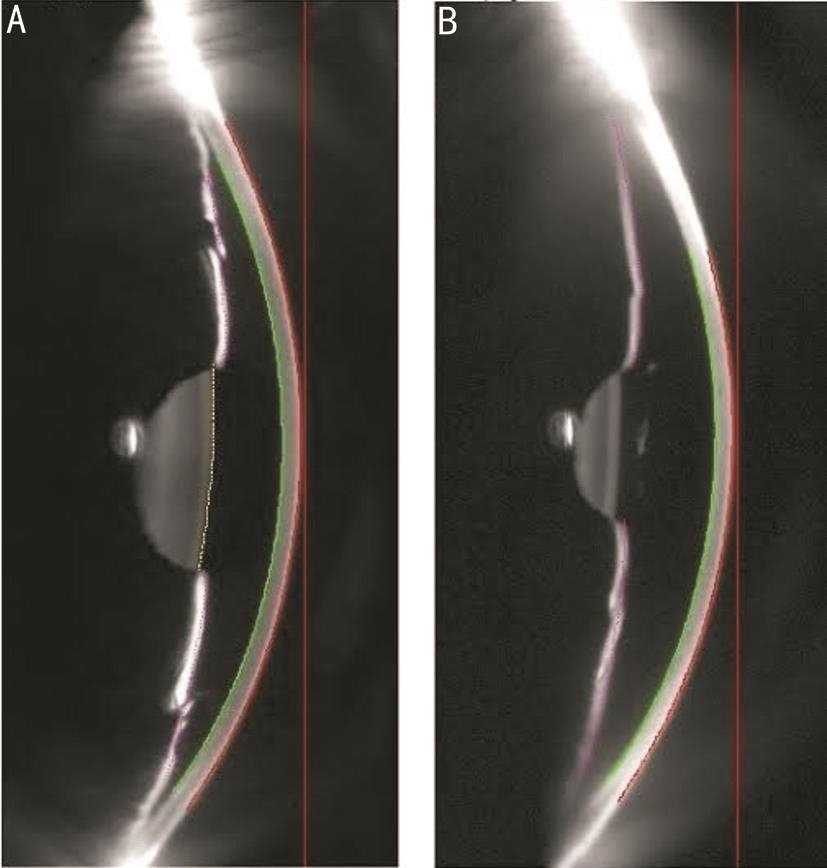
Figure 1 Galilei dual Scheimpflug image
A: Galilei image of a patient with narrow angle. The ACD by Galilei in this case was 1.97 mm and the ACA was 26.1°; B: Galilei image of a patient with open angle. The ACD was 3.04 mm and ACA was 31.0°.
T he World Health Organization (WHO) considers glaucoma an important cause of visual morbidity[1].Primary angle closure glaucoma (PACG) may be the cause for half of glaucoma cases, with high prevalence in Asia[2-3]. PACG is correlated with a faster glaucoma progression, representing an important public health issue[4]. The correct diagnosis of PACG relies on the correct identification of individuals with iridotrabecular contact. PACG requires initial treatment with laser peripheral iridotomy[5]. The assessment of anterior chamber angle (ACA) determinates the different types of glaucoma[6].
Gonioscopy is the gold standard for ACA assessment,however, it demands training and expertise, and is subjected to intraobserver bias[6-8]. Imaging devices, like anterior chamber segment optical coherence tomography (AS-OCT), ultrasound biomicroscopy (UBM), and Scheimpflug imaging, have been used to grant a more impersonal and precise assessment of the ACA[9-12].
The Galilei G6 Lens professional (Ziemer Ophthalmic Systems AG, Port, Switzerland) is an optical biometry instrument that combines a dual rotating Scheimpflug camera, a placido disc topographer, and an optical coherence tomographybased A scan. It performs axial biometry using light of 880 nm wavelength and which is based on low coherence interferometry[13-17].
Although some studies have correlated gonioscopy findings with anterior chamber data obtained with UBM, scanning peripheral anterior chamber depth (ACD) analyzer (SPAC),OCT and Pentacam[9-12], the efficacy of the Galilei dual Scheimpflug system in detecting narrow angles has never been previously analyzed. Even though Galilei dual Scheimpflug has been proved to acquire repeatable and accurate measurements of anterior segment parameters[13-17], it has not been clear whether the results of biometry and ACA are comparable with other imaging devices and whether they can be used interchangeably[14]. Therefore, this study aimed to compare the dual Scheimpflug analyzer with the traditional gonioscopy as a possible screening method for the detection of narrow ACA and to report the best cut-off values for this specific device.
