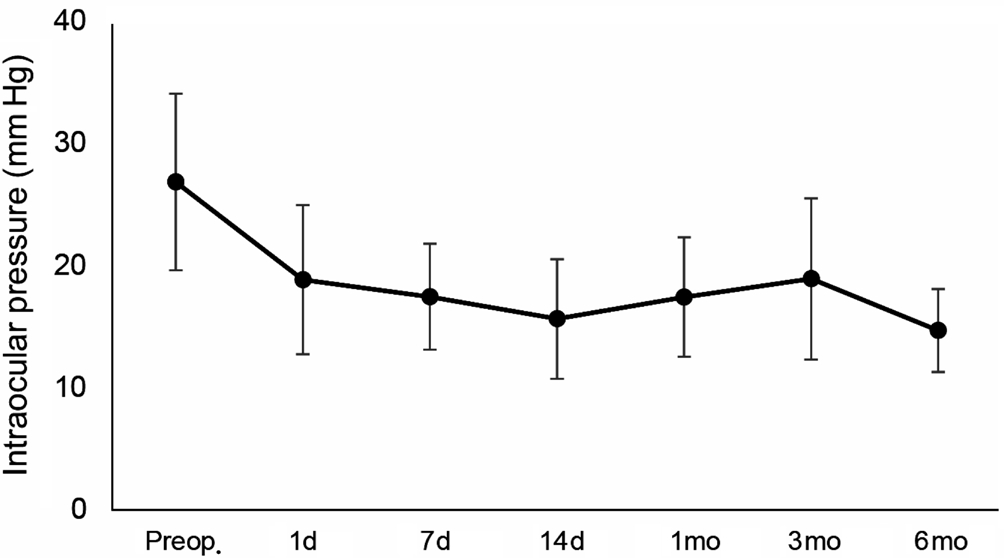
Figure 1 Preoperative and postoperative intraocular pressure at each follow-up visit.
Glaucoma represents one of the leading causes of visual impairment worldwide[1], and the reduction of intraocular pressure (IOP) represents the only treatment able to halt or slow the progression of visual field loss[2]. Many physical methods have been proposed in the past to partially destroy the ciliary body by means of diode and Nd:YAG laser, cryotherapy, and microwave heating[3-5]. However,cyclodestructive methods have two major drawbacks: they are non-selective for the ciliary body, potentially damaging also the adjacent structures; they have an unpredictable dose-effect relationship, with a high rate of complications.
Recently, a novel device using high-intensity focused ultrasound for the coagulation of the ciliary body, Ultrasound Cyclo Plasty (UCP), has been developed with the aim of achieving selective and controlled thermic effect on the ciliary body, thus limiting the damage to adjacent structures[6].
To date, there is no information available about the effects of UCP procedure on breakdown of blood-aqueous barrier, and thus on postoperative intraocular Inflammation.
The purpose of the study was to assess the postoperative course of intraocular inflammation by laser flare-cell photometry in glaucoma patients undergone UCP.
Ethical Approval This prospective interventional clinical study was conducted between January 2017 and June 2018 at S. Orsola-Malpighi University Hospital (Bologna, Italy).All participants provided both verbal and written informed consent before any study procedure. The study was approved by the local Institutional Review Board, and was carried out in accordance with the principles of the Declaration of Helsinki.
Methods Inclusion criteria were: age older than 18y; diagnosis of glaucoma with uncontrolled baseline IOP (≥ 21 mm Hg) under maximum treatment; intolerance to glaucoma medications despite well-controlled IOP. Exclusion criteria were: pregnancy;uveitic, traumatic or normal-tension glaucoma; history of laser or surgery within the previous 6mo; ocular surface disease; use of topical or systemic corticosteroid in the previous 3mo.
Patients were treated by UCP device (Eye OP1, Eye Tech Care, Rillieux-la-Pape, France). All of the procedures were performed under peribulbar anesthesia using the secondgeneration probes, as previously described[7]. After the procedure, patients were treated topically with tobramycin and dexamethasone combination eye drops (Tobradex, Alcon Inc.,Fort Worth, TX, USA) 4 times daily for 4wk. Hypotensive medications were interrupted after surgery, and then prescribed again only if postoperative IOP was ≥ 21 mm Hg at any visit.
