Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: a review
Yao-Wen Song, Zhi-Qiang Pan
Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology and Visual Science Key Laboratory, Beijing 100730, China
Abstract● Donor cornea shortage is a primary hurdle in the development of corneal transplantation. Of all species,porcine corneas are the ideal transplantation material for humans. However, the xenoimmune rejection induced by porcine corneal xenotransplantation compromises surgical efficacy. Although the binding of lgM/lgG in human serum to a genetically modified porcine cornea is significantly weaker than that of the wild type (WT), genetically modified porcine corneas do not display a prolonged graft survival time in vivo. Conversely, costimulatory blockade drugs,such as anti-CD40 antibodies, can reduce the xenoimmune response and prolong graft survival time in animal experiments. Moreover, porcine endothelial grafts can survive for more than 6mo with only the subconjunctival injection of a steroid-based immunosuppressants regime;therefore, they show great value for treating corneal endothelial disease. ln addition, zoonotic transmission is a primary concern of xenotransplantation. Porcine endogenous retrovirus (PERV) is the most significant virus assessed by ophthalmologists. PERV integrates into the porcine genome and infects human cells in vitro. Fortunately,no evidence from in vivo studies has yet shown that PERV can be transmitted to hosts.
INTRODUCTION
C orneal disease is one of the most common blindnessrelated eye diseases in the world, second only to cataracts. An estimated 100-150 million people are affected worldwide[1]. Corneal disease severely compromises patients'visual acuity. The most effective treatment for corneal blindness is corneal transplantation. Corneal transplantation has been performed for more than 100y, and has been proven to be safe and effective[2-4]. However, the lack of donors significantly restricts the application of corneal transplant surgery. Thus, some alternatives for human corneas must be explored. In fact, people have attempted to use animal corneas as a substitute for more than 100y[5]. Currently, pigs are considered suitable animals for the supply of heterologous organs, because their physiological structure is similar to that of humans. Genetic modifications can be easily performed in pigs, and the application of porcine organs avoids many ethical problems[6]. In addition, pigs are raised widely and accessed easily. Porcine corneas are also very similar to human corneas in terms of anatomy and biomechanical properties[7-8]. Therefore, some researchers have focused on using porcine corneas for corneal xenotransplantation since the late 20th century[9]. However, reducing porcine corneal xenotransplantation rejection remains a significant challenge.Brie fl y, corneal xenografts may trigger the recipients' immune system to synthesize corresponding antibodies and then mediate complement-dependent cytotoxic effects[10]. Moreover,xenografts rejection is induced by indirect pathways because of the significant difference in major histocompatibility complex (MHC) between donors and recipients[11]. Like allograft rejection, it may cause an inflammatory response,corneal opacity, edema and neovascularization, which damage corneal tissues and reduce graft survival time[12-13]. Xenograft rejection shows more serious signs than allograft rejection. It is crucial to decrease xenoantigens or adopt immune tolerance in recipients' bodies. We will discuss this topic in the following sections.
In addition, the safety of porcine organ xenotransplantation is also a major concern. After all, zoonotic transmission is intolerable regardless of whether the surgical outcomes are satisfactory in human clinical practice. Fortunately, designated pathogen-free (DPF)-pigs, which are obtained through cesarean section from special pathogen-free (SPF) species,have been designed as medical donors[14] because they are free of most bacteria, fungi, viruses and parasites. However,porcine endogenous retrovirus (PERV) remains a concern because the DPF technique can not eliminate this pathogen[15].This article reviews recent progress aiming to safely reduce porcine corneal graft rejection and zoonotic transmission,particularly for PERV.
STUDIES ON REDUCING PORCINE CORNEAL XENOTRANSPLANTATION REJECTION
Pathogenesis of Corneal Xenotransplantation Rejection Corneal xenotransplantation rejection pathogenesis does not differ so much different from allograft rejection. As we know,unlike other solid organs that include blood vessels, immune privilege shields the cornea and prevents it from developing hyperacute rejection. Because the central area of the cornea is verified as vascular-free, the aqueous humor maintains the anterior chamber-associated immune deviation (ACAID)due to the presence of free Fas ligand and negative immuneregulatory molecules such as IL-10 and TGF-β; moreover, tight junctions between corneal endothelial cells prevent the cells and proteins in the aqueous humor from entering the stroma.Corneal immune privilege is a prerequisite for performing xenografts transplantation. However, immune privilege may be defective or impaired in some cases, such as ocular Inflammation, corneal dystrophy, neovascularization, trauma,topical allergies, etc. and in such cases, the cornea would yield to rejection[16]. Antibody-dependent or complementmediated innate humoral immune responses induce hyperacute rejection, so comparatively, CD4+ T cells play a major role in corneal xenotransplantation rejection[16]. In addition, Reichart et al[17] have confirmed that the humoral and cellular immune responses both contribute to corneal xenotransplantation rejection. They also concluded that rejections are primarily correlated with corneal immune privilege, the extent of genetic homology between donors and recipients, and the dose of the immunosuppressants regime[17].
There are three major xenogeneic antigens, including galactose α 1,3-galactose (αGal), N-glycolylneuraminic acid (NeuGc)and β1,4 N-acetylgalactosaminyl transferase (B4GALNT2)that are expressed in pigs and may bind to the antibodies of humans or nonhuman primates (NHPs) to induce a humoral immune response[18]. Complement also damages porcine corneal grafts via antibody-dependent and -independent pathways because porcine corneal endothelial cells are highly sensitized to the cytotoxicity of complement binding to antibodies. In addition to the humoral immune response, the cellular immune response, particularly for the cytotoxic effect of CD4+ T cells on corneal endothelial cells, is also a main cause of corneal xenotransplantation rejection. If there are not sufficient CD4+ T cells, CD8+ T cells and dendritic cells will replace them.
Unlike corneal allograft rejection, dendritic cells in the corneal xenograft can directly bind to the recipient's T lymphocytes for antigen presentation and then cause a cellular immune response. In addition, porcine corneal endothelial cells continuously express costimulatory molecules (CD80 and CD86), which allows them to stimulate human T cells completely to induce an immune response by direct pathways[19].
