The Alexandria retinopathy of prematurity model (Alex-ROP): postnatal weight gain screening algorithm application in a developing country
Islam SH Ahmed1, Adham AO Badeeb2
1Department of Ophthalmology, Faculty of Medicine,Alexandria Main University Hospital, Alexandria 21121,Egypt
2Department of Pediatrics and Neonatology, Faculty of Medicine, Alexandria University maternity Hospital, Alexandria 21121, Egypt
Abstract● AlM: To suggest a novel retinopathy of prematurity (ROP)screening model in developing countries incorporating postnatal weight gain ratios (PWGR) to traditional parameters to maintain sensitivity and improve specificity in detecting ROP.● METHODS: Analysis of weekly PWGR of infants from one tertiary referral center during six months to determine the age at which the PWGR with the highest predictability for ROP development which was referred to as the postnatal net weight gain ratio (NWGR). NWGR was added to conventional criteria to describe a new model (The Alex-ROP model).● RESULTS: Of 560 infants were included. NWGR 28d after birth was the most predictive factor for the development of ROP. A new model Alex-ROP recommending screening infants with gestational age (GA) ≤33wk or birth weight≤1500 g and NWGR at 28d after birth <0.3 was suggested.A second screening model referred to as High-grade Alex-ROP (Hg Alex-ROP) model to detect worse grade ROP (Both type 1 and type 2) recommending a cutoff point of NWGR<0.15 between birth and 28th day.● CONCLUSlON: Both Alex-ROP and Hg Alex-ROP models are easy to apply to improve the specificity of ROP screening in developing countries while maintaining high sensitivity.
INTRODUCTION
T he fifirst report about retinopathy of prematurity (ROP)was done by Terry[1], afterwards, trying to detect an d treat the condition gained the interest of researchers. ROP is a preventable cause of visual disability in most of the affected infants[2-3].
ROP develops due to retinal vessels constriction in response to high oxygen levels due to supplemental oxygen therapy, this constriction will progress to permanent occlusion if arterial oxygen levels remain high enough[4].
Mostly, ROP is mild and regresses with no treatment and without visual sequelae. However, in some cases, the disease may progress to severe stages which causing visual disability and even blindness[5].
Currently, there are no clear national screening guidelines for recommend screening the infants at risk of ROP in Egypt, however there are some recommendations derived from previous studies witch advice for screening of infants with a birth weight ≤1500 g, gestational age (GA)≤33wk, or certain cases with a birth weight between 1500 and 2000 g or GA>33wk with an unstable clinical course[6].
Despite showing good sensitivity to detect ROP and ROP requiring treatment, these screening criteria alone still show low specificity as less than one-tenth of the screened infants require treatment, as indicated by previous studies[7].
Postnatal weight gain is considered a new variable which helps to predict the occurrence of ROP[7-14]. It is considered as an indirect indicator to the level of insulin growth factor-1 (IGF-1) which is more difficult to assess and whose deficiency is known to compromise fetal growth, vascular endothelia growth factor signaling, angiogenesis, and retinal vessel growth[10-12,15].Postnatal weight gain is not only easier to assess, cheaper but in addition, it gives a clue about the overall infant health and perinatal clinical course[8,10,14-16].
Several efforts were done trying to improve the sensitivity of ROP prediction with minimization of missing positive cases either by using “Weight and IGF-1 in Weight, IGF-1,Neonatal ROP (WINROP) algorithm”[9] or by incorporating“weekly postnatal weight gain, birth weight, and GA” in “The Children's Hospital of Philadelphia postnatal weight gain, birth weight, and GA ROP risk (CHOP-ROP) model”[7].
These models were successful to decrease the total number of babies requiring screening by 77% and 49%, respectively[7-8].And validation studies reported 20%-74.5% reduction in number of the screened infants[9,17-19].
In a busy neonatal intensive care unit (NICU), both models require a tedious weekly process of weight gain calculation and application of the model for every baby until an alarm for the need of fundus exam is triggered. Moreover, both models are designed to predict high-grade ROP, but not low-grade ROP detection which still may be associated with visual sequelae compared to infants without ROP. In addition, the need for screening can be triggered according to these models as late as 10wk or more postnatally[20-25].
In 2016, Cao et al[25] published “The Colorado-ROP model(CO-ROP): postnatal weight gain screening algorithm” which was developed in the USA describing a new user friendly model using birth weight, GA, and postnatal weight gain for ROP screening aiming to provide simple model to predict the development of any ROP grade while decreasing total number of babies needing ROP screening significantly[26].
There is evidence from many studies that ROP can develop in the developing world in older infants and those with larger birth weight than developed countries, in consequence,application of criteria of screening of western countries can lead to missing a portion of infants at risk of developing that disease if implemented in developing countries without modi fications[6,26].
