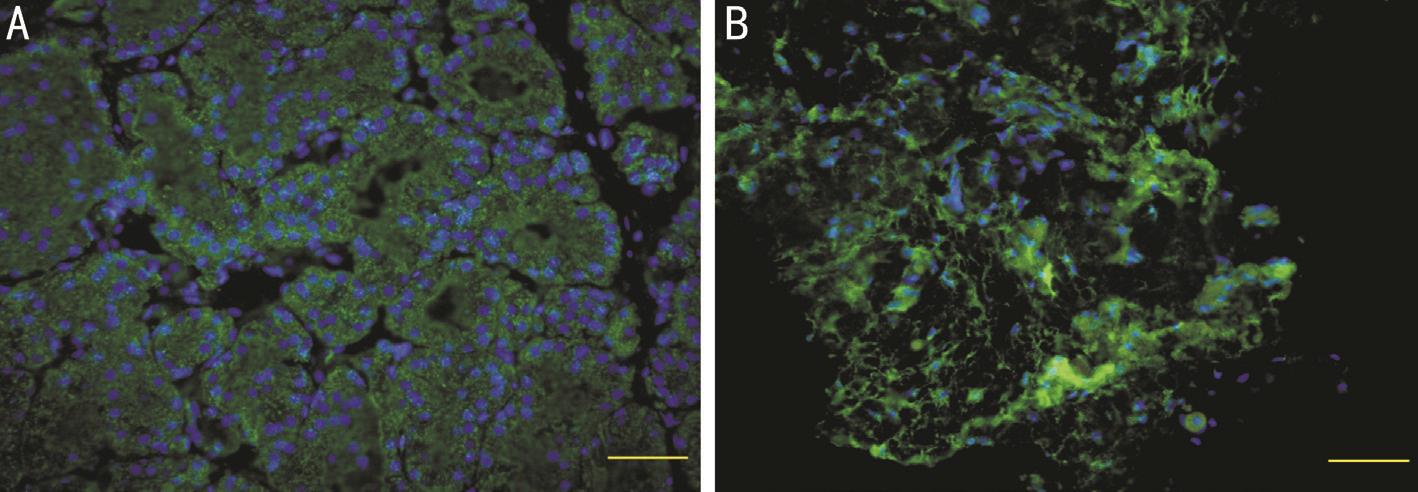
Figure 1 Tear protein lacritin expression
A: Lacrimal acinar cells (green fluorescein); B: Conjunctival surface (green fluorescence).
D ry eye is a multifactorial disease of the tear and ocular surface. Which causes discomfort, visual disturbances,and can even result in anxiety and irritability. Dry eye can affect people's lives and work efficiencies, even in mild cases not caused by organic disease. Moderate and severe cases of dry eye may result in corneal neovascularization, damage to the ocular surface, and ultimately irreversible vision loss. Dry eye has garnered more attention recently and treatments for dry eye have been proposed. Immunosuppressants are capable of stimulating tear production and may be a new option for dry eye treatment. They have been found to be effective in very severe cases of dry eye, however the mechanism by which immunosuppresents stimulate tear production remains unclear.The purpose of this study was to observe the effect of topical 0.05% cyclosporine A (CsA) on the ocular surface and tear protein lacritin in a botulinum B-induced rat model of dry eye and to provide an experimental basis for treating dry eye with 0.05% CsA.
Ethical Approval We confirmed adherence to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in this study and received approval from Wuhan University Institutional Animal Use and Care Committee.
A total of 36 female SD rats (8wk, 200-250 g), with normal eyes and no ocular pathologies, were randomly divided into 3 groups as follows: group A (n=15), group B (n=15) and a control group (n=6). Botulinum B was injected into the right lacrimal gland of all rats. Botulinum B for injection was obtained from Elan (USA). Tear test strips were purchased from Tianjin Jingming New Technology Development Co., Ltd. (China). Anti-rat tumor necrosis factor (TNF)-α monoclonal antibody was purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. (China). Goat anti-rat interleukin(IL)-6 monoclonal antibody was received from Pepro Tech(USA). Anti-rat lacritin protein antibody was obtained from Santa Cruz (USA). Tear protein lacritin expression were observed using photographic slit microscopy (SL-1E Topcon,Japan). Tear protein lacritin levels were detected using fluorescence microscopy (BX51 Olympus, Japan).