Corneal Xenotransplantation Using Genetically Modified Pigs
Genome editing techniques on pigs In recent years, there has been significant improvement in porcine genetically modified techniques aiming to eliminate xenoantigens. The fifirst generation of nuclease-mediated technique-zinc finger nucleases (ZFNs) was created, and zinc finger proteins were applied to recognize and bind specific DNA sequences in 2005. Combined with endonuclease Fok I, doublestranded DNA would then break[20]. The second generation of nuclease-mediated technique transcription activator-like effector nucleases (TALENs) was successfully applied to edit eukaryotic cells in 2012[21]. The TALEN technique identified DNA sequence specifically with TALE, and hydrolyzed DNA with the help of endonuclease Fok I[22]. Currently, clustered regularly interspaced short palindromic repeating associated nuclease 9 (CRISPR/Cas9) is an acquired immune system existing in bacteria and archaea that is one of the most advanced genome editing techniques. Under the guidance of guide RNA (gRNA), CRISPR/Cas9 can make nuclease Cas9 recognize and degrade exogenous DNA[23]. This technique is much simpler, more effective and more economical than ZFNs and TALENs, and it has been widely used to knock out the targeted genes of encoding porcine xenoantigens such as GGTA1/CMAH/B4GalNT2[24-26]. It is predicted that these genetically modified pigs for xenotransplantation could reduce xenoantigens-antibodies reactions to some extent.
In vitro studies on genetically modified porcine cornea αGal is a carbohydrate antigen mainly expressed in the porcine vascular endothelium; it is catalyzed and synthesized by α1,3-galactosyltransferase[27-28]. αGal can be recognized by antipig antibodies in primates and then causes hyperacute rejection in the recipient's body[29]. According to some reports, αGal is mildly expressed in the anterior stroma of the wild type (WT)porcine cornea, but it is not expressed in the epithelium or endothelium[30]. α 1,3-galactosyltransferase knockout (GTKO)pigs were successfully cultivated in 2002[31]. Hara et al[32]confirmed that the GTKO porcine cornea did not express αGal,and indicated that the immune response from the recipient to the GTKO porcine cornea was weaker than that to the WT after corneal xenotransplantation.
A primate's complement system is activated to form membrane attack complexes and different types of multiple active fragments after pig-primate xenotransplantation. The complement system would damage graft cells and mediate the inflammatory response[33]. The human complement-regulatory protein can interact with different complement components,enabling complement to mediate activation and suppression balanced reactions. Therefore, the expression of the human complements regulatory gene in donor pigs can effectively reduce the recipient's complement response[34]. Some researchers have successfully cultivated the transgenic pig expressing human complement-regulatory protein-CD46[35].Even transgenic GTKO/CD46 pigs expressing GTKO and CD46 simultaneously were also successfully bred and have become one of the major strains used for xenotransplantation research[36]. Hara et al[32] reported that human CD4+ T cells displayed a significantly weaker immune response against GTKO/CD46 porcine corneal endothelial cells than that of the WT in vitro. Moreover, CD55 and CD59 were also synthesized in some genetically modified pigs as transfection proteins. However,there were no reports showing that GTKO/CD55 and GTKO/CD59 species were used for corneal xenotransplantation.
Pigs also express some non-αGal antigens. Thus, there are still antibodies from the recipient that bind to non-αGal antigens in the porcine cornea even though αGal is knocked out through gene knockout techniques, which would cause immune rejection. A non-αGal antigen called NeuGc was discovered in 1990[37]. Subsequent studies confirmed that NeuGc was expressed in pigs, orangutans and rhesus monkeys, but not in humans[38]. Moreover, the anti-NeuGc antibody was expressed in humans. Given that NeuGc is expressed in some NHPs,research on investigating this antigen cannot draw support from pig-NHP models. Researchers had to perform some experiments on NeuGc in vitro. Cohen et al[30] demonstrated that NeuGc was strongly expressed in all layers of the corneas regardless of whether WT or GTKO strains were used, and IgM/IgG in human serum was positively expressed in the epithelium, anterior stroma and limbus of porcine corneas from WT and GTKO after binding to porcine xenoantigens.They also hypothesized that anti-NeuGc antibodies in human serum would bind to NeuGc in GTKO porcine corneas.Subsequently, some researchers attempted to cultivate a newly genetically engineered pig named NeuGcKO to eliminate NeuGc expression.
As we learned from articles reported by Gao et al[39],Wang et al[40] and other researchers in recent years, GTKO/NeuGcKO pigs have been successfully cultivated. It has been confirmed that the complement response in human serum binding to GTKO/NeuGcKO pig cells was weaker than that of GTKO in vitro. Moreover, Lee et al[41] indicated that there was no significant difference between IgM/IgG in human serum binding to xenoantigens in GTKO/NeuGcKO and GTKO porcine corneal endothelial cells. However, in another article published in the same year, they reported that the binding of IgM/IgG in human serum to GTKO/CD46/NeuGcKO porcine corneal tissue was weaker than that of GTKO/CD46[42]. The discordance between these two studies may attribute to the contents of xenoantigen in porcine corneal endothelial cells,which were minor and not sufficient to cause significant differences in the xenoimmune response between the GTKO/NeuGcKO and GTKO groups.
Byrne et al[43] characterized the porcine B4GALNT2 gene sequence, genomic organization and expression, which encoded another non-αGal antigen. Then Zhang et al[44] cultivated GTKO/NeuGcKO/ B4GALNT2KO pigs. Wang et al[45]investigated the xenoantigenicity of tissues and organs derived from GTKO/NeuGcKO/B4GALNT2KO pigs and found that compared to WT pigs, IgM/IgG in human serum binding to the cornea was significantly weaker in GTKO/NeuGcKO/B4GALNT2KO pigs. However, the difference between human serum IgM/IgG binding to GTKO/NeuGcKO/B4GALNT2KO and GTKO (or GTKO/NeuGcKO) strains has not been reported.
Corneal xenotransplantation using genetically modified pigsPorcine corneas have been applied to donors in corneal xenotransplantation for the past 10y. NHPs are considered highly homologous with human beings. Therefore, NHPs,particularly Macacus, which is classified as an old world monkey, were frequently used as recipients. Zhiqiang et al[46]fifirst performed pig-NHP corneal xenotransplantation. Their results showed that WT porcine corneal grafts could survive for 4mo by injecting betamethasone subconjunctivally after penetrating keratoplasty, and lamellar corneal xenografts could maintain transparency for 3mo without betamethasone. Dong et al[47] first attempt to perform penetrating keratoplasty with GTKO/CD46 and WT porcine corneal grafts in 2017. In their studies, the mean corneal graft survival time in GTKO/CD46 group was 100.8±59.11d (47-171d), whereas that in the WT group was 77.5±60.45d (28-157d). Unfortunately, compared to the WT porcine corneas, the GTKO/CD46 porcine corneas showed no correlation with prolonged corneal graft survival time or reduced immune response from the recipients' antibodies to the porcine xenoantigens. Based on the analysis of their report, we attributed this phenomenon to the complications that emerged in some xenografts such as anterior synechiae and retrocorneal membrane, which disrupt the postoperative efficacy. Lee et al[48] pointed out that the origin of retrocorneal membrane was the donor porcine corneal stroma, and young GTKO miniature porcine corneas should still be recommended for operation because their corneal thickness and biomechanical properties are closer to those of primates. Above all, GTKO/CD46 techniques alone may not improve porcine corneal survival status in vivo. Secondary postoperative complications and significant differences in corneal thickness between pig and rhesus monkey compromised corneal grafts survival.