To the best of our knowledge, no previous work was done in a developing country to study the postnatal weight gain in addition to the traditional criteria for improving the prediction of ROP while decreasing the number of babies needing ROP screening.
SUBJECTS AND METHODS
Ethical Approval We enrolled 560 eligible preterm infants indicated for ROP screening between December 2016 and May 2017. The Ethics Committee of the Faculty of Medicine,Alexandria University, Egypt reviewed and approved the study. The study was conducted in accordance with the tenets of the Declaration of Helsinki and the guidelines of good clinical practice. Informed consent was signed by the parent or the legal guardian of the infant before enrollment.
The study would be a prospective study carried out on the preterm infants in the NICU at the Alexandria University Hospital, Egypt, which is a largest NICU in the north of Egypt,during six months period from 1st of December 2016 to the end of May 2017 and for whom ROP screening was required according to the conventional ROP screening criteria in Egypt(GA≤33wk or with birth weight ≤1500 g or infants born with GA>33wk or birth weight >1500 g) who suffer “unstable clinical course” according to the treating neonatologist.
Weekly weight gain at day 7, 14, 21 and 28 after birth would be recorded from birth until the fifirst ROP examination.Calculation of the ratio of postnatal week gain each week to the initial birth weight would be done.
Review of the patients' medical records and collection of demographic data (gender, GA, associated comorbidities, etc.)would be done.
Initial fundus examination would be done after pupil dilation using indirect ophthalmoscope by a single ophthalmology consultant (Ahmed IS) at least 4wk after birth or 30wk chronological age (GA plus postnatal age), whichever came later unless the infant is too sick that the screening examination could endanger his/her safety as indicated by the attending neonatologist.
Fundus changes would be classified according to “The International Classification of Retinopathy of Prematurity”including “stage” and “zone” of the changes, and “presence of plus disease” at each examination, in addition to the determination whether any treatment would be required[27].
“Type 1 prethreshold ROP” requires treatment it includes“stage 1 or 2 ROP in zone I with plus disease, stage 3 ROP zone I with or without plus disease, or stage 2 or 3 ROP in zone II with plus disease”, and “type 2 prethroshold ROP”requires close follow up, it includes “stage 1 or 2 ROP in zone I without plus, or stage 3 ROP in zone II” as reported by “Early Treatment Retinopathy of Prematurity (ETROP) study”[4].
Infants would be classified into 3 groups according to the wofirst ROP grade noted during any screening: “high grade(prethreshold type 1 or 2 ROP), low grade (ROP less than prethreshold type 1 or 2 criteria), or no ROP”[4].
To detect the cutoff time point the weekly postnatal weight gain ratio giving best prediction of ROP development was detected from logistic regression by calculation of the unadjusted postnatal week and the postnatal week adjusted for GA and birth weight cutoffs with the highest area under the receiver operator characteristic (ROC) curve.
The value with 100% sensitivity for “high-grade ROP” and high sensitivity for “low-grade ROP” was chosen as a cutoff value for post-natal weight gain ratio.
Sensitivity and specificity calculation for detection of “highgrade”, “low-grade”, and overall ROP were done to assess the model performance.
RESULTS
The mean GA of screened infants was 31.6±1.8wk (range 27-35wk), and mean birth weight was 1212.7±247.2 g (range 700-1840 g), mean time between birth and 1st fundus examination was 35d (range 28-40d). All infants included in the study had a hispanic middle eastern ethnicity.