Ethical Approval This was a prospective, cross-sectional study, which had been approved by the Ethics Committee and followed the Declaration of Helsinki. Adult patients were enrolled from a general ophthalmology office, and all subjects signed a written informed consent form.
Although we intended to include patients with both open and narrow ACAs, no previous evaluation was done to determine the actual morphology of the angle. The exclusion criteria were any history of intraocular surgery, anterior segment laser treatment or patients with limbal defects, which limited the observation of the peripheral anatomy of the anterior segment.If both eyes were eligible for the study, only the right eyes were analyzed.
Gonioscopy was performed with a Goldmann 3-mirror lens in the dark in all cases by a single examiner (Beatriz Fiuza Gomes) masked to dual Scheimpflug findings. Gonioscopy examinations (at 16× magnification) were realized with a 1 mm beam and a very narrow slit with lowest illumination to allow appropriate identification of the structures. Only topical anesthetic drops and hydroxyethil cellulose were applied prior to the procedure. The ACA in every quadrant was classified based on the Shaffer grading system. A narrow-angle was defined when we could not visualize the posterior trabecular meshwork in more than 180 degrees before indentation(Shaffer 2 or less).
The dual Scheimpflug analyzer Galilei (Galilei G6, Ziemer Group, Port, Switzerland) was used to automatically measure the mean ACA, anterior chamber volume (ACV) and ACD.These measurements were also performed in a dark room, after allowing for dark adaptation (about 30s), and in accordance with the manufacturer's guidelines, until acceptable quality values were obtained.
Statistical Analysis Statistical analysis was done using the JMP statistical software (version 8.0; SAS Institute, Inc.,Cary, NC, USA). Continuous variables were reported as the mean±standard deviation (SD). Student's t-test (Wilcoxon test for skewed distributions) was used to compare quantitative variables. Receiving operator characteristic (ROC) curves were adopted for studying the efficacy of the Galilei measurements in screening for open and narrow-angle eyes.

Figure 1 Galilei dual Scheimpflug image
A: Galilei image of a patient with narrow angle. The ACD by Galilei in this case was 1.97 mm and the ACA was 26.1°; B: Galilei image of a patient with open angle. The ACD was 3.04 mm and ACA was 31.0°.
Partition analysis was the chosen method to determine the most efficient parameter of Galilei and its threshold value to distinguish between open and narrow-angles. ACA, ACV,and ACD were individually interpreted in those analyses.The correlation coefficient among parameters obtained by Galilei and Shaffer's grade, determined by gonioscopy, was assessed with spearman's correlation coefficient. Agreement(kappa statistics), sensitivity, specificity, positive predictive value, negative predictive value, accuracy, likelihood ratio for positive test for detecting an angle as narrow according to Galilei parameters compared with gold standard (gonioscopy)were also evaluated. For this study, a P-value below 0.05 was acknowledged statistically significant.
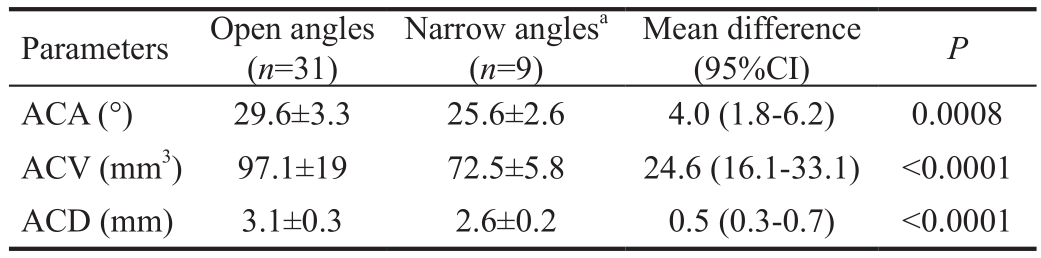
Forty eyes of 40 patients were prospectively enrolled. The study included 31 (78%) eyes of patients graded as open-angle and 9 (23%) eyes as narrow-angle, based on gonioscopy. The mean age of the patients was 61±11y (range, 20-86 years old).The female-male ratio was 3:1. The average ACA according to noncontact morphometry with dual Scheimpflug (Galilei G6)was 28.7°±3.5° (range, 21.4°-35°). For specific groups ACA mean was 29.6°±3.3° for open-angle eyes and 25.6°±2.6° for narrow-angle (P=0.0008). Figure 1 illustrates the Galilei image for both open and narrow angles. ACV and ACD measures were also statistically distinct among the 2 groups (P<0.0001;Table 1).