Conversely, some researchers investigated to reduce the cellular immune response. There is a major costimulatory molecule named B7/CD28. B7-1 (CD80) and B7-2 (CD86)are expressed on the surface of antigen presenting cells(APCs) and bind to CD28 expressed on the surface of T cells. They then induce CD28 to transmit signals into T cells to activate a response. B cells also express B7 when they recognize antigens. Another receptor of the B7 group named cytotoxic lymphocyte associated antigen-4 (CTLA-4) was discovered later. Both CTLA-4 and CD28 are expressed on the transmembrane receptors of the surface of T cells, and they are similar in structure, particularly in the intracellular region. Therefore, CTLA-4 binding to B7 can naturally inhibit the combination of B7 and CD28. CTLA-4/B7 interactions can transmit inhibitory signals into activated T cells, therefore reducing T cells-mediated immune responses. Vabres et al[49]used porcine corneas from transgenic pigs that secreted human CTLA4Ig (hCTLA4Ig), GTKO and WT pigs, respectively,for corneal xenotransplantation in rhesus monkeys. Before transplantation, hCTLA4Ig was mainly expressed in the porcine corneal stroma and moderately expressed in the epithelium and endothelium. After surgery, hCTLA4Ig was still expressed in the porcine corneal grafts. Although the corneal grafts were eventually rejected in the hCTLA4Ig group, the quantity of inflammatory, T and B cells in porcine corneal grafts was less than that in the WT group. The longest final rejection was 120d in the hCTLA4Ig group whereas there was no significant difference in mean final rejection days between the hCTLA4Ig and WT groups (P=0.12).
Corneal Xenotransplantation Using Wild Type PigsGiven that there were no advantages in prolonging corneal grafts survival days with genetically modified pigs, researchers tend to utilize WT pigs to reduce experimental costs. One feasible method in current research that involves blocking the costimulation pathway has gradually gained more attention than before; this method is intended to enhanced recipients'immunological tolerance to WT porcine corneal grafts[6,50].Briefly, naive T cells activation requires two different extracellular signals, which both worked well. The first signal is derived from the major MHC-antigen peptide complex on the surface of APCs, this type of APC can interact and bind with T cell receptors (TCRs). The second signal is derived from the costimulatory molecule, which is the innate immune component that responds to the microorganism. As we know,the second signaling molecules and their ligands include B7/CD28, ICAM-1/FFA-1, LFA-3/CD2 and CD40/CD40L.Normal tissues and residual APCs may or may not express costimulatory molecules at low levels. The second signal deficiency can place autoreactive T cells in an anergic state,which is conducive to maintaining autoimmune tolerance.Therefore, the blocking activity of costimulatory molecules is expected to reduce rejection and prolong corneal graft survival time.
Some researchers have blocked CD40/CD40L pathways in animal experiments. The interaction between CD40L on the surface of T cells and CD40 on the surface of APCs can enhance the T cells response. The reaction mechanism involves the activation of APCs, which transmit intracellular signals that promote B7 expression and increase IL-12. CD40 is also expressed on the surface of B cells and binds to CD40L expressed on the surface of activated CD4Th+ cells. The CD40/CD40L complex stimulates quiescent B cells into the proliferation cycle and is the strongest costimulatory molecule that enables the activation of B cells. As early as 2001, some researchers attempted to block CD40/CD40L to prolong the corneal graft survival time after allograft transplantation.Subsequently, Choi et al[51] performed pig-NHP corneal xenotransplantation on 4 rhesus monkeys and administered intravenous immunoglobulin (IVIG) and anti-CD40L antibodies within 6mo after surgery. Compared to the control group, the corneal xenograft survival time in recipients who received anti-CD40L antibodies was significantly longer (192-933d), and administrating anti-CD40L antibodies significantly reduced the deposition of inflammatory cells in corneal tissues.Conversely, some researchers have attempted to use anti-CD40 antibodies to reduce the T cells immune response. Kim et al[52] performed deep lamellar corneal xenotransplantation on 5 rhesus monkeys. After administrating IVIG and anti-CD40 antibodies within 6mo after surgery, the xenografts survival time in recipients who received anti-CD40 antibodies was significantly longer than that of the control group(61d-12mo). The local expansion and activation of central and effector memory T cells were also effectively suppressed. Kim et al[53] further compared anti-CD40 antibody efficacy with a commercially available immunosuppressants regime (anti-CD20/basiliximab/tacrolimus). They performed full-thickness corneal xenotransplantation on 13 rhesus monkeys. In the anti-CD40 antibodies group, graft survival time ranged from 41 to 511d, whereas that in the anti-CD20 antibodies group ranged from 97 to 470d. There was no significant difference in the concentration of T cells in the recipients' blood, antiαGal antibodies in the recipients' plasma and αGal expression in porcine corneal grafts. Unfortunately, 3 rhesus monkeys in the anti-CD20 antibodies group suffered from adverse events (anorexia/pneumonitis/shigellosis) after receiving an immunosuppressants regime. Combined with Choi and Kim's studies, we can conclude that costimulatory blockade drugs may significantly improve the corneal xenograft survival time.However, some reports found that anti-CD40L antibodies may cause thrombosis when treating immune-related diseases.Consequently, anti-CD40L antibodies are not recommended to patients in clinical practice. We believe that it is also necessary to pay close attention to the safety of anti-CD40 antibodies.