Methods Animals were anesthetized via intraperitoneal injection of 10% chloral hydrate (4 mL/Kg). The right lacrimal gland of each rat was exposed under a surgical microscope and injected with 0.1 mL (20 milliunits, mU) of botulinum-B.Animals were fed a regular diet. The animals had free access to food and water and they were on a 12h light/dark cycle. Group A rats were treated with 0.05% CsA eye drops three times daily and group B were treated with 0.1% sodium hyaluronate eye drops three times daily, beginning three days after injection.Control group rats were not treated.
Rats were sedated using basic anesthesia and basal tear fl ow was measured with Schirmer strips on days 1, 3, 7, 14, and 42 post-injection. The Shirmer strip was placed inside the lower eyelid for 5min, taking care to avoid corneal stimulation, and the amount of wetting was measured in millimeters.
Corneal fluoresce in staining was evaluated 1min after fluoresce in instillation using a slit lamp with a cobalt blue light. Corneal staining was scored from 0 to 4 as follows: 0) no fluorescein stain; 1) ≤1/8 of corneal surface stained; 2) ≤1/4 of corneal surface stained; 3) ≤1/2 of corneal surface stained; 4)>1/2 of corneal surface stained.
Rats were randomly euthanized at 3, 7, 28, and 42d postprocedure (specific numbers of rats from each group). The lacrimal glands were immediately removed and fixed in 4%paraformaldehyde for 24h. The lacrimal glands were then paraffin-embedded, sectioned and immunohistochemically stained, followed the instructions. Rabbit anti-lacritin (Santa Cruz), diluted 1:50 in PBS-T; mouse nestin (Chemicon),diluted 1:200 in PBS-T; goat DCX (Santa Cruz), diluted 1:100 in PBS-T; mouse NeuN (Chemicon), diluted 1:300 in PBS-T;and mouse GFAP (Chemicon), diluted 1:500 in PBS-T were added step by step and the sections incubated overnight at 4℃.Biotin-labeled rabbit IgG (Vector, US) secondary antibody,diluted 1:200 in PBS-T, was then added and the sections were incubated for 1h at room temperature. ABC complex (Vector)was prepared 1h before use, then added to the sections and the sections were incubated for 1h at room temperature and developed using DAB (Zhongshan Jinqiao Biotechnology Co., Ltd., China). The sections were dehydrated, cleaned, and sealed with a neutral gum. Immuno fluorescence staining using the primary antibody was performed as above.
The working concentration of FITC-labeled secondary antibody (Zhongshan) was 1:200. The cells were incubated in the dark at room temperature for 2h. The nuclear fuel DAPI was added before mounting. The target site was photographed using a BX51 fluorescence microscope for qualitative observation of lacritin protein expression. After coloration of each tissue section, the target area was selected under the microscope, keeping the brightness of the light source constant. The white balance was set using a blank area of the tissue section and the resolution, magnification, and scale size of the photograph were recorded. Fluorescent stained sections were photographed using a BX51 fluorescence microscope(Olympus) and the black balance was set using a non-tissue site. The principle of the slice was the same as above.
Rats were euthanized and then the lacrimal glands were quickly removed and placed on ice. An appropriate amount of lysate was added (100 μL of lysate per 5 mg of tissue). The tissue was cut into pieces using an ophthalmic scissors and crushed with a mechanical tissue crusher. Lastly, the tissue was placed in an ice bath and completely crushed using a sonicator (72 kJ, 20% amplitude, ultrasonic 5s, intermittent 25s, total 5min) and centrifuged at 12 000 g for 10min at 4℃.The supernatant was then transferred to another pre-chilled Eppendorf tube and stored at -80℃.