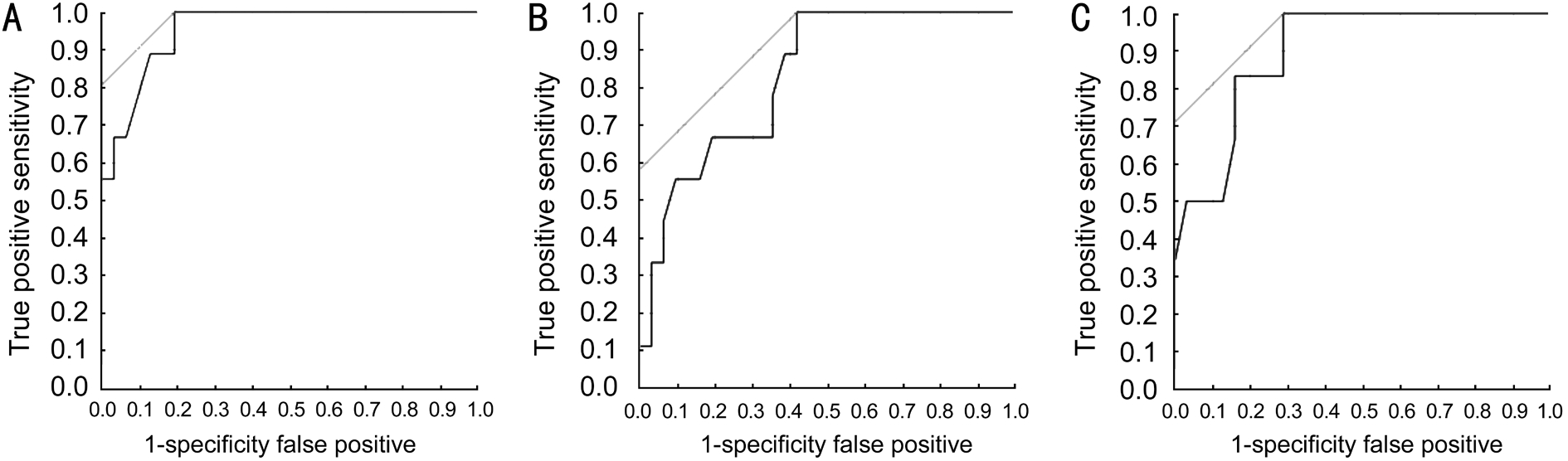
Figure 2 ROC curves of the Galilei parameters
A: Central ACD; B: ACV; C: ACA to discriminate eyes with a narrow angle (an angle width of Shaffer's grade 2 or less in 3 or more quadrants).
Table 1 Comparison of Galilei parameters between eyes with gonioscopically narrow and open ACA mean±SD
ACA: Anterior chamber angle; ACV: Anterior chamber volume;ACD: Anterior chamber depth.aNarrow-angle was defined as an eye where less than 180 degrees of the filtering trabecular meshwork was visible before indentation in gonioscopy.
Parameters Open angles(n=31)Narrow anglesa(n=9)Mean difference(95%CI) P ACA (°) 29.6±3.3 25.6±2.6 4.0 (1.8-6.2) 0.0008 ACV (mm3) 97.1±19 72.5±5.8 24.6 (16.1-33.1) <0.0001 ACD (mm) 3.1±0.3 2.6±0.2 0.5 (0.3-0.7) <0.0001
Shaffer's grade significantly correlated with all of the parameters: ACA (r=0.6, P<0.001), ACV (r=0.52, P<0.001)and ACD (r=0.71, P<0.001). The efficacy of the Galilei parameters to screen out the narrow angle eyes, as defined above, was analyzed using ROC curves (Figure 2). The ROC analysis revealed very good discriminant power of all Galilei parameters in detecting narrow angles. The areas under the curve (AUC) were 0.82 for ACA, 0.90 for ACV and 0.95 for ACD. According to the partition analysis, the narrow angles were most effectively partitioned with an ACD threshold of 2.86 mm with 100% sensitivity, 80% specificity, 60% positive predicted value and 100% of negative predictive value (Table 2).Fifteen patients were tested positive and 25 negative for narrow angle considering ACD. All 25 subjects who were negative for ACD (ACD≥2.86 mm) evaluation had open angle by gonioscopy. Out of 15 patients with ACD<2.86 mm, 6 had open angle by gonioscopy.
