
·Letter to the Editor·
Fluorescein
angiography findings in both eyes of a unilateral retinoblastoma case during
intra-arterial chemotherapy with melphalan
Cem Ozgonul1, Neeraj Chaudhary2,
Raymond Hutchinson3, Steven M. Archer1,
Hakan Demirci1
1Department of Ophthalmology and
Visual Sciences, W.K. Kellogg Eye Center, MI 48105, USA
2Department of Radiology, University
of Michigan, MI 48109, USA
3Department of Pediatric
Hematology/Oncology, University of Michigan, MI 48109, USA
Correspondence to: Hakan Demirci. Department of
Ophthalmology and Visual Science, W.K. Kellogg Eye Center, 1000 Wall St, Ann
Arbor, MI 48105, USA. hdemirci@med.umich.edu
Received:
DOI:10.18240/ijo.2019.12.24
Citation: Ozgonul
C, Chaudhary N, Hutchinson R, Archer SM, Demirci H. Fluorescein angiography
findings in both eyes of a unilateral retinoblastoma case during intra-arterial
chemotherapy with melphalan. Int J Ophthalmol 2019;12(12):1987-1989
Dear Editor,
Intra-arterial chemotherapy (IAC) is
a treatment for retinoblastoma that involves direct injection of
chemotherapeutic agent into the ophthalmic artery. The main advantage of this
method is the ability to deliver high drug concentration in the tumor with low
systemic toxicity[1-2]. However,
it has the potential to cause vascular-related ocular side effects of vitreous
hemorrhage, branch retinal artery obstruction, ophthalmic artery spasm with
reperfusion or obstruction, and choroidal ischemia[3].
To further understand the underlying mechanisms of these vascular side
effects, we report the fluorescein angiography (FA) findings of the treated and
untreated eyes in a unilateral retinoblastoma patient during IAC with
melphalan. A 13-month-old boy was referred with leukocoria in his left eye.
Informed consent form was signed by patient’s mother. Fundus examination of the
left eye showed a retinoblastoma with surrounding localized vitreous seeds,
measuring 16×6×
IAC was performed by the
neuro-interventional radiology team under general anesthesia. A Magic 1.5 Fr
BALT microcatheter was inserted into the left femoral artery, advanced into the
internal carotid and up to the origin of the ophthalmic artery. Once the
catheter tip position was confirmed at the origin of the ophthalmic artery by
fluoroscopy, 5 mg melphalan was infused in a pulsatile fashion over 30min. There
was no anatomical variant of orbital vascular structure. During the 2nd
IAC, following the infusion of melphalan, sodium fluorescein dye at a dose of
7.7 mg/kg was injected through the same microcatheter. Real-time FA was
recorded by using the RetCam III (Clarity Medical Systems, Pleasanton,
California). FA was repeated 4wk later during the 3rd IAC in the
same manner, before infusion of the chemotherapy. In both sessions, there was
no catheterization or injection of contrast material into the untreated carotid
and ophthalmic artery. During both procedures, vital signs and pulmonary
compliance values were within the normal range.
We evaluated the FA of both eyes
after the 2nd and before the 3rd cycles of IAC. In the
first FA, following IAC, the early phase showed delayed choroidal perfusion in
the treated left eye. Diffuse retinal arterial narrowing, hypoperfusion of the
tumor and less leakage of intra-tumoral vessels were observed in the mid and
late phases compared to the second FA before IAC (Figure 1B
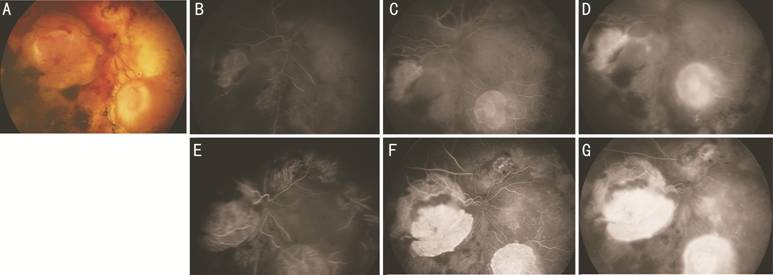
Figure 1 Treated left eye A: Color fundus image. B-D: Early and
late phases of the first FA following the 2nd cycle of IAC.
Choroidal hypoperfusion in the early phases, diffuse retinal arterial
narrowing, and hypoperfusion of the tumor are visible. E-G: Early and late
phases of the second FA before the 3rd cycle of IAC.
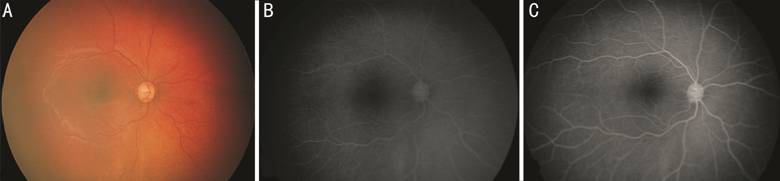
Figure 2 Untreated right eye A: Color fundus image. B: Late phase of
the first FA following the 2rd cycle of IAC. Diffuse retinal
arterial narrowing and diffuse hypoperfusion of choroid are noted. C: Late
phase of the 2rd FA before the 3rd cycle of IAC.
In a non-human primate model, Wilson
et al[4] reported local vascular
complications of IAC with melphalan including retinal artery narrowing, retinal
edema, retinal artery precipitates and choroidal hypoperfusion. Moreover, in a
study that evaluated FA findings after IAC by Bianciotto et al[5], the authors concluded that vascular perfusion of the
retina and the choroid can be compromised after IAC. The retinal abnormalities
that they found were similar to those seen by Wilson et al[4], including ophthalmic artery obstruction in 4% of
cases, choroidal perfusion abnormalities in 25%, central and branch retinal
artery obstruction in 4% and 13% of cases, respectively. However, these studies
did not describe untreated fellow eyes. In our case, the untreated fellow eye
demonstrated FA findings similar to the treated eye. Vasoconstriction of
retinal vessels was observed in both eyes.
To our knowledge, this is the first
case report to demonstrate evidence of vascular changes in the untreated fellow
eye of a unilateral retinoblastoma patient. Vascular complications following
IAC have been proposed to be due to the catheter-related vascular insult,
endothelial cell toxicity of melphalan or foreign body embolization[6]. In an animal model, Steinle et al[7] showed that melphalan caused endothelial cell
inflammation and leukostasis of the ophthalmic artery following 3 IAC with
melphalan. Kato et al[8] demonstrated that
29% of patients experienced a severe pulmonary compliance event during IAC by
analyzing peak inspiratory pressure (PIP), positive end expiratory pressure
(PEEP), tidal volume (TV), oxygen saturation (SpO2), and end tidal
CO2 (EtCO2). The decrease in pulmonary compliance values
might play a role in vascular changes during IAC. However, in our case,
pulmonary compliance values were within normal limits. Our finding of vascular
spasm in the untreated fellow eye might suggest that vascular spasm might occur
in both treated and untreated eyes during the IAC despite the normal pulmonary
compliance and multiple factors might play role in the etiology.
ACKNOWLEDGEMENTS
Conflicts of Interest: Ozgonul C, None; Chaudhary N, None;
Hutchinson R, None; Archer SM, None; Demirci H, None.
REFERENCES