The extracted homogenate supernatant was subjected to protein quantification using the Bradford method. Here, 15 μg samples,mixed with an equal volume of 2×loading buffer, were bathed in boiling water at 100℃ for 2min. Samples were loaded on a polyacrylamide gel, electrophoresed at 200 V, and transferred to a PVDF membrane. Blots were blocked for 1h at room temperature, incubated with anti-rat lacritin protein antibody,diluted 1:300 in the blocking solution, overnight at room temperature, rinsed with PBS-T (3×10min), and incubated with goat anti-rat IL-6 monoclonal antibody (PeproTech,USA) diluted 1:2000 in PBS-T for 2h at room temperature.Blots were rinsed with PBS-T (3×10min) and developed via chemiluminescence using Western blotting substrate. The experiment was repeated 3 times and β-actin was used as an internal reference. The results of the Western blot were analyzed using the Gene Tools software of UK Syn gene.
Statistical Analysis Data are expressed as mean±standard deviation. Variance analysis of multiple comparisons between multiple samples was used for each group. Corneal fluorescence staining was analyzed via the Mann-Whitney method using SPSS 16.0 (USA). P<0.05 was considered statistically significant.
Tear secretion in all rats was significantly reduced on days 3 and 7 compared with preoperative levels (P<0.05). Tear secretion increased in group A on day 14 and had recovered to the preoperative level on day 42. Tear secretion was lower in group B and control rats on days 14 and 42 compared to preoperative levels (P<0.05; Table 1).

Figure 1 Tear protein lacritin expression
A: Lacrimal acinar cells (green fluorescein); B: Conjunctival surface (green fluorescence).
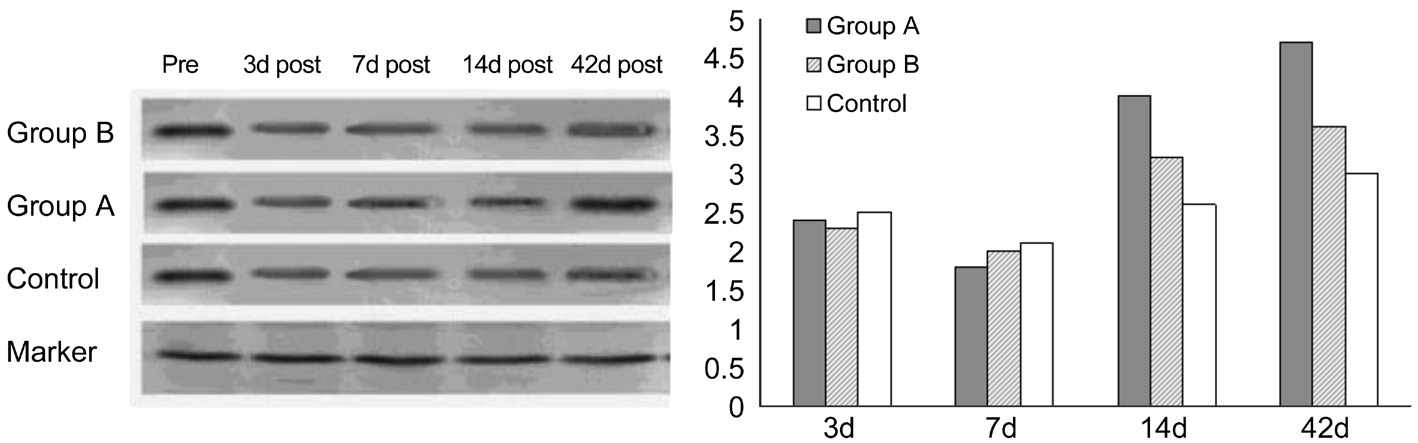
Figure 2 Fluctuation of tear protein lacritin levels of groups at all time points.
Table 1 Tear secretion in each group mm
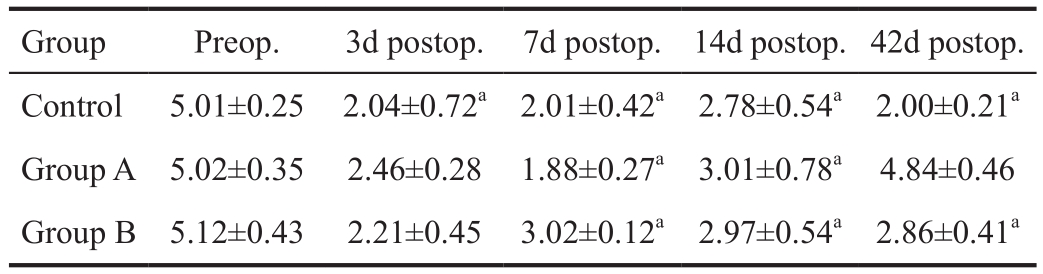
aP<0.05 vs preoporative.