The cutoffs chosen for each measurement were: 2.86 mm for ACD, 29.0° for ACA and 93.1 mm3 for ACV (Tables 2-4 for 2×2 contingency tables). Agreement statistics revealed kappa of 0.20 (P=0.04) for ACA, 0.42 (P=0.001) for ACV and 0.52(P=0.0002) for ACD.
Table 3 shows results with screening test for narrow angles with ACV with 44% of sensitivity and 55% of specificity and Table 4 shows results with screening test for narrow angles with ACA with 89% sensitivity and 58% specificity.
To the best of our knowledge, this is the first study to evaluate the performance of dual Scheimpflug analyzer as a screening method for narrow angles.
The current gold standard method for ACA assessment is gonioscopy, and its findings remain indispensable for thecorrect evaluation of the iridocorneal anatomic characteristics and the diagnosis of occludable ACAs[6]. However, this procedure demands not only a trained examiner, but also involves direct contact with the cornea, compromising its reproducibility and efficiency as a screening method for a large population[6-8,11].
Table 2 Calculate the diagnostic sensitivity and specificity of ACD using gonioscopy as the gold standard
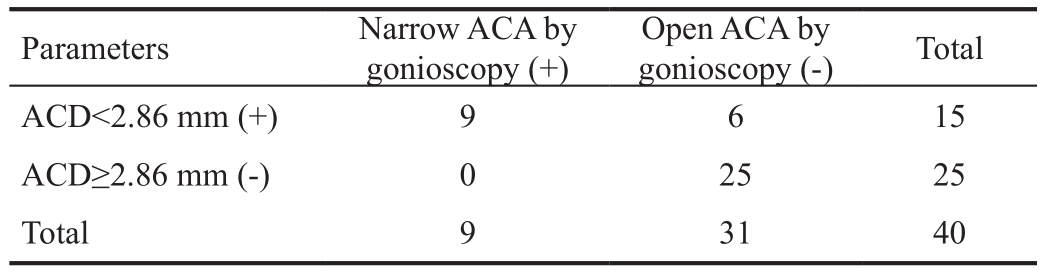
ACD: Anterior chamber depth; ACA: Anterior chamber angle.Sensitivity (true positive rate) =100%; specificity (true negative rate) =80%; Positive predictive value =60%; Negative predictive value =100%; Accuracy =9.63; Likelihood ratio for positive test =5.17. A cut-off value of 2.86 mm was used for ACD.
Parameters Narrow ACA by gonioscopy (+)Open ACA by gonioscopy (-) Total ACD<2.86 mm (+) 9 6 15 ACD≥2.86 mm (-) 0 25 25 Total 9 31 40
Table 3 Calculate the diagnostic sensitivity and specificity of ACV using gonioscopy as the gold standard
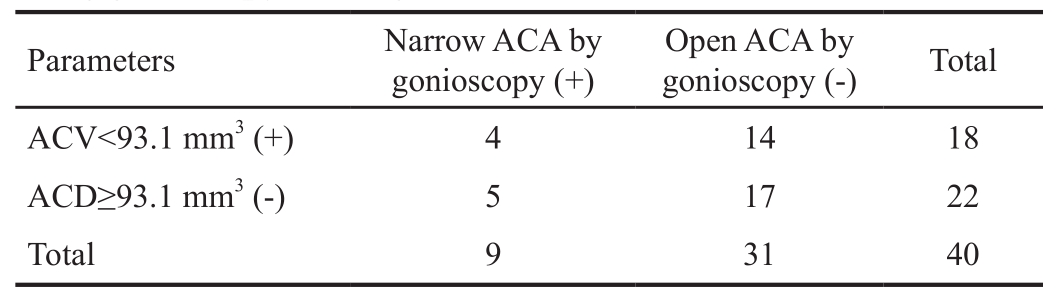
ACV: Anterior chamber volume; ACA: Anterior chamber angle.Sensitivity (true positive rate) =44%; Specificity (true negative rate) =55%; Positive predictive value =22%; Negative predictive value =77%; Accuracy =4.42; Likelihood ratio for positive test =0.98.A cut-off value of 93.1 mm3 was used for ACV.