Table 1 Characteristics of cohorts with variable ROP severities n (%)
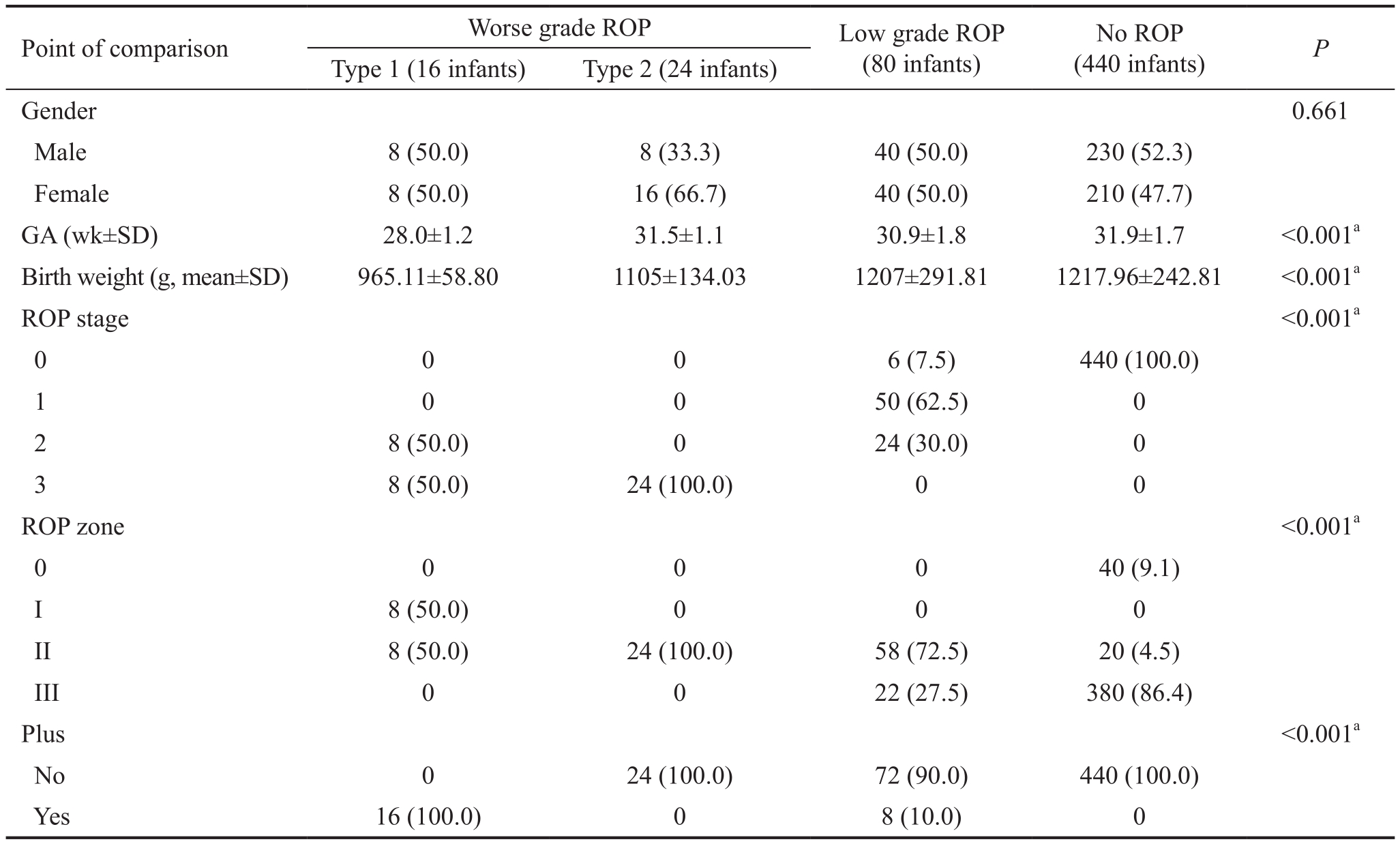
ROP: Retinopathy of prematurity.aStatistically significant difference.
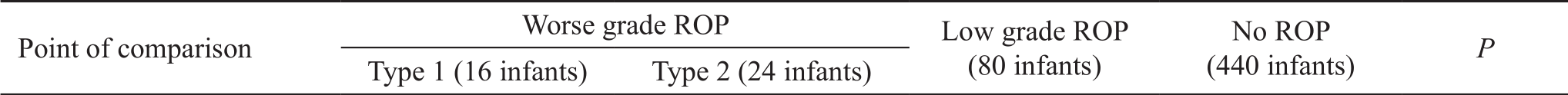 Gender 0.661 Male 8 (50.0) 8 (33.3) 40 (50.0) 230 (52.3)Female 8 (50.0) 16 (66.7) 40 (50.0) 210 (47.7)GA (wk±SD) 28.0±1.2 31.5±1.1 30.9±1.8 31.9±1.7 <0.001a Birth weight (g, mean±SD) 965.11±58.80 1105±134.03 1207±291.81 1217.96±242.81 <0.001a ROP stage <0.001a 0 0 0 6 (7.5) 440 (100.0)1 0 0 50 (62.5) 0 2 8 (50.0) 0 24 (30.0) 0 3 8 (50.0) 24 (100.0) 0 0 ROP zone<0.001a 0 0 0 0 40 (9.1)I 8 (50.0) 0 0 0 II 8 (50.0) 24 (100.0) 58 (72.5) 20 (4.5)III 0 0 22 (27.5) 380 (86.4)Plus<0.001a No 0 24 (100.0) 72 (90.0) 440 (100.0)Yes 16 (100.0) 0 8 (10.0) 0
Gender 0.661 Male 8 (50.0) 8 (33.3) 40 (50.0) 230 (52.3)Female 8 (50.0) 16 (66.7) 40 (50.0) 210 (47.7)GA (wk±SD) 28.0±1.2 31.5±1.1 30.9±1.8 31.9±1.7 <0.001a Birth weight (g, mean±SD) 965.11±58.80 1105±134.03 1207±291.81 1217.96±242.81 <0.001a ROP stage <0.001a 0 0 0 6 (7.5) 440 (100.0)1 0 0 50 (62.5) 0 2 8 (50.0) 0 24 (30.0) 0 3 8 (50.0) 24 (100.0) 0 0 ROP zone<0.001a 0 0 0 0 40 (9.1)I 8 (50.0) 0 0 0 II 8 (50.0) 24 (100.0) 58 (72.5) 20 (4.5)III 0 0 22 (27.5) 380 (86.4)Plus<0.001a No 0 24 (100.0) 72 (90.0) 440 (100.0)Yes 16 (100.0) 0 8 (10.0) 0
To facilitate the analysis of the results we divided the screened infants into 3 groups based on the wofirst ROP condition diagnosed in either eye during any screening fundus examination;worse grade ROP (subdivided into type 1 and type 2 prethreshold ROP and indicated for treatment or close follow up according to the ETROP study), low grade ROP (any ROP less severe than type 1 or type 2 prethreshold ROP), or infants with no ROP.
