozoites to
the anterior chamber of hamster eyes. This was supported through a robust
neutrophilic reaction in these eyes. This also supports the hypothesis, that
choroid and retinal inflammation is rather immune-mediated and not related to
the presence of the Acanthamoeba. However, there might be a difference
in human and animal immune response.
Iovieno et al[15] described that in case of AK-related mild
scleritis/limbitis, treatment with topical steroids and oral non-steroidal
antiinflammatory drugs may be sufficient. However, moderate/severe scleritis
requires systemic immunosuppressive therapy (cyclosporine or
mycophenolat-mophetil) over months (about 7mo)[16].
Monitoring scleritic pain may help to decide on the length of the immunosuppressive
treatment[16]. Acanthamoeba sclerokeratitis
is associated with poor clinical outcomes, but management of Acanthamoeba
sclerokeratitis with anti-inflammatory/immunosuppressive treatment is usually
effective in reducing scleral inflammation and symptoms and the number of
enucleations[15-16].
Previous studies have shown that
polyhexamethilen-biguanide and propamidin-isethionat may be cytotoxic for human
corneal cells in clinically relevant concentrations[25].
It has also been suggested, that posterior segment inflammation may be related
to toxicity of topical treatment used in AK, however, previous studies also
reported AK patients with long-lasting topical treatment and absence of
posterior pole inflammation, which contradicts this theory. Nevertheless,
mature cataract formation in both patients could be related to toxicity of
biguanides. These can then disrupt the lens surface, provoke lenticular
oxidative or osmotic stress, and contribute to cataract formation by altering
lipid membranes, damaging lens fibers, and inducing electrolyte imbalance[26-27].
In our study, enucleation was
performed at the end of patient histories with repeat (intraocular) surgeries.
The most conspicuous finding of the histological analysis is that there were no
trophozoites or cysts in both enucleated eyes. Although there were Acanthamoeba
trophozoites and cysts in the explanted corneal buttons of PKPs and repeat PKPs
previously, these were not persisting in corneal and ocular tissues
subsequently. However, intraocular inflammation with CRAO/CRVO developed.
Therefore, we hypothesize that Acanthamoeba or the long-lasting
triple-therapy triggered an immune response, which was persisting without
microorganisms.
In case of uveitis or retinal
vasculitis in AK patients, a systemic immune-suppression for a longer period of
time should be initiated in order to avoid the potentially blinding syndrome of
the posterior part of the eye, most probably related to immune-mediated
processes.
In summary, in long-standing,
recalcitrant AK, uveitis, retinal vasculitis and scleritis may occur and result
in blindness, even without further persistence of Acanthamoeba
trophozoites or cysts. The etiology of these inflammatory complications is
unclear, but may be explained with molecular mimicry or type III
immune-reaction. Therefore, in late stage of AK, systemic immune suppression
may be necessary for a longer period of time.
ACKNOWLEDGEMENTS
Conflicts of Interest: Shi L, None; Hager T, None; Fries
FN, None; Daas L, None; Holbach L, None; Hofmann-Rummelt
C, None; Zemova E, None; Seitz B, None; Szentmáry N, None.
REFERENCES