Parameters Narrow ACA byOpen ACA byTotal gonioscopy (+)gonioscopy (-)ACV<93.1 mm3 (+) 4 14 18 ACD≥93.1 mm3 (-) 5 17 22 Total 9 31 40
Table 4 Calculate the diagnostic sensitivity and specificity of ACA using gonioscopy as the gold standard
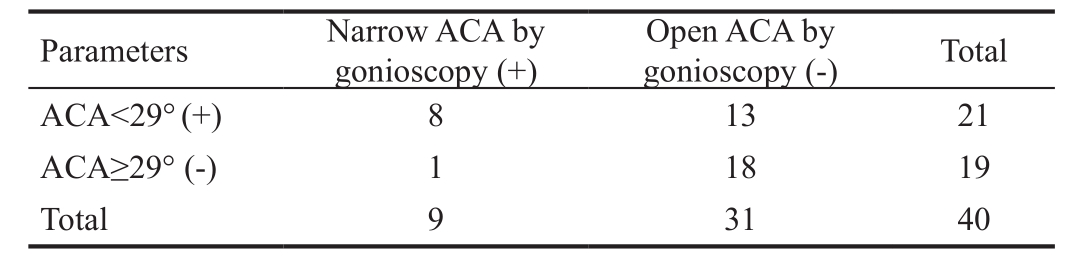
ACA: Anterior chamber angle. Sensitivity (true positive rate) =89%;specificity (true negative rate) =58%; Positive predictive value =38%;Negative predictive value =94%; Accuracy =8.45; Likelihood ratio for positive test =2.11. A cut-off value of 29° was used for ACA.
 ACA<29° (+) 8 13 21 ACA≥29° (-) 1 18 19 Total 9 31 40
ACA<29° (+) 8 13 21 ACA≥29° (-) 1 18 19 Total 9 31 40
The Galilei dual Scheimpflug analyzer is a noninvasive optical system that incorporates two principles: Placido rings and a dual Scheimpflug camera, for the study of the anterior segment of the eye[13,18-19]. This technology allows a three-dimensional and 360 degrees evaluation of the anterior segment and captures different data of a large range of structures, such as corneal thickness, anterior and posterior corneal curvature,anterior and posterior corneal topography, corneal volume,ACD, and horizontal and vertical distance from limbus to limbus[20]. Some recent previous studies have demonstrated good reproducibility of the parameters acquired with Galilei,which offers advantages for being more unbiased, repeatable,objective and a noncontact tool, besides generating and saving fast imaging and quantitative analysis[15-17,20-22].
However, with Scheimpflug systems, the light is incapable to pass through the angle recess, preventing the direct visualization of the ACA (most available systems have automated programs to analyze the angle). Moreover, on contrary to the gonioscopic evaluation, the details of the ciliary body and iris-ciliary body relationship cannot be assessed with this exam. It is also unable to detect peripheral anterior synechiae and discriminate from nonsynechial appositional closure, or evaluate the level of pigmentation of the angle. Therefore, like other technologies,dual Scheimpflug images are not able to completely replace the direct study of the ACA anatomy with gonioscopy.
Galilei imaging systems intent to reduce the subjectivity related to the assessment of ACA information by using entirely automated analysis. The present study revealed that all Galilei parameters analyzed (ACA, volume and depth) correlated with the gonioscopy Schaffer grade and had strong efficacy in detecting narrow angles (Schaffer II or less). These findings correspond to previous studies, in which the same parameters were analyzed using the Pentacam[10-11].