Worse grade ROP was found in 40 infants (7.3% of screened infants). Sixteen infants (2.9%) developed type 1 ROP requiring treatment, twenty-four infants (4.3%) developed type 2 ROP, eighty infants (14.2%) developed low-grade ROP and 440 infants (78.6%) did not show signs of ROP, the baseline demographics of each cohort are shown in Table 1.
By analysis of the weekly postnatal weight gain ratios in infants without ROP against those who developed the disease,we found that the mean weight gain ratio 28d after birth was the most predictive factor for developing ROP and referred to it as the postnatal weight gain ratio (PWGR), both as a sole factor and when adjusted to the GA and birth weight, the area under the ROC curve was highest for combination of birth weight+GA+PWGR at 28d as shown in Table 2.
Table 2 AUC and 95%CIs of factors related to the prediction of ROP

AUC: Area under the ROC curve; CI: Confidence interval; GA:Gestational age.
?
The PWGR at 28d was significantly lower in infants both with worse grade ROP (type 1 & 2 prethreshold disease) and lower grade ROP than in those with absent ROP as shown in Table 3.We proposed 2 new screening models in view of these results aiming at improving the prediction of development of ROP by adding the PWGR at 28d after birth (weight at day 28-birth weight/birth weight) to the previously established screening criteria of the GA and birth weight. And we referred to the first model as the (Alex-ROP model) designed to detect any stageof ROP which recommends screening infants with GA≤33wk or birth weight ≤ 1500 g and PWGR at 28d after birth <0.3.
Table 3 Comparison of PWGR in infants without ROP and infants with different grades of ROP
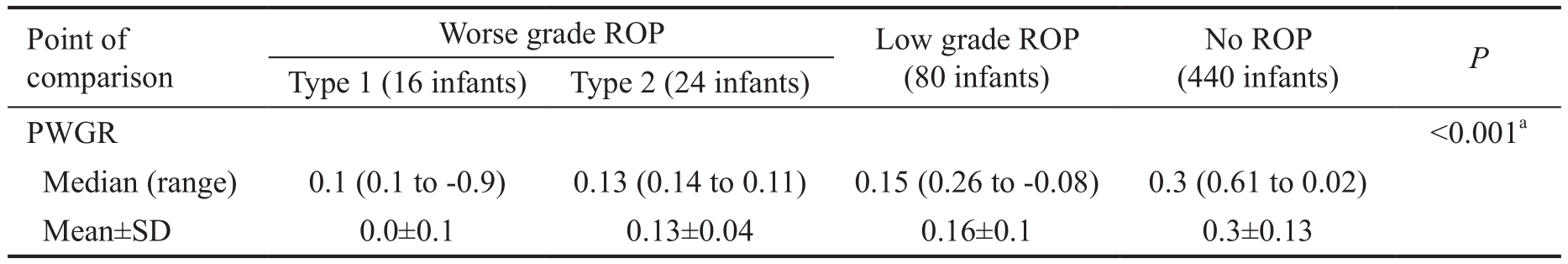
PWGR: Postnatal weight gain ratios; ROP: Retinopathy of prematurity.aStatistically significant difference.
 <0.001a Median (range) 0.1 (0.1 to -0.9) 0.13 (0.14 to 0.11) 0.15 (0.26 to -0.08) 0.3 (0.61 to 0.02)Mean±SD 0.0±0.1 0.13±0.04 0.16±0.1 0.3±0.13 PWGR
<0.001a Median (range) 0.1 (0.1 to -0.9) 0.13 (0.14 to 0.11) 0.15 (0.26 to -0.08) 0.3 (0.61 to 0.02)Mean±SD 0.0±0.1 0.13±0.04 0.16±0.1 0.3±0.13 PWGR
Table 4 Specificities and sensitivities of Alex ROP and Hg Alex ROP models %

ROP: Retinopathy of prematurity; Hg Alex-ROP: High-grade Alex-ROP.