?
Corneal fluoresce in staining was positive in all groups on day 3. Corneal fluorescein staining in group A increased after beginning eye drops, worsening from a spot to a sheet on day 7, but gradually improved by day 14 and was fully recovered on day 42. Corneal fluorescein staining improved in group B,but was not fully recovered on day 42. Corneal fluoresce in staining in control group rats slowly enlarged to a sheet and was worse compared with group A and group B rats on days 7,14 and 42 (Table 2).
Tear protein lacritin expression was mainly restricted to lacrimal acinar cells, and was not detected in other lacrimal gland tissues or duct sections (Figure 1).
Tear protein lacritin levels began to decrease on day 3 and reached the lowest level on day 7 in all groups. In group A, tear protein lacritin increased on day 14 and had recovered on day 42, however tear protein lacritin did not fully recover in group B. Tear protein lacritin level was lower than pre-procedure levels in the control group at all time points (Figure 2).
Dry eye is one of the most common ocular surface diseases.The lacrimal functional unit (LFU) is defined as an integrated system that is comprised of tearfilm, lacrimal glands, corneal and conjunctival epithelium, meibomian glands, lids, and the sensory and motor nerves that connect them[1]. The LFUplays a vital role in maintaining a healthy ocular surface[2].Dry eye is a complex multifactorial disease associated with inflammation, tear film instability, tear hyperosmolarity,changes in sex hormones[3], and dysfunction of the nervous system. It is characterized by symptoms of ocular surface discomfort and irritation. Although the causes for dry eye are various[4-6], Inflammation is the most critical factor in the pathogenesis and progression of dry eye[7-8]. Furthermore,inflammation involves every component of the LFU[9-10].Immune-related inflammation is attributed to lymphocytic infiltration of the lacrimal and ocular surface tissue and the release of inflammatory factors. inflammatory factors damage the neuromodulation of tear secretion, subsequently affecting the quality and quantity of tears, which ultimately becomes a malignant cycle[11]. Thus, a chronic pseudo-immune rat model of dry eye, similar to that observed in humans, was established in the current study as an approach to treating the Inflammation associated with dry eye.
Table 2 Comparison of corneal staining of groups

aP<0.05 vs preoporative.
Group Preop. 3d postop. 7d postop.14d postop.42d postop.Control 0.01±0.23 1.04±0.42 2.41±0.95 2.54±0.54 3.05±0.26 Group A 0.02±0.32 1.35±0.72a2.01±0.86a 1.41±0.78a 0.04±0.46a Group B 0.01±0.33 1.12±0.76a1.04±0.42a 2.47±0.37a 1.06±0.42a
Botulinum-B blocked the neuromuscular and cholinergic connections of the sweat glands and lacrimal glands, and inhibited tear and sweat secretion. The chronic pseudo-immune rat model of dry eye in this study reflected the pathological mechanism of dry eyes in humans. Corneal fluorescence staining was persistent in group B rats, which were treated with artificial tears and no anti-Inflammatories. This suggests that Inflammation has always existed in dry eye since the tear secretion was normal in group B rats. Previous studies have shown that, unlike in humans, there is no difference in the effects of botulinum-A and botulinum-B in rats[11]. botulinum-B has a more extensive target and causes a more rapid response,thus botulinum-B was chosen to develop the rat model of dry eye used in this study.
All rats showed signs of dry eye on day 3, such as decreased tear secretion and corneal epithelial defects. These signs were persistent for 6-8wk, even though tear secretion had recovered.Corneal epithelial defects were still present on day 42. No corneal necrosis or ulceration was observed in any subject during the course of the study.
