Table 1 Baseline characteristics before conbercept injection in all subjects

?
A ge-related macular degeneration (AMD) is the leading cause of irreversible blindness in people 50 years of age or older in the developed world[1]. There are more than 5 million patients in our country, as the aging of population, the incidence is still rising[2]. AMD can be divided into dry and neovascular types according to the retina performance, and the latter is responsible for approximately 90% of cases of severe vision loss due to AMD[3]. Vascular endothelial growth factor(VEGF) is an important factor to promote the neovascular formation. Inhibition of VEGF can effectively inhibit the formation of neovascular and reduce leakage of blood vessels.Pharmaceutical agents that block VEGF have revolutionized the management of neovascular AMD and most exudative retinal diseases[4]. At present, anti-angiogenesis drugs are divided into two types, monoclonal antibodies including ranibizumab(Lucentis; Genentech, Inc and Novartis International AG,Basel, Switzerland) and bevacizumab (Avastin; Genentech,South San Francisco, CA, USA), fusion proteins including aflibercept (Eylea; Regeneron, Tarrytown, NY, USA and VEGF-Trap Eye; Bayer AG, Leverkusen, Germany) and conbercept (Lumitin; Chengdu Kang Hong Biotech Co, Ltd,Sichuan, China), the latter is a fusion protein composed of the extracellular domain 2 of VEGF receptor 1 and extracellular domains 3 and 4 of VEGF receptor 2 combined with the Fc portion of the human immunoglobulin G1, and which with independent intellectual property rights in our country.In numerous studies, fusion proteins had superiority than monoclonal antibodies, it decreased the number of injections and central macular thickness (CMT)[5-6]. The present retrospective and non-comparative study was to assess the efficacy and safety of conbercept intravitreal injection in neovascular AMD in China.
Ethical Approval This study was approved by local Ethics Committee of Xi'an No.1 Hospital. All patients gave written informed consent of intravitreal injection of conbercept and were aware of the treatment protocol [3+ prorenata (PRN)treatment].
Patient Information A retrospective chart review of 66 eyes of 63 patients with neovascular AMD who were confirmed through related inspections in the Department of Ophthalmology of Xi'an No.1 Hospital between October 2014 and May 2017 was conducted. All subjects were selected according to the inclusion criteria and exclusion criteria.
All patients received intravitreal injection of conbercept(0.5 mg/0.05 mL) on 3 consecutive monthly schedule and then as needed with monthly evaluation. PRN retreatment was performed if one of the following changes were observed between visits: a loss of one line (logMAR) in conjunction with fluid in the macula as detected by optical coherence tomography (OCT); an increase in OCT CMT of at least 100 μm[7]; new-onset classic choroidal neovascularization (CNV);new macular hemorrhage or persistent macular fluid detected by OCT at least one month after the previous injection.
Inclusion criteria were as follows: 1) persistent intraretinal or subretinal fluid with or without pigment epithelial detachment at the baseline; 2) no injections with ranibizumab, bevacizumab or aflibercept before conbercept initiation; 3) at least 3mo of follow-up on a monthly basis. Exclusion criteria were: 1)vision worse than 3.0 (logMAR); 2) a diagnosis of retinal angiomatous proliferation; 3) combined with any other ocular disease that could affect the best-corrected visual acuity(BCVA) in a short period of time; 4) a history of intraocular surgery (except cataract surgery); 5) any systemic condition contraindicating the use of intravitreal anti-VEGF agents.
Methods The initial data collections include: patient age,gender, diagnosis, coexisting ocular conditions, the history of systemic disease and surgery. Before and after intravitreal conbercept injection, all patients had to have a complete ophthalmic examination, including BCVA measurement,applanation tonometry, slit-lamp evaluation, dilated binocular indirect ophthalmoscopy and OCT. BCVA was measured using “E” snellen chart at 5 m distance and transformed into logMAR. Heidelberg OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) was used for OCT examination, active eye tracking and automatic follow-up scan were used to enable point-to-point correspondence between consecutive follow-up scans. A 20° 6-radial line scan protocol,centered on the fovea, with at least 50 frames averaged for each scan was performed for each eye. From one scan of the radial protocol, with the most serious lesion, we evaluated the presence or absence of intraretinal fluid, subretinal fluid, and pigment epithelial detachment. CMT was also measured on the same scan from the inner limiting membrane to the retinal pigment epithelium band. Repeat the fast scan 3 times, select and store the satisfactory results for analysis.