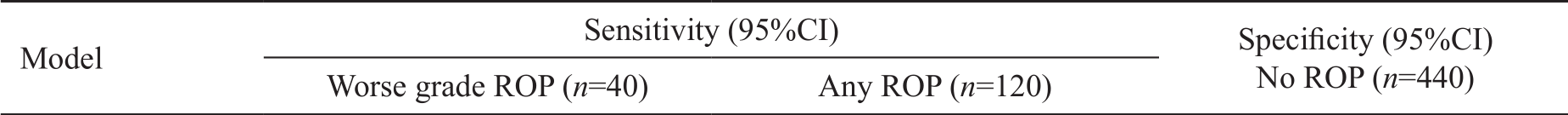 Alex ROP 100 (91.2-100.0) 100 (94.04-100.0) 50.45 (45.5-53.7)Hg Alex ROP 100 (91.2-100.0) 57.83 (55.4-63.5) 71.07 (68.1-76.2)
Alex ROP 100 (91.2-100.0) 100 (94.04-100.0) 50.45 (45.5-53.7)Hg Alex ROP 100 (91.2-100.0) 57.83 (55.4-63.5) 71.07 (68.1-76.2)
Table 5 Number of infants indicated for screening as per different screening models
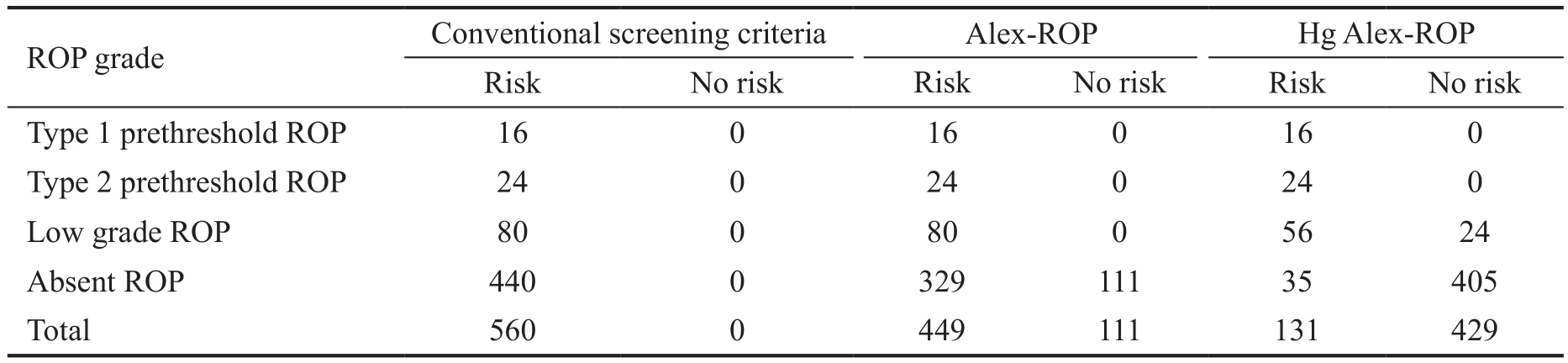
ROP: Retinopathy of prematurity; Hg Alex-ROP: High-grade Alex-ROP.
 Type 1 prethreshold ROP 16 0 16 0 16 0 Type 2 prethreshold ROP 24 0 24 0 24 0 Low grade ROP 80 0 80 0 56 24 Absent ROP 440 0 329 111 35 405 Total 560 0 449 111 131 429
Type 1 prethreshold ROP 16 0 16 0 16 0 Type 2 prethreshold ROP 24 0 24 0 24 0 Low grade ROP 80 0 80 0 56 24 Absent ROP 440 0 329 111 35 405 Total 560 0 449 111 131 429
In addition and according to the analysis of the criteria of the infants suffered worse grade ROP, we developed a second screening model referred to as High-grade Alex-ROP (Hg Alex-ROP) model recommending a cutoff point of PWGR<0.15 between birth and 28th day.
Table 4 shows that the sensitivity of the Alex-ROP model,when applied on the cohort of infants of the current study was 100% for both types of ROP (low-grade and high-grade ROP)and a specificity of 50.45%. On the other hand, the sensitivity of the Hg Alex-ROP was 100% for high-grade ROP, and 91.67% for any ROP (none of cases not indicated for screening developed high-grade ROP), and a specificity of 71.07%.