Earlier experiments have confirmed that the levels of ocular surface inflammatory factors, such as IL-1β, TNF-α, and macrophage migration inhibitory factor are increased in the rat model of dry eye[12]. Although it is still not clear what proteins make up the human tear proteome, over 400 proteins have been reported. It is thought that no more than 5% of these participate in ocular surface disease, of which lacritin is the only one apparently capable of promoting tear production[13]. Lacritin is wide-spread in the LFU and is an important component of tears[14]. In addition, it has been found that lacritin, as a growth factor protein, plays an important role in the occurrence and development of dry eye[15-16], though the mechanism for this is still not clear.
Lacritin is mainly secreted by the lacrimal gland and flows through ducts to target corneal epithelial cells[17]. Lacritin promotes mitosis, corneal epithelial cell proliferation, and tear secretion[18]. In dry eyes, lacritin is decreased due to the effects of inflammatory factor IL-2, particularly in cases of severe dry eye[19-22]. Therefore, the current study lacritin was chosen as the target to investigate the efficacy of topical 0.05% CsA for the treatment of ocular surface Inflammation. Lacritin had increased on day14 and recovered by day 42 in rats treated with topical 0.05% CsA. However, lacritin had still not returned to normal on day 42 in rats treated with 0.1% sodium hyaluronate. The results of this study, suggest that 0.05% CsA inhibited the production of IL-2 in cells and subsequently blocked T-cell proliferation. Cyclosporine can inhibit T cellinduced immune responses by selectively inhibiting the proliferation of T cells[23-24], thus increasing lacritin levels in the dry eye rat model. Lacritin also promotes corneal wound healing and tear secretion[25]. In this study lacritin had decreased in the control group on day 3 and remained at a low level for 6wk. This coincided with the decrease in tear secretion and damage to the ocular surface confirming that the level of lacritin is linked to tear secretion, ocular surface damage and dry eye severity.
In the current study, the lacrimal gland tissue structure remained intact and no T cell infiltration was found in pathological sections. This indicates that the lacritin was secreted by lacrimal acinar cells flowed through ducts to the ocular surface. Lacritin is mainly secreted by the lacrimal gland and meibomian gland with a small amount secreted by the cornea. The detection of lacritin in tear or conjunctiva can be used as an objective index to reflect lacrimal function and also as a measure of drug efficacy and/or complications.Larger trials are needed to study the association between lacritin protein and inflammatory factors as well as the specific function and mechanisms of action of lacritin. In summary, in this study 0.05% CsA improved ocular surface repair and micro-environment by reducing the production of inflammatory cytokines. Lacritin in tears can be used as a marker to assess the progression of dry eye and the efficacy of the drugs used to treat dry eye.
Foundations: Supported by Natural Science Foundation of Shaanxi Province (No.S2010JC376); Xi'an Science and Technology Program Funding Project [No.SF1022(1)].
Conflicts of Interest: Zhang FD, None; Hao ZQ, None; Gao W, None; Xing YQ, None.
1 McKown RL, Wang NN, Raab RW, Karnati R, Zhang YH, Williams PB, Laurie GW. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res 2009;88(5):848-858.
2 Moss SE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 2000;118(9):1264.
3 Ayaki M, Kawashima M, Uchino M, Tsubota K, Negishi K. Gender differences in adolescent dry eye disease: a health problem in girls. Int J Ophthalmol 2018;11(2):301-307.
4 Xu L, Zhang W, Zhu XY, Suo T, Fan XQ, Fu Y. Smoking and the risk of dry eye: a Meta-analysis. Int J Ophthalmol 2016;9(10):1480-1486.
5 You YS, Qu NB, Yu XN. Alcohol consumption and dry eye syndrome: a Meta-analysis. Int J Ophthalmol 2016;9(10):1487-1492.
6 Villani E, Rabbiolo G, Nucci P. Ocular allergy as a risk factor for dry eye in adults and children. Curr Opin Allergy Clin Immunol 2018;18(5):398-403.