Statistical Analysis SPSS 22.0 statistical software was used for statistical analysis. Fifirst adopting variance analysis forrepeated measurement data to analysis of the averages in different times; second the paired t-test analysis method was adopted to compare the change of BCVA and CMT before and after the conbercept injection (mean values±standard deviation), with P values<0.05 for the difference was statistically significant.
Table 1 Baseline characteristics before conbercept injection in all subjects

?
Sixty-six eyes of 63 patients were treated with intravitreal conbercept injections for neovascular AMD during the study period. All subjects were treated with a loading dose of 3 monthly conbercept injections followed by a PRN protocol and no patients were lost in the core period of treatment. Baseline characteristics of the patients at the time of conbercept initiation are summarized in Table 1. The average time from illness onset to the injection was 7.4±9.4mo, with different length of 1wk to 4y. There were 11 patients combined with diabetes (without obvious diabetic retinopathy), 17 patients had high blood pressure, 6 people had both of diabetes and high blood pressure, 1 patient underwent cardiac surgery and 3 cases had cataract surgery.
All 63 patients completed the first 3mo of the treatment. After 3mo some patients were lost. Patients may have been lost to follow-up because the vision improved and they did not think they needed further treatment or because the vision worsened and they lost confidence and did not want more treatment.Others may have been lost to follow-up due to transportation or other medical issues. There were 57 eyes in 6mo with the mean injection time of 3.6±0.5, 52 eyes in 9mo and 48 eyes in 1y, the injection times were 4.3±0.9 and 4.8±1.7 respectively.No serious complications were observed.
Best-corrected Visual Acuity and Central Macular Thickness Outcomes of Sixty-six Eyes The mean BCVA(logMAR) before conbercept initiation was 1.11±0.60. After 1wk of the fifirst conbercept injection, the average BCVA was 0.80±0.49 (P<0.05) and after 1mo it was 0.73±0.38(P<0.05), the improvement was significant (Figure 1). At 3mo after conbercept initiation, the mean BCVA were 0.68±0.38(P<0.05). The mean CMT of 66 eyes was 533.20±219.95 µm at baseline, after 1wk, 1 and 3mo of conbercept injection were 396.88±172.61, 359.03±147.12 and 310.28±125.60 µm,respectively, which were all significantly changed compared with the baseline (P<0.05).

Figure 1 BCVA and CMT outcomes after conbercept injection
A: The outcomes after injection of 66 eyes; B: The outcomes of 48 eyes.
Table 2 BCVA and CMT after conbercept injection in different times of 48 eyes
t-test, and P<0.05 means difference was significant.
Characteristics Baseline 1wk 1mo 3mo 6mo 9mo 12mo BCVA 0.83±0.46 0.59±0.45 0.57±0.40 0.55±0.41 0.52±0.42 0.49±0.31 0.55±0.51 CMT 547.59±196.77 359.71±108.23 351.35±142.21 318.24±141.29 345.64±153.84 354.56±181.30 333.87±173.25
Best-corrected Visual Acuity and Central Macular Thickness Outcomes of Forty-eight Eyes Forty-eight patients completed the whole period of follow-up at least 1y.Visual acuity and anatomical outcomes of these subjects are shown in Table 2. The differences of BCVA and CMT between each point of follow-up and baseline were significantly(P<0.05).