Baseline evaluation included best-corrected visual acuity(BCVA), slit lamp biomicroscopy examination, gonioscopy,fundus examination, IOP measurement by Goldmann applanation tonometry, optical biometry (Lenstar LS 900, Haag-Streit,Koeniz, Switzerland) and laser fl are-cell photometry (FM-500,Kowa, Tokyo, Japan). Slit lamp biomicroscopy, BCVA, IOP measurements and laser flare-cell photometry were repeated postoperatively at 1, 7, and 14d, 1, 3 and 6mo.
Statistical Analysis The SPSS statistical software (SPSS Inc, Chicago, Illinois, USA) was used for data analysis. The homogeneity of variances was assessed by the Levene test.Variables following a normal distribution were presented as the mean±SD. Paired t tests were used to compare pre- and postoperative IOP and aqueous fl are values. One-way ANOVA tests were conducted to assess the differences of aqueous fl are values among glaucoma types and lens status. The associations of the aqueous flare values with the IOP and the biometric parameters were calculated using Pearson correlation analysis.A P<0.05 was considered statistically significant.
Eighteen eyes of 18 consecutive patients undergone UCP procedure were included in the study. The baseline characteristics of patients are summarized in Table 1.
One day postoperatively, the mean IOP significantly decreased from 26.8±7.2 to 18.8±6.1 mm Hg (P<0.001). At 6mo postoperatively, the mean IOP was still significantly reduced compared to preoperative values (14.7±3.4 mm Hg; P<0.001).Figure 1 shows the reduction of IOP over the time, expressed as both mean value and percentage of reduction.
Mean BCVA remained statistically unchanged (1.54±1.18 vs 1.59±1.31 logMAR; P=0.234), and no major intraoperative or postoperative complications occurred. Before surgery, the mean laser flare-cell photometry value was 12.1±7.5 ph/ms.After surgery, fl are values steeply increased to 64.1±53.9 ph/ms(P=0.001) at day 1, and then progressively decreased to respectively 60.6±49.7 at day 7, 43.5±38.5 at day 14 and 28.2±18.3 at month 1 (all P<0.05). Flare photometry values were above 100 ph/ms in 6 patients (33.3%) at day 7. Three and 6mo after surgery, the mean laser fl are-cell value further reduced to levels similar to baseline ones (respectively 16.7±6.2 and 12.8±10.2, both P>0.05; Figure 2).
The increase of flare values was not significantly different among patients with different glaucoma types and lens status(both P>0.05). A significant negative correlation was found between the increase of aqueous fl are photometry values one day postoperatively and anterior chamber depth (R=-0.568;P=0.014; Figure 3).
Conversely, no significant correlation was found between the increase of fl are values and the IOP reduction (all P>0.05).

Figure 1 Preoperative and postoperative intraocular pressure at each follow-up visit.
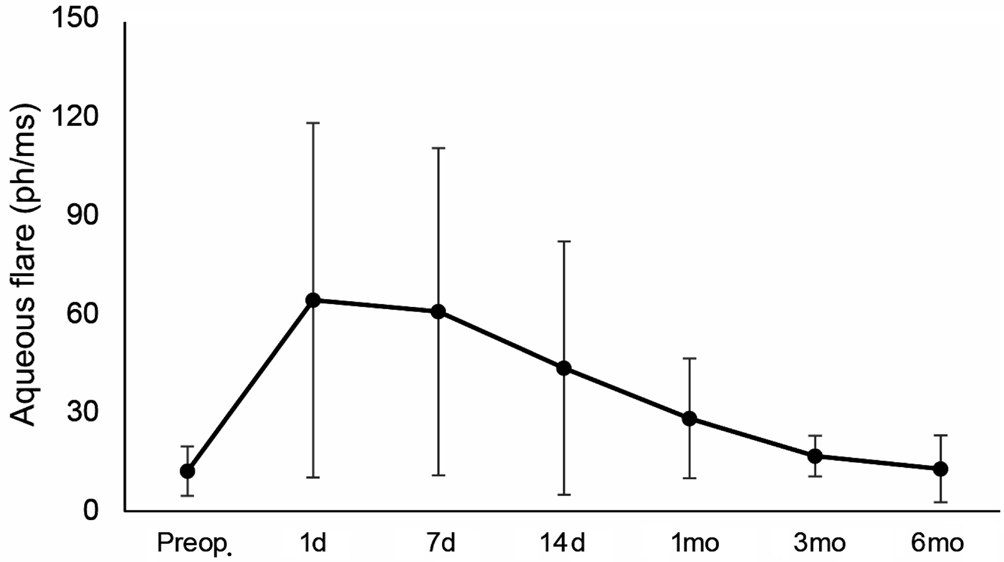
Figure 2 Preoperative and postoperative anterior chamber aqueous fl are measured by laser fl are-cell photometry at each follow-up visit.