Table 5 shows that application of the Alex-ROP model application of the cohort of infants of the current study decreased the infants needing screening for by 19.82% and the application of the Hg Alex-ROP model reduced this number by 76.61%.
DISCUSSION
With the improvement of the neonatal care in the developing countries, the ROP started to become under the spot of interest of both the neonatologists and the ophthalmologists.
The screening protocols vary from one country to another, and it was observed that the both low-grade ROP and high grades of the disease can occur in infants with larger GA and birth weight in developing countries than those in developed ones,and thus application of the screening protocols used in the developed countries on infants in developing countries without modifications can lead to missing of some infants who may develop this potentially blinding disease.
There are no official screening criteria for infants requiring ROP screening in Egypt, however, most practitioners would agree to screen infants with birth weight ≤1500 g or GA≤33wk.Fundus examination for ROP screening puts stress on the infant and comprises a burden on the health service and consumes doctors time and effort, thus avoiding an unnecessary fundus exam without diminishing the sensitivity of disease detection will be a virtue.
There is growing interest around the world to improve the criteria of inclusion for ROP screening without decreasing positive cases detection sensitivity. For example, measuring IGF-1 levels which is thought to be a mediator of great importance in the pathogenesis of ROP, however, it's difficult and costly to assess its levels, and post natal weight gain was thought of as an indirect indicator about the IGF-1 levels and thus to improve the prediction of ROP.
Previous models focusing on the postnatal weight gain include CHOP-ROP and WINROP studies, however the calculations for triggering the alarm for ROP screening were tedious and time-consuming, the alarm could be triggered as late as 10wk after birth, and they were designed for detection of worse grade ROP but not for the low grades. Then, another model was proposed by Cao et al[25], it was simple to apply especially in a busy NICU and designed to detect both low grade and highgrade ROP, it added the criterion of NWG to the conventional criteria of GA and birth weight.
In the present study, we tried to observe the rates of postnatal PWGR in infants in Egypt as an example of the developing countries and to suggest cut of points to improve the prediction of development of both any ROP and worse grade ROP.
When applied on infants, the Alex-ROP model showed 100%sensitivity both low grade and worse grade ROP and decreased the infants needed to be screened by almost about 20% while the application of Hg Alex-ROP showed sensitivity of 100%to detect type 1 and 2 prethreshold ROP, and maintains good sensitivity of more than 55% for any ROP and decreases the total number of infants needed to be screened by more than 75%.
We noticed that the postnatal weight gain in infants in the current study is less. In comparison to the infants in studies from developed countries, we think that this reflects a difference in the quality of the neonatal care especially the total parenteral nutrition, which may be reflected in the level of some factors like the IGF-1, this may explain the fact that ROP can develop in infants with larger GA and birth weight in developing countries in comparison to those in developed countries who usually have better postnatal weight gain.
In 2018, the “Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study” was published, it was conducted on 6351 premature infants in USA and Canada to validate the performance of the CO-ROP model, it reported that despite maintaining high sensitivity, the CO-ROP model failed to predict the condition in 23 (3.1%) infants who developed severe ROP[28]. It worth noticing that all the infants included in that study enjoyed the high quality care in that part of the developed world, and taking into consideration the due to the difference in natural history of ROP in infants in developing countries, implementing the CO-ROP study in a developing country may lead to missing of even more cases with severe ROP.The main drawbacks of the current study include the small number of infants suffering worse grade ROP (prethreshold type 1 and type 2), we need to make sure the proposed cutoff points won't miss a single infant with high-grade ROP especially those who require treatment, this requires larger studies to increase the confidence interval lower limit for worse grade ROP.
In addition, the study was conducted in one tertiary referral center, and the cohort studied may not be a true representation of the infants in the whole community, but anyway, the ROP is more common to occur in young sick premature infants who are usually referred to such centers.
Moreover, some infants may show abnormally higher levels of postnatal weight gain due to a disease process (sepsis,hydrocephalus, oedema, etc.) those infants may be missed if the proposed model was implemented, it should be mentioned that the WIN-ROP recommended screening of infants gaining>400 g in a single week.
We hope that this study will be a step towards improving the prediction of the ROP screening, albeit the fact that the results of which cannot give solid evidence to modify the current agreed upon screening criteria, yet it may be an initiative for conduction of larger multicentral studies.
ACKNOWLEDGEMENTS
Conflicts of Interest:Ahmed IS, None; Badeeb AO, None.
REFERENCES
1 Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely: II. report of cases-clinical aspects. Trans Am Ophthalmol Soc 1942;40:262-284.
2 Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB,Kinney M, Lawn J, Born Too Soon Preterm Birth Action Group. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013;10(Suppl 1):S2.
3 Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Pretermassociated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74(Suppl 1):35-49.