Some researchers chose not to administer a systemic immunosuppressants regime in corneal xenotransplantation to avoid side effects. This expectation might result in endothelial keratoplasty. Currently, corneal endothelial keratoplasty has been widely developed. It is considered to be an effective therapy for bullous keratopathy, Fuchs corneal endothelial dystrophy and corneal endothelial dysfunction. With the rapid development of phacoemulsification in recent years,the incidence of corneal endothelial dysfunction is expected to increase significantly[54]. Rejection risks after pig-NHP endothelial keratoplasty are estimated to be significantly lower than those after penetrating keratoplasty because surgeons make minor incisions without sutures, xenoantigens in the porcine corneal endothelium are scarce[41] and porcine endothelial grafts are fully protected by ACAID. Liu et al[55]fifirst studied the application of porcine corneal endothelial grafts. They successfully prepared porcine corneal endothelial grafts for endothelial keratoplasty through mechanical stripping and liquid bubble techniques, respectively. There was no significant difference in the success rate between these two methods. The liquid bubble technique was less timeconsuming than mechanical stripping, but the former method led to significantly higher endothelial cell loss.
Subsequently, Liu et al[56] performed descement's stripping automated endothelial keratoplasty (DSAEK) on 7 rhesus monkeys. They adopt a mechanical stripping technique for the preparation of porcine endothelial grafts. Their results showed that 5 of 7 corneal grafts became transparent within 30d postoperatively, and 4 corneal grafts survived more than 180d (180-270d) with only injecting betamethasone subconjunctivally. In addition, immunohistochemistry showed that there was no obvious inflammatory cell infiltration in the survival grafts. Immunofluorescence showed that αGal expression was very scarce, and both anti-pig IgG and complement C3 were not found in the surviving endothelial grafts. Above all, the long-term survival of porcine corneal endothelial grafts may not depend on the systemic immunosuppressants regime. However, we could not find other studies about the utilization of porcine endothelial grafts, so more rigorous animal studies are necessary to strengthen Liu's conclusion.
We summarized the characteristics of pig-NHP corneal xenotransplantation mentioned above in Table 1.
Safety Issues with Porcine Endogenous RetrovirusThe microorganism safety of xenotransplantation is also an important concern. Donor animals should be examined before xenotransplantation to determine whether they carry pathogenic microorganisms. Like other animals, many microorganisms cling to the porcine alimentary canal and skin. Tissues and organs for transplantation, including porcine corneas, should be obtained under aseptic conditions[57]. Some reviews on microorganism safety of xenotransplantation reported that many microorganisms could infect humans through transplantation and result in zoonotic diseases.However, it was unclear which types of microorganisms should be monitored before operation. Currently, hepatitis E virus(HEV), porcine cytomegalovirus (PCMV), porcine circovirus(PCV), porcine lymphotropic herpesviruses (PLHV) and PERV are considered the primary contagious viruses[58]. Some strategies for eliminating these high-risk pathogens have been described in previous detailed reports (except for PERV)[59-60].Briefly, screening for zoonotic virus carriers in all cultivated pigs and administrating caesarean sections, antiviral treatment or vaccines to the qualified pigs (or those that may be carriers but with values lower than the baseline) help identify virus-free pigs that can be bred and further used for xenotransplantation.We reviewed published articles to date and found that ophthalmologists mainly focused on the risk of PERV transmission in corneal xenotransplantation[61-63]. Therefore,we will emphasize the relevant contents as follows. PERV is characterized as a retrovirus that transmitted across species and integrated into the porcine genome. It is classified as a C-type retrovirus, γ1 subgroup and RNA virus[64]. PERV exists as a previral formation that is integrated and exists in the host genome. Its genetic monomeric length is approximately 7-9 kb,which is composed of 5'LTR, 3'LTR and a middle region encoding the gag, pol, and env genes. Gag and pol display high homology even when obtained from different sources of PERV; however, env varies significantly[64]. Because of env gene differences and cellular tropism in vitro, PERV can be divided into three different subtypes: PERV-A, PERV-B and PERV-C. PERV-A and B are expressed in all porcine genomes and possess different copy levels, whereas PERV-C cannot be integrated into all porcine genomes. PERV-A and B display polytropism and infect humans' and other species' cells;PERV-C only infects porcine cells[65]. Since it is difficult to remove PERV from porcine genomes, effective strategies arenecessary overcome the infection. Fifirst, pigs with low copy numbers and low PERV expression should be preferentially assigned. Second, to avoid PERV-A/C recombination, PERV-C negative pigs should be utilized as much as possible. Third,genetically modified pigs that express low or even negative PERV should be cultivated to reduce the possibility of PERV transmission. Finally, vaccines should be used to block PERV transmission[66-67].
Table 1 Characteristics of pig-NHP corneal xenotransplantation
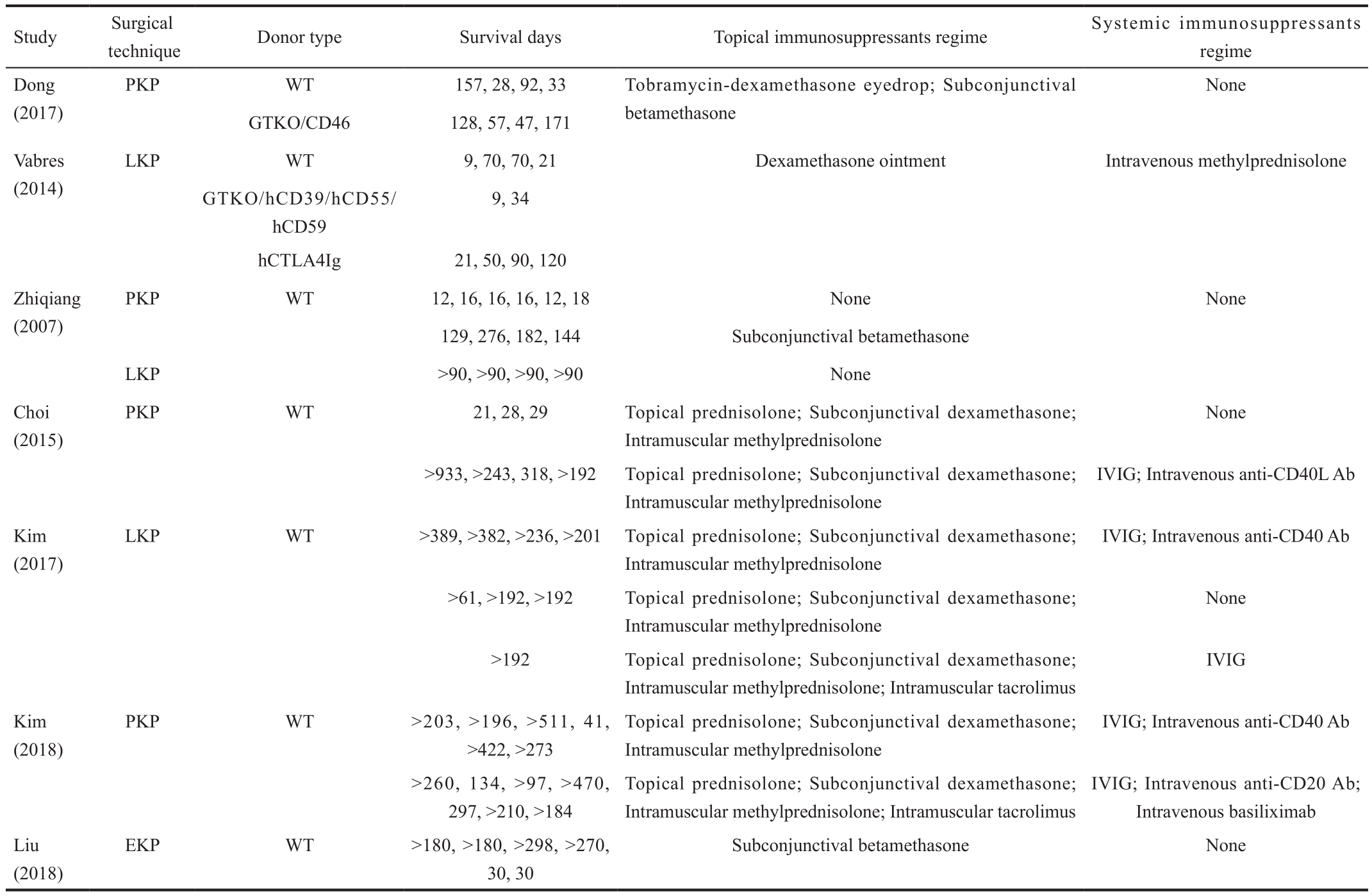
PKP: Penetrating keratoplasty; LKP: Lamellar keratoplasty; EKP: Endothelial keratoplasty; WT: Wild type; GTKO: α 1,3-galactosyltransferase knockout; hCTLA4Ig:Human cytotoxic lymphocyte associated antigen-4 immunoglobulin; IVIG: Intravenous immunoglobulin; Ab: Antibody.