Table 1 Demographical and clinical characteristics of patients treated with Ultrasound Cyclo Plasty
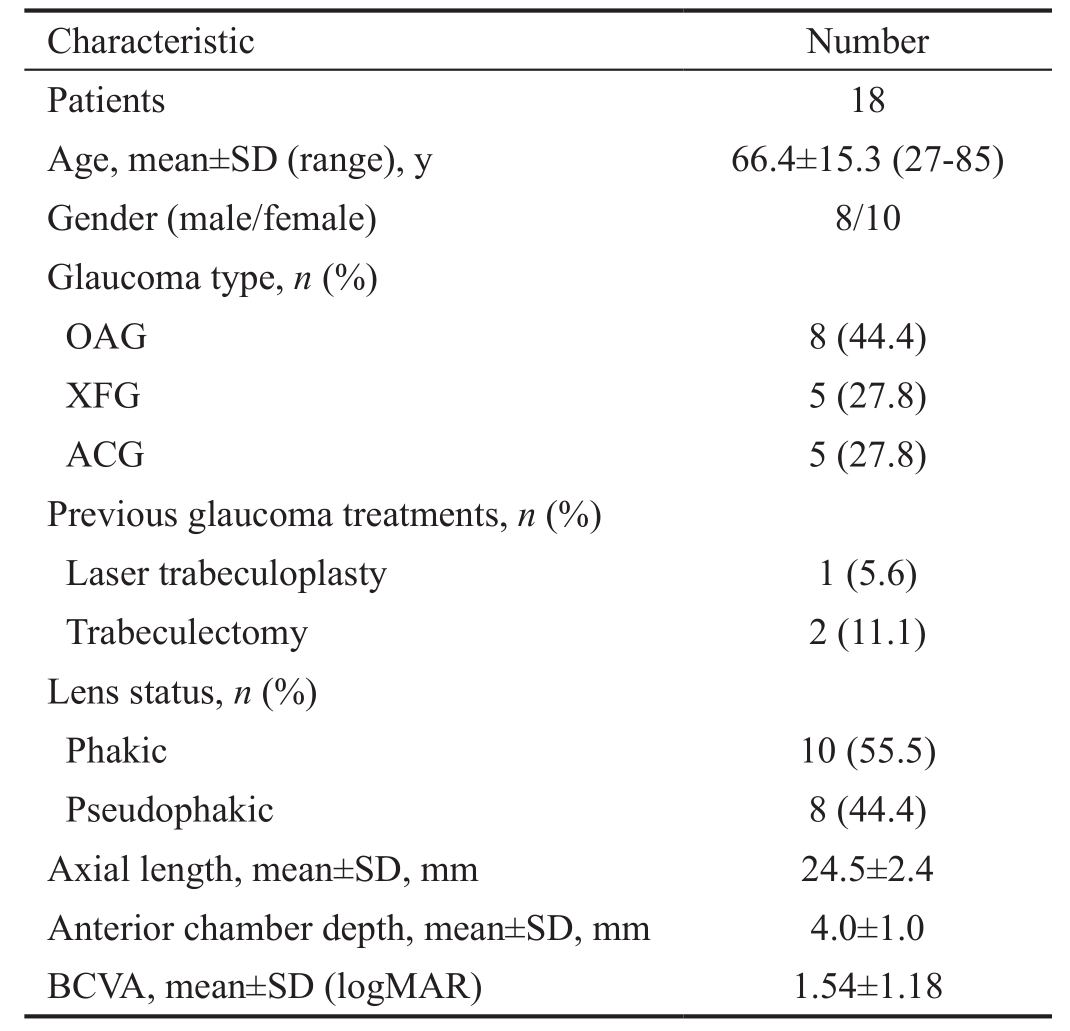
ACG: Angle-closure glaucoma; BCVA: Best-corrected visual acuity;IOP: Intraocular pressure; OAG: Open angle glaucoma; SD: Standard deviation; XFG: Exfoliative glaucoma.