4 Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1995;1(10):1024-1028.
5 Solebo AL, Rahi J. Epidemiology, aetiology and management of visual impairment in children. Arch Dis Child 2014;99(4):375-379.
6 Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol 2013;7:831-837.
7 Binenbaum G, Ying GS, Quinn GE, Huang J, Dreiseitl S, Antigua J,Foroughi N, Abbasi S. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol 2012;130(12):1560-1565.
8 Löfqvist C, Andersson E, Sigurdsson J, Engström E, Hård A, Aimon Niklasson A, Smith LES, Hellström A. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol 2006;124(12):1711-1718.
9 Hellström A, Hård AL, Engström E, Niklasson A, Andersson E,Smith L, Löfqvist C. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics 2009;123(4):e638-e645.
10 Hellström A, Engström E, Hård AL, Albertsson-Wikland K, Carlsson B, Niklasson A, Löfqvist C, Svensson E, Holm S, Ewald U, Holmström G, Smith LE. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003;112(5):1016-1020.
11 Stahl A, Hellstrom A, Smith LE. Insulin-like growth factor-1 and antivascular endothelial growth factor in retinopathy of prematurity: has the time come? Neonatology 2014;106(3):254-260.
12 Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL,Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D,Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A 2001;98(10):5804-5808.
13 Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol 2009;247(6):831-836.
14 Aydemir O, Sarikabadayi YU, Aydemir C, Tunay ZO, Tok L, Erdeve O,Oguz SS, Uras N, Dilmen U. Adjusted poor weight gain for birth weight and gestational age as a predictor of severe ROP in VLBW infants. Eye(Lond) 2011;25(6):725-729.
15 Binenbaum G. Algorithms for the prediction of retinopathy of prematurity based on postnatal weight gain. Clin Perinatol 2013;40(2):261-270.
16 Wu C, Löfqvist C, Smith LE, VanderVeen DK, Hellström A, WINROP Consortium. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol 2012;130(8):992-999.
17 Wu C, Vanderveen DK, Hellström A, Löfqvist C, Smith LE.Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol 2010;128(4):443-447.
18 Hård AL, Löfqvist C, Fortes Filho JB, Procianoy RS, Smith L, Ann Hellström A. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch Ophthalmol 2010;128(11):1432-1436.
19 Zepeda-Romero LC, Hård AL, Gomez-Ruiz LM, Gutierrez-Padilla JA, Angulo-Castellanos E, Barrera-de-Leon JC, Ramirez-Valdivia JM,Gonzalez-Bernal C, Valtierra-Santiago CI, Garnica-Garcia E, Löfqvist C,Hellström A. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol 2012;130(6):720-723.
20 O'Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ,Ratib S, Ng Y, Fielder AR. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics 2002;109(1):12-18.
21 Gursoy H, Basmak H, Bilgin B, Erol N, Colak E. The effects of mildto-severe retinopathy of prematurity on the development of refractive errors and strabismus. Strabismus 2014;22(2):68-73.
22 Fielder A, Blencowe H, O'Connor A, Gilbert C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch Dis Child Fetal Neonatal Ed 2015;100(2):F179-F184.
23 Tufail A, Singh AJ, Haynes RJ, Dodd CR, McLeod D, Charteris DG. Late onset vitreoretinal complications of regressed retinopathy of prematurity. Br J Ophthalmol 2004;88(2):243-246.
24 Smith BT, Tasman WS. Retinopathy of prematurity: late complications in the baby boomer generation (1946-1964). Trans Am Ophthalmol Soc 2005;103:225-234.
25 Cao JH, Wagner BD, McCourt EA, Cerda A, Sillau S, Palestine A, Enzenauer RW, Mets-Halgrimson RB, Paciuc-Beja M, Gralla J,Braverman RS, Lynch A. The Colorado-retinopathy of prematurity model (CO-ROP): postnatal weight gain screening algorithm. J AAPOS 2016;20(1):19-24.
26 Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A, International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics 2005;115(5):e518-e525.
27 International Committee for the Classification of Retinopathy of Prematurity. The international classi fication of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123(7):991-999.
28 McCourt EA, Ying GS, Lynch AM, Palestine AG, Wagner BD,Wymore E, Tomlinson LA, Binenbaum G, G-ROP Study Group.Validation of the colorado retinopathy of prematurity screening model.JAMA Ophthalmol 2018;136(4):409-416.