We observed the functional and anatomic response of patients with neovascular AMD after conbercept injection. Our results at 3mo of 66 eyes showed that conbercept treated eyes maintained stable visual acuity and improved retinal lesions,which had significant improvement compared with baseline(Figure 1A). In the subsequent follow-up times, some subjects were lost, mostly correlated with the length of disease and the status of lesion. Fortunately there were 48 eyes insisted and complted the observation time for more than one year. Results show that after treatment, most patients could have stable vision in the best level without obvious drop or decreased of retinal thickness accompanied by lesion atrophy (Figure 1B).Since the discovery of the importance of VEGF-A in the pathophysiology of neovascular AMD, anti-VEGF drugs became the standard treatment. Rofagha et al[8] summarized approximately 7y after ranibizumab therapy in the ANCHOR or MARINA trials, one third of patients demonstrated good visual outcomes, whereas another third had poor outcomes.Compared with baseline, almost half of eyes were stable,whereas one third declined by 15 letters or more. Even at this late stage in the therapeutic course, exudative AMD patients remain at risk for substantial visual decline. In contrast to bevacizumab and ranibizumab, which only bind to VEGF-A,a flibercept also binds to VEGF-B and placental growth factor(P1GF)[9]. Various pharmacokinetic studies have demonstrated that a flibercept has a higher affinity for VEGF-A and a longer half-life than ranibizumab and bevacizumab[10-12]. Aflibercept appears to have clinical advantages in the treatment of neovascular AMD and may be useful in patients with persistent fluid despite treatment with ranibizumab or bevacizumab[5-6,13].So doctors would transit to aflicercept for patients on prior ranibizumab or bevacizumab treatment for neovascular AMD[14].In this paper, the use of conbercept, as a flibercept, all belong to the fusion protein drug of anti-angiogenesis drugs, also has the characteristics of multiple targets, and compared with the single drug class has the advantage of good therapeutic effect (Figures 2, 3). Intravitreal administration of conbercept has been shown to successfully prevent lesion growth and leakage of CNV in a nonhuman primate model[15-16]. In a retrospective case series study, thirty patients (30 eyes) with wet AMD were enrolled to receive intravitreal injections of ranibizumab (0.5 mg) on 3 consecutive monthly schedule and 28 patients (30 eyes) with wet AMD were enrolled to receive intravitreal injections of conbercept (0.5 mg) on 3 consecutive monthly schedule. BCVA, OCT measurement were compared at 1mo after injections. One month after every injection, the BCVA increased while CMT decreased compared with those before treatment in both groups A and B (P<0.05). BCVA and CMT changes between two groups were no statistically different (P>0.05). So the author thought that ranibizumab and conbercept therapy can control the prognosis of wet AMD and improve the vision effectively. There is no statistical difference on the curative effect between two drugs for 3mo[17].Li et al[7] enrolled 122 patients in a randomized double-mask,multicenter study, and they concluded that the significant gains in BCVA at 3mo were the same or better at 12mo in all conbercept dosing groups of neovascular AMD patients.Unfortunately, there is a lack of long-term contrast of the curative effect between the two drugs.
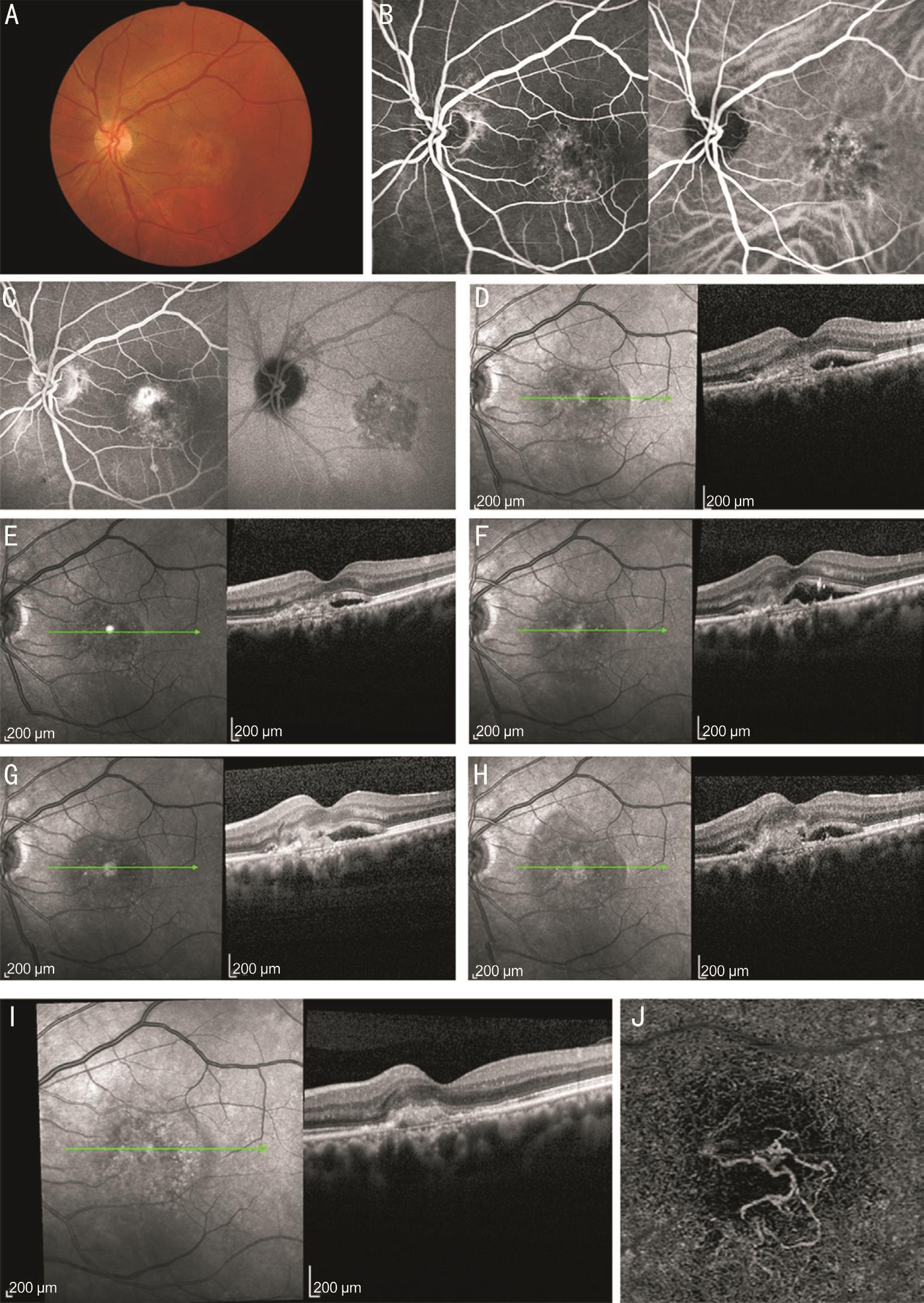
Figure 2 Patient 1 before and after injection
A-C: Study eye color fundus photograph with early and late-phase fluorescein angiographic images at baseline; D-I: Horizontal OCT showed CMT at baseline (370 µm), 1st month (310 µm), 3rd month (388 µm), 6th month (337 µm), 11th month (372 µm) and 12th month (238 µm); J: An en-face OCTA image at the 12th month of the outer retina.
We took of treatment protocol as 3+PRN for all neovascular AMD, with the same treatment were used in a number of other studies. The study has shown that each patient received intravitreal injection of conbercept monthly up to 3mo, followed by monthly evaluation and injection PRN to month, intravitreal conbercept can reduce injection times and frequency of monitoring and effective to treat neovascular AMD. Also the treatment protocol of 3+PRN were widely adopted in anti angiogenesis drug treat of other diseases, such as conbercept in the treatment of macular edema due to retinal vein occlusion (RVO)[18].
Recent research has shown that the fusion protein drugs to other retinal vascular diseases had good therapeutic effect and without obvious adverse reactions. Intravitreal aflibercept 2 mg was effective for treatment of myopic CNVwith clinically important visual and anatomic benefits achieved with a limited number of injections given in the first 8wk of treatment[19]. Converting eyes with refractory macular edema due to central RVO to a flibercept can result in stabilization of the vision, improved macular anatomy, and extension of the injection interval[20]. In a prospective, phase II clinical trial,intravitreal injections of conbercept was performed on 60 patients with macular edema secondary to RVO, each patient received intravitreal injections of conbercept monthly up to 3mo, followed by monthly evaluation and injection PRN to month 9. The results showed that conbercept bringing a generally favorable safety and tolerability profile as well as efficacy in the treatment of macular edema due to RVO[20].

Figure 3 Patient 2 before and after injection
A-C: Study eye color fundus photograph with early and late-phase fluorescein angiographic images at baseline; D-F: 6mo after the first injection; G-J: Vertical OCT and CMT measurement at baseline (665 µm), 2nd week (520 µm), 1st month (322 µm), and 4th month (290 µm).