7 Kwon JW, Choi JA, Shin EY, La TY, Jee DH, Chung YW, Cho YK.Effect of trapping vascular endothelial growth factor-A in a murine model of dry eye with inflammatory neovascularization. Int J Ophthalmol 2016;9(11):1541-1548.
8 Lee SY, Han SJ, Nam SM, Yoon SC, Ahn JM, Kim TI, Kim EK,Seo KY. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients.Am J Ophthalmol 2013;156(2):247-253.e1.
9 Ratay ML, Balmert SC, Acharya AP, Greene AC, Meyyappan T, Little SR. TRI Microspheres prevent key signs of dry eye disease in a murine,inflammatory model. Sci Rep 2017;7(1):17527.
10 Yagci A, Gurdal C. The role and treatment of Inflammation in dry eye disease. Int Ophthalmol 2014;34(6):1291-1301.
11 Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA,Benítez-del-Castillo J, Boboridis KG, Merayo-Lloves J, Rolando M,Labetoulle M. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol 2016;100(3):300-306.
12 Zhu HF, Hao ZQ, Cheng Y, Gao W. Evaluation criterions and establishment of dry eye model of rats induced by BTX-B. Guoji Yanke Zazhi (Int Eye Sci) 2015;15(9):1512-1515
13 Fujii A, Morimoto-Tochigi A, Walkup RD, Shearer TR, Azuma M.Lacritin-induced secretion of tear proteins from cultured monkey lacrimal acinar cells. Invest Ophthalmol Vis Sci 2013;54(4):2533-2540.
14 Ma PS, Wang NN, McKown RL, Raab RW, Laurie GW. Focus on molecules: lacritin. Exp Eye Res 2008;86(3):457-458.
15 Nakajima T, Walkup RD, Tochigi A, Shearer TR, Azuma M.Establishment of an appropriate animal model for lacritin studies:cloning and characterization of lacritin in monkey eyes. Exp Eye Res 2007;85(5):651-658.
16 Wang W, Jashnani A, Aluri SR, Gustafson JA, Hsueh PY, Yarber F, McKown RL, Laurie GW, Hamm-Alvarez SF, MacKay JA. A thermo-responsive protein treatment for dry eyes. J Control Release 2015;199:156-167.
17 Seifert K, Gandia NC, Wilburn JK, Bower KS, Sia RK, Ryan DS,Deaton ML, Still KM, Vassilev VC, Laurie GW, McKown RL. Tear lacritin levels by age, sex, and time of day in healthy adults. Invest Ophthalmol Vis Sci 2012;53(10):6610-6616.
18 Karnati R, Talla V, Peterson K, Laurie GW. Lacritin and other autophagy associated proteins in ocular surface health. Exp Eye Res 2016;144:4-13.
19 McNamara NA, Ge SK, Lee SM, Enghauser AM, Kuehl L, Chen FY, Gallup M, McKown RL. Reduced levels of tear lacritin are associated with corneal neuropathy in patients with the ocular component of sjögren's syndrome. Invest Ophthalmol Vis Sci 2016;57(13):5237-5243.
20 Gurumurthy S, Iyer G, Srinivasan B, Agarwal S, Angayarkanni N.Ocular surface cytokine profile in chronic Stevens-Johnson syndrome and its response to mucous membrane grafting for lid margin keratinisation.Br J Ophthalmol 2018;102(2):169-176.
21 Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol 2016;10:1083-1094.
22 Pinto-Fraga J, Enríquez-de-Salamanca A, Calonge M, González-García MJ, López-Miguel A, López-de la Rosa A, García-Vázquez C, Calder V,Stern ME, Fernández I. Severity, therapeutic, and activity tear biomarkers in dry eye disease: an analysis from a phase III clinical trial. Ocul Surf 2018;16(3):368-376.
23 Arman A, Demirseren DD, Takmaz T. Treatment of ocular rosacea:comparative study of topical cyclosporine and oral doxycycline. Int J Ophthalmol 2015;8(3):544-549.
24 Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res 2014;9(2):240-250.
25 Vijmasi T, Chen FY, Balasubbu S, Gallup M, McKown RL, Laurie GW, McNamara NA. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Invest Ophthalmol Vis Sci 2014;55(8):5401-5409.