In this study, the most accurate parameter for screening narrow angles with dual Scheimpflug analyzer was the ACD,using a threshold of 2.86 mm with 100% sensitivity and 80%specificity, according to the partition analysis. The AUC(0.82 for ACA, 0.90 for ACV and 0.95 for ACD) reaffirm that data. Kurita et al[11] found similar results when analyzing the Pentacam measurements: ACD showed very high sensitivity(100%) and reasonable specificity (87.1%) for screening eyes with narrow angles (ACD threshold of 2.58 mm)[11]. The evidence that ACD and ACV provided better AUC, compared to the ACA, with both Pentacam and Galilei devices is probably due to the fact that they are nearly independent on the anatomy of the most peripheral part of the anterior chamber[11].Other imaging devices, like AS-OCT, UBM, and Scheimpflug imaging (Pentacam), have been used to grant a more impersonal and precise assessment of the ACA. All these technologies are important and additive in clinical practice,particularly when one technique is hard to apply or the obtained results are doubtful. UBM can visualize structures behind the iris, which is an advantage comparing to others imaging modalities, especially for the investigation of the mechanisms behind angle closure. However, it requires contact of transducer with eye and the associated discomfort and the need for experienced staff. Optical coherence tomography is comparable to UBM in quantitative ACA measurement and screening for narrow angles, both showed good screening habilities in previous study with areas under the ROC all in the range of 0.96 to 0.98[23].
We believe that this study has some limitations. Our classification of the data assessed were all correlated with gonioscopy; which, although the current gold standard, is a subjective exam and may induce misclassi fication. In fact, the elimination of the subjectiveness by automatic acquisitions and data analysis is one of the most relevant qualities of Galilei.The principal study design for assessing the accuracy of diagnostic tests is a nonexperimental cross-sectional study that compares a test's classi fication of a diagnosis with a reference standard's classification and this was the reason why it was compared with gonioscopy (gold standard).
The sample size of 40 eyes was appropriate for the purpose of the study as it has been shown that the magnitude of the bias in sensitivity and specificity using data-driven selection of optimal cutoff values is estimated to be about 5% in studies with a sample size of 40, which is acceptable[24]. In addition,we believe that the findings of our study are relevant for several other reasons. For example, the diagnostic test was evaluated in an appropriate spectrum of patients, like those in whom we would use it in the every day practice.
Moreover, the results of this study indicate that the pre-test probability of narrow angles (which is the prevalence of narrow angles in this sample) was 22.5% and for ACD<2.86 mm the posttest probability (positive predictive value) of narrow angle was 60%, while for ACD≥2.86 mm the post-test probability(negative predictive value) of narrow angle was 100%.Therefore, within the present study, the uncertainty regarding narrow angle has shifted from the initial 22.5% to probabilities of either 60% or 100%, which appear to be clinically significant variations.
In summary, this study provides data on the efficacy of the Galilei G6 System (dual Scheimpflug analyzer), a noncontact,safe and objective method for detecting narrow ACA, which may be used to enhance screening approaches in order to detect and prevent PACG. However it does not provide sufficient information about the ACA anatomy to be considered a substitute for gonioscopy. Future studies on different populations are needed to develop worldwide-accepted cutoff values to screen for narrow/occludable angles with Galilei dual Scheimpflug analyzer and to validate these results. Studies comparing Galilei with others anterior segment imaging technologies in detecting narrow angles are also welcomed.
Conflicts of Interest:Bessa NM, None; Souza RA, None;Santhiago MR is a consultant of Alcon, Ziemer; Moraes Jr HV, None; Gomes BF, None.
1 Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010.Br J Ophthalmol 2012;96(5):614-618.
2 Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in north China: the Beijing eye study. Am J Ophthalmol 2010;150(6):917-924.
3 Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol 2001;85(11):1277-1282.
4 Song PG, Wang JW, Bucan K, Theodoratou E, Rudan I, Chan KY.National and subnational prevalence and burden of glaucoma in China: A systematic analysis. J Glob Health 2017;7(2):020705.
5 Chung HJ, Park HY, Kim SY. Comparison of laser iridotomy using short duration 532-nm Nd: YAG laser (PASCAL) vs conventional laser in dark irides. Int J Ophthalmol 2015;8(2):288-291.
6 Smith SD, Singh K, Lin SC, Chen PP, Chen TC, Francis BA, Jampel HD. Evaluation of the anterior chamber angle in glaucoma: a report by the american academy of ophthalmology. Ophthalmology 2013;120(10):1985-1997.