This study was a retrospective case analysis, had shown that intravitreal injections of conbercept was efficacy and safety in the treatment of neovascular AMD. It can control and stabilize the condition and improve the patient's vision, no serious adverse reactions and complications, worthy of clinical promotion. But it needs further prospective randomized double-blind trials to confirm the differences in efficacy and safety between fusion protein drugs and single drug class and different fusion protein drugs for different diseases.
Conflicts of Interest: Wu BH, None; Wang B, None; Wu HQ, None; Chang Q, None; Lu HQ, None.
1 Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med 2008;358(24):2606-2617.
2 Ambati J. Age-related macular degeneration and the other double helix.The Cogan Lecture. Invest Ophthalmol Vis Sci 2011;52(5):2165-2619.
3 Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration. Drugs 2008;68(8):1029-1036.
4 Wykoff CC, Brown DM, Maldonado ME, Croft DE. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections(TURF trial). Br J Ophthalmol 2014;98(7):951-955.
5 Thorell MR, Nunes RP, Chen GW, Doshi RR, Dugar J, George MK,Kim BT, Lowrance MD, Modi D, Nahas Z, Gregori G, Yehoshua Z, Feuer W, Rosenfeld PJ. Response to a flibercept after frequent re-treatment with bevacizumab or ranibizumab in eyes with neovascular AMD. Ophthalmic Surg Lasers Imaging Retina 2014;45(6):526-533.
6 Pinheiro-Costa J, Costa JM, Beato JN, Freitas-da-Costa P, Brandão E,Falcão-Reis F, Carneiro AM. Switch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practice. Ophthalmologica 2015;233(3-4):155-161.
7 Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, Zhang F, Zhang J, Tang L, Wu Q, Luo D, Ke X; AURORA Study Group. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA Study. Ophthalmology 2014;121(9):1740-1747.
8 Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVENUP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study(SEVEN-UP). Ophthalmology 2013;120(11):2292-2299.
9 Shinkai A, Ito M, Anazawa H, Yamaguchi S, Shitara K, Shibuya M.Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J Biol Chem 1998;273(47):31283-31288.
10 Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15(2):171-185.
11 Semeraro F, Morescalchi F, Duse S, Parmeggiani F, Gambicorti E,Costagliola C. Aflibercept in wet AMD: specific role and optimal use.Drug Des Devel Ther 2013;7:711-722.
12 Elshout M, van der Reis MI, Webers CA, Schouten JS. The costutility of a flibercept for the treatment of age-related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefes Arch Clin Exp Ophthalmol 2014;252(12):1911-1920.
13 Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 2013;97(8):1032-1035.
14 Chan CK, Jain A, Sadda S, Varshney N. Optical coherence tomographic and visual results at six months after transitioning to aflibercept for patients on prior ranibizumab or bevacizumab treatment for exudative age-related macular degeneration (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc 2014;112:160-198.
15 Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, Li H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res 2009;26(1):204-210.
16 Zhang M, Zhang J, Yan M, Li H, Yang C, Yu D. Recombinant antivascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis 2008;14(4):37-49.
17 Cai XA, Peng H. Efficacy of two anti-VEGF drugs for age-related macular degeneration. Guoji Yanke Zazhi (Int Eye Sci) 2016;16(8):1501-1503.
18 Sun Z, Zhou H, Lin B, Jiao X, Luo Y, Zhang F, Tao S, Wu Q, Ke Z,Liu X. efficacy and safety of intravitreal conbercept injections in macular dedma secondary to retianl vein occlusion. Retina 2016;37(9):1723-1730.
19 Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, Stemper B, Asmus F, Zeitz O, Ishibashi T; MYRROR Investigators. Intravitreal a flibercept injection in patients with myopic choroidal neovascularization:the MYRROR Study. Ophthalmology 2015;122(6):1220-1227.
20 Papakostas TD, Lim L, van Zyl T, Miller JB, Modjtahedi BS, Andreoli CM, Wu D, Young LH, Kim IK, Vavvas DG, Esmaili DD, Husain D,Eliott D, Kim LA. Intravitreal a flibercept for macular oedema secondary to central retinal vein occlusion in patients with prior treatment with bevacizumab or ranibizumab. Eye (Lond) 2016;30(1):79-84.