Study Surgical technique Donor type Survival days Topical immunosuppressants regime Systemic immunosuppressants regime Dong(2017)None GTKO/CD46 128, 57, 47, 171 Vabres(2014)PKP WT 157, 28, 92, 33 Tobramycin-dexamethasone eyedrop; Subconjunctival betamethasone LKP WT 9, 70, 70, 21 Dexamethasone ointment Intravenous methylprednisolone GTKO/hCD39/hCD55/hCD59 9, 34 hCTLA4Ig 21, 50, 90, 120 Zhiqiang(2007)PKP WT 12, 16, 16, 16, 12, 18 None None 129, 276, 182, 144 Subconjunctival betamethasone LKP >90, >90, >90, >90 None Choi(2015)PKP WT 21, 28, 29 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone None>933, >243, 318, >192 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone IVIG; Intravenous anti-CD40L Ab Kim(2017)LKP WT >389, >382, >236, >201 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone IVIG; Intravenous anti-CD40 Ab>61, >192, >192 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone>192 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone; Intramuscular tacrolimus None IVIG Kim(2018)PKP WT >203, >196, >511, 41,>422, >273 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone IVIG; Intravenous anti-CD40 Ab IVIG; Intravenous anti-CD20 Ab;Intravenous basiliximab Liu(2018)>260, 134, >97, >470,297, >210, >184 Topical prednisolone; Subconjunctival dexamethasone;Intramuscular methylprednisolone; Intramuscular tacrolimus EKP WT >180, >180, >298, >270,30, 30 Subconjunctival betamethasone None
In fact, some researchers have suppressed or eliminated the PERV gene with aforementioned gene editing technology. In brief, Yang et al[66] at Harvard University adopted CRISPR/Cas9 technology to knock out all 62 copies of the PERV pol(polymerase) gene in the porcine kidney cell line (PK15),which could reduce endogenous viral transmission risks in pig-recipient by 1/1000. Niu et al[68] analyzed the porcine fetal fibroblast (PFF) genome sequence. They then found 25 copies of the PERV gene and inactivated them by utilizing CRISPR/Cas9. In addition, they successfully cloned fifirst endogenous retrovirus-inactivated pigs in the world with somatic cell nuclear transfer (SCNT) technology. Moreover,aforementioned reports[15] showed that the integrase inhibitors might be the most efficient inhibitors of PERV. Of course, their potency against PERV infection in the recipient body should continue to be analyzed.
PERV can infect a variety of human cell lines, such as stem cells, bone marrow cells, NK cells and kidney cell lines in vitro[69]. However, Meije et al[70] demonstrated that it was difficult for “endogenous ‘retroviral restriction factors'” such as intracellular proteins and components of the innate immune system to take effect in vitro environment. In some in vivo studies, Kim et al[71] found that no transmission of PERV was detected in the process of PERV-producing porcine cells transplanted into mice unless the murine cells were pretreated with PERV. Denner[72] confirmed this finding that although recombinations between the human tropic PERV-A and the ecotropic PERV-C in pigs showed high replication activity,recombinant PERV-A/C failed to infect pigs.
Some ophthalmologists also investigated the possibility of PERV transmission. Choi et al[62] cultured porcine keratocytes with the human embryonic kidney cell line-HEK-293 to explore PERV infectivity in vitro. They performed pig-NHP corneal xenotransplantation on 13 monkeys. Some monkeys were administered a steroid-based immunosuppressants regime(n=3) or anti-CD40 antibodies (n=4) postoperatively. Their results showed that neither PERV pol nor pig mitochondrial cytochrome oxidase II were detected in cocultured cell lines after 41 and 92d, respectively. Moreover, 257 rhesus monkeys'peripheral blood mononuclear cell (PBMC) samples, 34 serial plasma samples and 282 tissue samples were detected by polymerase chain reaction (PCR) and real time-PCR,respectively. As a result, no positive signal of PERV DNA and RNA were found. Li et al[61] cultured porcine aortic endothelial cells (PAECs) with monkey vein endothelial cells(MVECs) and performed pig-NHP corneal xenotransplantation on 10 rhesus monkeys. Their results showed that PERV was transmitted from PAEC to MVEC in vitro. PCR and RT-PCR showed that positive signals of PERV-A and B were present in porcine corneas. Fortunately, no evidence showed that PERV was transmitted into the recipients in vivo.