Correspondence to:Islam SH Ahmed. Department of Ophthalmology, Faculty of Medicine, Alexandria University,Champollion Street, Alexandria 21121, Egypt. islamshereen@gmail.com
Received: 2018-03-15 Accepted: 2018-08-29
● KEYWORDS: screening; retinopathy of prematurity;postnatal weight gain
DOl:10.18240/ijo.2019.02.18
Citation:Ahmed IS, Badeeb AO. The Alexandria retinopathy of prematurity model (Alex-ROP): postnatal weight gain screening algorithm application in a developing country. Int J Ophthalmol 2019;12(2):296-301

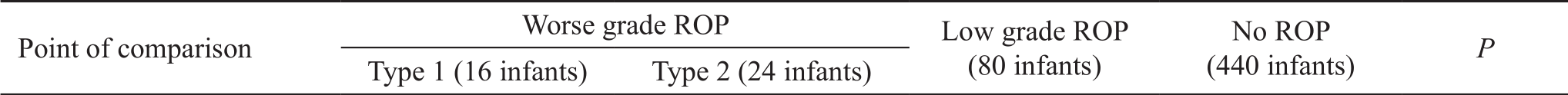 Gender 0.661 Male 8 (50.0) 8 (33.3) 40 (50.0) 230 (52.3)Female 8 (50.0) 16 (66.7) 40 (50.0) 210 (47.7)GA (wk±SD) 28.0±1.2 31.5±1.1 30.9±1.8 31.9±1.7 <0.001a Birth weight (g, mean±SD) 965.11±58.80 1105±134.03 1207±291.81 1217.96±242.81 <0.001a ROP stage <0.001a 0 0 0 6 (7.5) 440 (100.0)1 0 0 50 (62.5) 0 2 8 (50.0) 0 24 (30.0) 0 3 8 (50.0) 24 (100.0) 0 0 ROP zone<0.001a 0 0 0 0 40 (9.1)I 8 (50.0) 0 0 0 II 8 (50.0) 24 (100.0) 58 (72.5) 20 (4.5)III 0 0 22 (27.5) 380 (86.4)Plus<0.001a No 0 24 (100.0) 72 (90.0) 440 (100.0)Yes 16 (100.0) 0 8 (10.0) 0
Gender 0.661 Male 8 (50.0) 8 (33.3) 40 (50.0) 230 (52.3)Female 8 (50.0) 16 (66.7) 40 (50.0) 210 (47.7)GA (wk±SD) 28.0±1.2 31.5±1.1 30.9±1.8 31.9±1.7 <0.001a Birth weight (g, mean±SD) 965.11±58.80 1105±134.03 1207±291.81 1217.96±242.81 <0.001a ROP stage <0.001a 0 0 0 6 (7.5) 440 (100.0)1 0 0 50 (62.5) 0 2 8 (50.0) 0 24 (30.0) 0 3 8 (50.0) 24 (100.0) 0 0 ROP zone<0.001a 0 0 0 0 40 (9.1)I 8 (50.0) 0 0 0 II 8 (50.0) 24 (100.0) 58 (72.5) 20 (4.5)III 0 0 22 (27.5) 380 (86.4)Plus<0.001a No 0 24 (100.0) 72 (90.0) 440 (100.0)Yes 16 (100.0) 0 8 (10.0) 0

 <0.001
<0.001
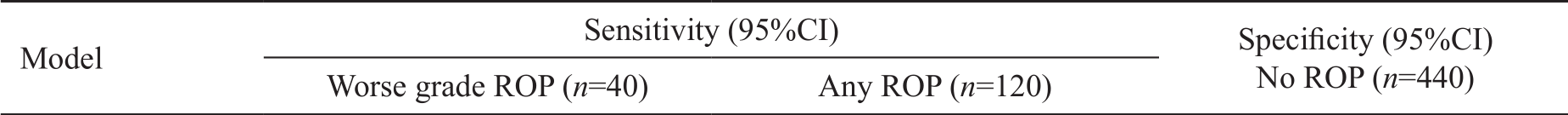 Alex ROP 100 (91.2-100.0) 100 (94.04-100.0) 50.45 (45.5-53.7)Hg Alex ROP 100 (91.2-100.0) 57.83 (55.4-63.5) 71.07 (68.1-76.2)
Alex ROP 100 (91.2-100.0) 100 (94.04-100.0) 50.45 (45.5-53.7)Hg Alex ROP 100 (91.2-100.0) 57.83 (55.4-63.5) 71.07 (68.1-76.2)
 Type 1 prethreshold ROP 16 0 16 0 16 0 Type 2 prethreshold ROP 24 0 24 0 24 0 Low grade ROP 80 0 80 0 56 24 Absent ROP 440 0 329 111 35 405 Total 560 0 449 111 131 429
Type 1 prethreshold ROP 16 0 16 0 16 0 Type 2 prethreshold ROP 24 0 24 0 24 0 Low grade ROP 80 0 80 0 56 24 Absent ROP 440 0 329 111 35 405 Total 560 0 449 111 131 429