7 Dorairaj S, Liebmann JM, Ritch R. Quantitative evaluation of anterior segment parameters in the era of imaging. Trans Am Ophthalmol Soc 2007;105:99-110.
8 Devereux JG, Foster PJ, Baasanhu J, Uranchimeg D, Lee PS,Erdenbeleig T, Machin D, Johnson GJ, Alsbirk PH. Anterior chamber depth measurement as a screening tool for primary angle-closure glaucoma in an East Asian population. Arch Ophthalmol 2000;118(2):257-263.
9 Baskaran M, Oen FT, Chan YH, Hoh ST, Ho CL, Kashiwagi K, Foster PJ, Aung T. Comparison of the scanning peripheral anterior chamber depth analyzer and the modified van Herick grading system in the assessment of angle closure. Ophthalmology 2007;114(3):501-506.
10 Rossi GC, Scudeller L, Delfino A, Raimondi M, Pezzotta S, Maccarone M, Antoniazzi E, Pasinetti GM, Bianchi PE. Pentacam sensitivity and specificity in detecting occludable angles. Eur J Ophthalmol 2012;22(5):701-708.
11 Kurita N, Mayama C, Tomidokoro A, Aihara M, Araie M. Potential of the pentacam in screening for primary angle closure and primary angle closure suspect. J Glaucoma 2009;18(7):506-512.
12 Dabasia PL, Edgar DF, Murdoch IE, Lawrenson JG. Noncontact screening methods for the detection of narrow anterior chamber angles.Invest Ophthalmol Vis Sci 2015;56(6):3929-3935.
13 Anayol MA, Güler E, Yağc R, Şekeroğlu MA, Ylmazoğlu M, Trhş H,Kulak AE, Ylmazbaş P. Comparison of central corneal thickness, thinnest corneal thickness, anterior chamber depth, and simulated keratometry using galilei, pentacam, and sirius devices. Cornea 2014;33(6):582-586.
14 Dervişoğulları MS, Totan Y, Gürağaç B. Comparison of anterior chamber depth measurements of nidek AL-scan and galilei dual Scheimpflug analyzer. Cont Lens Anterior Eye 2015;38(2):85-88.
15 Fahd DC, Cherfan CG, Raad C, Asouad M, Awwad ST. Assessment of anterior and posterior corneal indices using two Scheimpflug analyzers.Arq Bras Oftalmol 2014;77(1):17-20.
16 Cerviño A, Dominguez-Vicent A, Ferrer-Blasco T, García-Lázaro S, Albarrán-Diego C. Intrasubject repeatability of corneal power,thickness, and wavefront aberrations with a new version of a dual rotating Scheimpflug-Placido system. J Cataract Refract Surg 2015;41(1):186-192.
17 Menassa N, Kaufmann C, Goggin M, Job OM, Bachmann LM, Thiel MA. Comparison and reproducibility of corneal thickness and curvature readings obtained by the Galilei and the Orbscan II analysis systems. J Cataract Refract Surg 2008;34(10):1742-1747.
18 Shirayama M, Wang L, Weikert MP, Koch DD. Comparison of corneal powers obtained from 4 different devices. Am J Ophthalmol 2009;148(4):528-535.e1.
19 Dinc UA, Oncel B, Gorgun E, Yalvac IS. Assessment of anterior chamber angle using Visante OCT, slit-lamp OCT, and Pentacam. Eur J Ophthalmol 2010;20(3):531-537.
20 Rabsilber TM, Khoramnia R, Auffarth GU. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J Cataract Refract Surg 2006;32(3):456-459.
21 Altıparmak Z, Yağcı R, Güler E, Arslanyılmaz Z, Canbal M, Hepşen İF. Repeatability and reproducibility of anterior segment measurements in normal eyes using dual scheimpflug analyzer. Turk J Ophthalmol 2015;45(6):243-248.
22 Lopez de la Fuente C, Sanchez-Cano A, Segura F, Fuentes-Broto L,Pinilla I. Repeatability of ocular measurements with a dual-Scheimpflug analyzer in healthy eyes. Biomed Res Int 2014;2014:808646.
23 Radhakrishnan S, Goldsmith J, Huang D, Westphal V, Dueker DK,Rollins AM, Izatt JA, Smith SD. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol 2005;123(8):1053-1059.
24 Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem 2008;54(4):729-737.