CONCLUSION
The global severe shortage of donor corneas will not be improved for a long time. Thus, porcine corneas as a replacement for humans is a foreseeable future possibility.Although genetically modified pigs have been successfully cultivated and raised, their corneal grafts did not survive longer than those of the WT in vivo. Some newly genetic engineered pigs, such as GTKO/NeuGcKO and GTKO/NeuGcKO/ B4GALNT2KO strains, have not yet been applied to animal experiments. Costimulatory blockade drugs can reduce the immune response that targets WT porcine corneal grafts and prolong their survival time. Aoyagi et al[73] studied the pharmacodynamic effects of a novel anti-CD40 antibody-4D11, which could mitigate some side effects including thrombosis. Of course, more preclinical experiments should be performed to ensure that a reasonable dose is used. WT porcine endothelial grafts could survive for months without a systemic immunosuppressants regime due to their low level of xenoimmunity and moderate complications. More comprehensive studies must be performed to verify their efficacy and safety to progress into clinical trials.
Obviously, there are no cases on PERV transmission during pig-NHP corneal xenotransplantation. However, it is indicated that there is no receptor for PERV-C in NHPs[74]; therefore,non-crossover into humans cannot be 100% assured even though pig-NHP studies showed no PERV infection. Thus,effective PERV detection must be performed in porcine corneal grafts before implanting them into human eyes in the future. It is better to use low level or negative PERV pigs as donors.
ACKNOWLEDGEMENTS
Foundation: Supported by National Natural Science Foundation of China (No.81470608).
Conflicts of Interest: Song YW, None; Pan ZQ, None.
REFERENCES
1 Liu Y, Zhang YN, Liu Y, Zhang J, Li AP, Liang QF, Pan ZQ.Demographic characteristics of voluntary donors registered in Beijing Tongren hospital eye bank of China: a retrospective study from 2007 to 2016. Transplant Proc 2017;49(8):1712-1718.
2 Murube J. Ramon Castroviejo centenary: a life dedicated to corneal transplantation. Surv Ophthalmol 2005;50(2):215-225.
3 Kubota T, Seitz B, Tetsumoto K, Naumann GO. Lamellar excimer laser keratoplasty: reproducible photoablation of corneal tissue. A laboratory study. Doc Ophthalmol 1992;82(3):193-200.
4 Binder PS. Selective suture removal can reduce postkeratoplasty astigmatism. Ophthalmology 1985;92(10):1412-1416.
5 Tost F. Arthur von Hippel: 100y motorized trepanation. Klin Monbl Augenheilkd 1992;201(1):55-58.
6 Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev 2014;258(1):241-258.
7 Kunzmann BC, Hellwinkel OJC, Klameth C, Wenzel D, Bartz-Schmidt KU, Spitzer MS, Schultheiss M. Establishment of a porcine corneal endothelial organ culture model for research purposes. Cell Tissue Bank 2018;19(3):269-276.
8 Hatami-Marbini H, Etebu E, Rahimi A. Swelling pressure and hydration behavior of porcine corneal stroma. Curr Eye Res 2013;38(11):1124-1132.
9 Cooper DKC, Gaston R, Eckhoff D, Ladowski J, Yamamoto T, Wang L, Iwase H, Hara H, Tector M, Tector AJ. Xenotransplantation-the current status and prospects. Br Med Bull 2018;125(1):5-14.
10 Lenčová A, Pokorná K, Zajícová A, Krulová M, Filipec M, Holáň V.Graft survival and cytokine production profile after limbal transplantation in the experimental mouse model. Transpl Immunol 2011;24(3):189-194.
11 Cooper DK, Bottino R. Recent advances in undefirstanding xenotransplantation: implications for the clinic. Expert Rev Clin Immunol 2015;11(12):1379-1390.
12 Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol 2016;238(2):288-299.
13 Kim MK, Wee WR, Park CG, Kim SJ. Xenocorneal transplantation.Curr Opin Organ Transplant 2011;16(2):231-236.
14 Fishman JA. Infectious disease risks in xenotransplantation. Am J Transplant 2018;18(8):1857-1864.
15 Denner J. Can antiretroviral drugs be used to treat porcine endogenous retrovirus (PERV) infection after xenotransplantation? Viruses 2017;9(8):E213.
16 Lacerda RP, Peña Gimenez MT, Laguna F, Costa D, Ríos J, Leiva M. Corneal grafting for the treatment of full-thickness corneal defects in dogs: a review of 50 cases. Vet Ophthalmol 2017;20(3):222-231.
17 Reichart B, Guethoff S, Brenner P, Poettinger T, Wolf E, Ludwig B,Kind A, Mayr T, Abicht JM. Xenotransplantation of cells, tissues, organs and the german research foundation transregio collaborative research centre 127. Adv Exp Med Biol 2015;865:143-155.
18 Marquez-Curtis LA, McGann LE, Elliott JAW. Expansion and cryopreservation of porcine and human corneal endothelial cells.Cryobiology 2017;77:1-13.
19 Hara H, Witt W, Crossley T, Long C, Isse K, Fan LM, Phelps CJ,Ayares D, Cooper DK, Dai YF, Starzl TE. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology 2013;140(1):39-46.
20 Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases.Genetics 2002;161(3):1169-1175.
21 Nieuwenhuysen TV, Vleminckx K. Targeted genome engineering in xenopus using the transcription activator-like effector nuclease (TALEN)technology. Methods Mol Biol 2018;1865:55-65.
22 Liu JY, Chen YX, Jiao RJ. TALEN-mediated Drosophila genome editing: protocols and applications. Methods 2014;69(1):22-31.
23 Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339(6121):819-823.
24 Reyes LM, Estrada JL, Wang ZY, Blosser RJ, Smith RF, Sidner RA, Paris LL, Blankenship RL, Ray CN, Miner AC, Tector M, Tector AJ. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol 2014;193(11):5751-5757.
25 Ryu J, Prather RS, Lee K. Use of gene-editing technology to introduce targeted modi fications in pigs. J Anim Sci Biotechnol 2018;9:5.
26 Martens GR, Reyes LM, Li P, Butler JR, Ladowski JM, Estrada JL,Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2,and SLA class I knockout pigs. Transplantation 2017;101(4):e86-e92.
27 Elder S, Chenault H, Gloth P, Webb K, Recinos R, Wright E, Moran D,Butler J, Borazjani A, Cooley A. Effects of antigen removal on a porcine osteochondral xenograft for articular cartilage repair. J Biomed Mater Res A 2018;106(8):2251-2260.
28 Choi K, Shim J, Ko N, Eom H, Kim J, Lee JW, Jin DI, Kim H.Production of heterozygous alpha 1, 3-galactosyltransferase (GGTA1)knock-out transgenic miniature pigs expressing human CD39. Transgenic Res 2017;26(2):209-224.
29 Huai GL, Qi P, Yang HJ, Wang Y. Characteristics of α-Gal epitope,anti-Gal antibody, α1, 3 galactosyltransferase and its clinical exploitation(Review). Int J Mol Med 2016;37(1):11-20.
30 Cohen D, Miyagawa Y, Mehra R, Lee W, Isse K, Long C, Ayares DL, Cooper DK, Hara H. Distribution of non-gal antigens in pig cornea:relevance to corneal xenotransplantation. Cornea 2014;33(4):390-397.
31 Nottle MB, Salvaris EJ, Fisicaro N, McIlfatrick S, Vassiliev I,Hawthorne WJ, O'Connell PJ, Brady JL, Lew AM, Cowan PJ. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI-dCas9. Sci Rep 2017;7(1):8383.
32 Hara H, Koike N, Long C, Piluek J, Roh DS, SundarRaj N, Funderburgh JL, Mizuguchi Y, Isse K, Phelps CJ, Ball SF, Ayares DL, Cooper DK. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci 2011;52(8):5278-5286.
33 Crowley MA, Delgado O, Will-Orrego A, Buchanan NM, Anderson K,Jaffee BD, Dryja TP, Liao SM. Induction of ocular complement activation by inflammatory stimuli and intraocular inhibition of complement factor d in animal models. Invest Ophthalmol Vis Sci 2018;59(2):940-951.
34 Liu FJ, Liu JJ, Yuan ZM, Qing YB, Li HH, Xu KX, Zhu WY, Zhao H,Jia BY, Pan WR, Guo JX, Zhang XZ, Cheng WM, Wang W, Zhao HY,Wei HJ. Generation of GTKO diannan miniature pig expressing human complementary regulator proteins hCD55 and hCD59 via T2A peptidebased bicistronic vectors and SCNT. Mol Biotechnol 2018.
35 Lee SE, Mehra R, Fujita M, Roh DS, Long C, Lee W, Funderburgh JL, Ayares DL, Cooper DK, Hara H. Characterization of porcine corneal endothelium for xenotransplantation. Semin Ophthalmol 2014;29(3):127-135.
36 Li J, Andreyev O, Chen M, Marco M, Iwase H, Long C, Ayares D,Shen ZY, Cooper DK, Ezzelarab MB. Human T cells upregulate CD69 after coculture with xenogeneic genetically-modified pig mesenchymal stromal cells. Cell Immunol 2013;285(1-2):23-30.
37 Rodriguez-Zhurbenko N, Rabade-Chediak M, Martinez D, Griñan T,Hernandez AM. Anti-NeuGcGM3 reactivity: a possible role of natural antibodies and B-1 cells in tumor immunosurveillance. Ann N Y Acad Sci 2015;1362:224-238.
38 Park HM, Kim YW, Kim KJ, Kim YJ, Yang YH, Jin JM, Kim YH,Kim BG, Shim H, Kim YG. Comparative N-linked glycan analysis of wild-type and α1, 3-galactosyltransferase gene knock-out pig fibroblasts using mass spectrometry approaches. Mol Cells 2015;38(1):65-74.
39 Gao HC, Zhao CJ, Xiang X, Li Y, Zhao YL, Li ZS, Pan DK,Dai YF, Hara H, Cooper DK, Cai ZM, Mou LS. Production of α1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene double-deficient pigs by CRISPR/Cas9 and handmade cloning. J Reprod Dev 2017;63(1):17-26.
40 Wang KK, Tang XC, Xie ZC, Zou XD, Li MJ, Yuan HM, Guo NN,Ouyang HS, Jiao HP, Pang DX. CRISPR/Cas9-mediated knockout of myostatin in Chinese indigenous Erhualian pigs. Transgenic Res 2017;26(6):799-805.
41 Lee W, Miyagawa Y, Long C, Ekser B, Walters E, Ramsoondar J,Ayares D, Tector AJ, Cooper DK, Hara H. Expression of NeuGc on pig corneas and its potential significance in pig corneal xenotransplantation.Cornea 2016;35(1):105-113.
42 Lee W, Hara H, Ezzelarab MB, Iwase H, Bottino R, Long C, Ramsoondar J, Ayares D, Cooper DK. Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs. Xenotransplantation 2016;23(2):137-150.
43 Byrne GW, McGregor CGA, Breimer ME. Recent investigations into pig antigen and anti-pig antibody expression. Int J Surg 2015;23(Pt B):223-228.
44 Zhang RJ, Wang Y, Chen L, Wang RG, Li C, Li XX, Fang B, Ren XY,Ruan MM, Liu JY, Xiong Q, Zhang LN, Jin Y, Zhang ML, Liu XR, Li L, Chen Q, Pan DK, Li RF, Cooper DKC, Yang HY, Dai YF. Reducing immunoreactivity of porcine bioprosthetic heart valves by geneticallydeleting three major glycan antigens, GGTA1/β4GalNT2/CMAH. Acta Biomater 2018;72:196-205.
45 Wang RG, Ruan MM, Zhang RJ, Chen L, Li XX, Fang B, Li C,Ren XY, Liu JY, Xiong Q, Zhang LN, Jin Y, Li L, Li RF, Wang Y, Yang HY, Dai YF. Antigenicity of tissues and organs from GGTA1/CMAH/β4GalNT2 triple gene knockout pigs. J Biomed Res 2018.
46 Zhiqiang P, Cun S, Ying J, Ningli W, Li W. WZS-pig is a potential donor alternative in corneal xenotransplantation. Xenotransplantation 2007;14(6):603-611.
47 Dong XJ, Hara H, Wang Y, Wang L, Zhang YN, Cooper DK, Dai YF, Pan ZQ. Initial study of α1, 3-galactosyltransferase gene-knockout/CD46 pig full-thickness corneal xenografts in rhesus monkeys.Xenotransplantation 2017;24(1).
48 Lee W, Mammen A, Dhaliwal DK, Long C, Miyagawa Y, Ayares D,Cooper DK, Hara H. Development of retrocorneal membrane following pig-to-monkey penetrating keratoplasty. Xenotransplantation 2017;24(1).
49 Vabres B, Le Bas-Bernardet S, Riochet D, Chérel Y, Minault D,Hervouet J, Ducournau Y, Moreau A, Daguin V, Coulon F, Pallier A,Brouard S, Robson SC, Nottle MB, Cowan PJ, Venturi E, Mermillod P,Brachet P, Galli C, Lagutina I, Duchi R, Bach JM, Blancho G, Soulillou JP, Vanhove B. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation 2014;21(5):431-443.
50 Di Zazzo A, Kheirkhah A, Abud TB, Goyal S, Dana RZ. Management of high-risk corneal transplantation. Surv Ophthalmol 1993;62(6):816-827.
51 Choi HJ, Lee JJ, Kim DH, Kim MK, Lee HJ, Ko AY, Kang HJ, Park C, Wee WR. Blockade of CD40-CD154 costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am J Transplant 2015;15(3):628-641.
52 Kim J, Kim DH, Choi HJ, Lee HJ, Kang HJ, Park CG, Hwang ES, Kim MK, Wee WR. Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates. Xenotransplantation 2017;24(3).
53 Kim J, Choi SH, Lee HJ, Kim HP, Kang HJ, Kim JM, Hwang ES, Park CG, Kim MK. Comparative efficacy of anti-CD40 antibody-mediated costimulation blockade on long-term survival of full-thickness porcine corneal grafts in nonhuman primates. Am J Transplant 2018;18(9):2330-2341.
54 Okumura N, Kinoshita S, Koizumi N. The role of rho kinase inhibitors in corneal endothelial dysfunction. Curr Pharm Des 2017;23(4):660-666.
55 Liu Y, Zhang J, Zhang YN, Yin MY, Miao S, Liang QF, Pan ZQ.The feasibility and efficacy of preparing porcine Descemet's membrane endothelial keratoplasty (DMEK) grafts by two techniques: an ex-vivo investigation for future xeno-DMEK. Xenotransplantation 2018;25(5):e12407.
56 Liu Y, Zhang YN, Liang QF, Yan C, Wang L, Zhang J, Pan ZQ.Porcine endothelial grafts could survive for a long term without using systemic immunosuppressors: an investigation of feasibility and efficacy of xeno-Descemet's stripping automated endothelial keratoplasty from WZS-pig to rhesus monkey. Xenotransplantation 2018:e12433.
57 Hu J, Chen LL, Tang YM, Xie CL, Xu BY, Shi M, Zheng WY, Zhou SY, Wang XK, Liu L, Yan YQ, Yang T, Niu YR, Hou QL, Xu XF, Yan XH. Standardized preparation for fecal microbiota transplantation in pigs.Front Microbiol 2018;9:1328.
58 Jarchum I. Getting rid of PERVs. Nat Biotechnol 2016;34(1):46.
59 Tao R, Fang LR, Bai DC, Ke WT, Zhou YR, Wang D, Xiao SB.Porcine reproductive and respiratory syndrome virus nonstructural protein 4 cleaves porcine DCP1a to attenuate its antiviral activity. J Immunol 2018;201(8):2345-2353.
60 Linhares DC, Cano JP, Wetzell T, Nerem J, Torremorell M, Dee SA.Effect of modified-live porcine reproductive and respiratory syndrome virus (PRRSv) vaccine on the shedding of wild-type virus from an infected population of growing pigs. Vaccine 2012;30(2):407-413.
61 Li A, Zhang Y, Liu Y, Pan Z. Corneal xenotransplantation from pig to rhesus monkey: no signs of transmission of endogenous porcine retroviruses. Transplant Proc 2017;49(9):2209-2214.
62 Choi HJ, Kim J, Kim JY, Lee HJ, Wee WR, Kim MK, Hwang ES.Long-term safety from transmission of porcine endogenous retrovirus after pig-to-non-human primate corneal transplantation. Xenotransplantation 2017;24(4).
63 Diao YM, Hong J. Feasibility and safety of porcine Descemet's membrane as a carrier for generating tissue-engineered corneal endothelium. Mol Med Rep 2015;12(2):1929-1934.
64 Denner J. Recent progress in xenotransplantation, with emphasis on virological safety. Ann Transplant 2016;21:717-727.
65 Denner J. The porcine virome and xenotransplantation. Virol J 2017;14(1):171.
66 Yang LH, Güell M, Niu D, George H, Lesha E, Grishin D, Aach J,Shrock E, Xu WH, Poci J, Cortazio R, Wilkinson RA, Fishman JA,Church G. Genome-wide inactivation of porcine endogenous retroviruses(PERVs). Science 2015;350(6264):1101-1104.
67 Denner J, Mihica D, Kaulitz D, Schmidt CM. Increased titers of neutralizing antibodies after immunization with both envelope proteins of the porcine endogenous retroviruses (PERVs). Virol J 2012;9:260.
68 Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan YN, Shrock E, Lesha E, Wang G, Luo YL, Qing YB, Jiao DL,Zhao H, Zhou XY, Wang SQ, Wei H, Güell M, Church GM, Yang LH.Inactivation of porcine endogenous retrovirus in pigs using CRISPRCas9. Science 2017;357(6357):1303-1307.
69 Kuddus RH, Gandhi CR, Rehman KK, Guo FL, Watkins SC, Valdivia LA, Fung JJ. Some morphological, growth, and genomic properties of human cells chronically infected with porcine endogenous retrovirus(PERV). Genome 2003;46(5):858-869.
70 Meije Y, Tönjes RR, Fishman JA. Retroviral restriction factors and infectious risk in xenotransplantation. Am J Transplant 2010;10(7):1511-1516.
71 Kim N, Choi J, Kim S, Gwon YD, Cho Y, Yang JM, Oh YK, Kim YB.Transmission of porcine endogenous retrovirus produced from different recipient cells in vivo. PLoS One 2016;11(11):e0165156.
72 Denner J. How active are porcine endogenous retroviruses (PERVs)?Viruses 2016;8(8):E215.
73 Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M,Shimamura T, Takahashi N, Miura T, Okimura K, Itoh T, Shimizu A,Furukawa H, Todo S. A human anti-CD40 monoclonal antibody, 4D11,for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant 2009;9(8):1732-1741.
74 Denner J. Is porcine endogenous retrovirus (PERV) transmission still relevant? Transplant Proc 2008;40(2):587-589.
Correspondence to:Zhi-Qiang Pan. Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University,Beijing Ophthalmology and Visual Science Key Laboratory,Dongjiaominxiang 1#, Dongcheng District, Beijing 100730,China. panzq2016@163.com
Received: 2018-05-15 Accepted: 2018-11-28
● KEYWORDS:corneal xenotransplantation; genetically modified pigs; wild type pigs; costimulatory blockade drugs;porcine endothelial grafts; porcine endogenous retrovirus safety
DOl:10.18240/ijo.2019.02.21
Citation: Song YW, Pan ZQ. Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: a review. Int J Ophthalmol 2019;12(2):324-332