The present study confirms existing data about the safety and efficacy of UCP procedure[8-15], with no major complications and an IOP reduction of almost fifty percent after 6mo of follow-up.
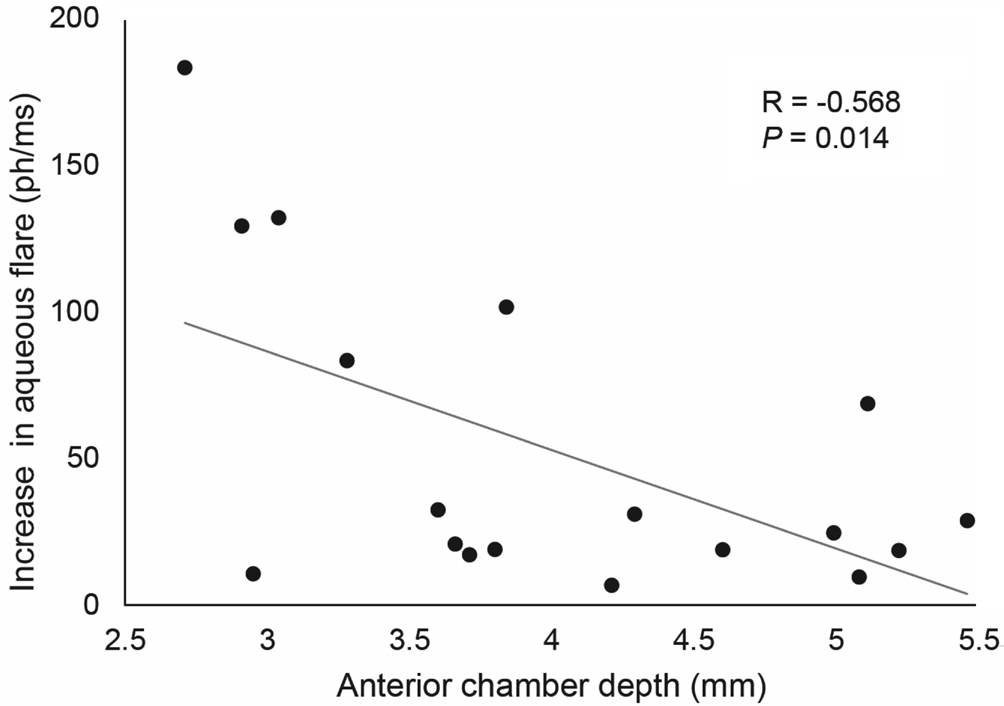
Figure 3 Correlation between the increase of anterior chamber aqueous fl are one day postoperatively and anterior chamber depth.
Despite previous studies have shown increased fl are values after various surgical procedures, including phacoemulsification,penetrating keratoplasty, refractive surgery, trabeculectomy and deep sclerectomy[16-22], poor information are available regarding postoperative inflammation in patients undergone cyclodestructive procedures[23]. To the best of our knowledge,no research has yet addressed the post-operative Inflammation following UCP. In fact, despite advances in ocular surgical techniques, some amount of post-operative intraocular Inflammation is unavoidable, even after uneventful minimally invasive surgery, like UCP[24]. Traditionally, inflammation in the anterior chamber is evaluated with the slit-lamp in a semiquantitative method; conversely, laser flare-cell photometry provides an objective, non-invasive and quantitative method for measuring the aqueous fl are.
In the present study, flare photometry values showed a significant increase at day 1 postoperatively, with one third of the patients having a flare higher than 100 ph/ms in the fifirst post-operative week. Then, flare values gradually decreased,and recovered to preoperative levels by 3mo after surgery. The increase of flare values after UCP appears lower compared to values recorded after traditional cyclophotocoagulation in the only paper available in the literature[24]. Postoperative inflammatory reaction may be secondary to the direct damage of the ciliary epithelium, which is a key component of the blood-aqueous barrier. Postoperative Inflammation has major implications in daily clinical practice. It has been hypothesized that intraocular Inflammation may be related, at least in part,to IOP-lowering effect of cyclodestructive procedures[25-26]. In fact, some inflammatory mediators synthetized and released after surgery, i.e. prostaglandins, may enhance aqueous drainage through the uveoscleral pathway, with consequent IOP reduction[27]. On the other hand, other mediators may lead to trabecular meshwork swelling with reduced aqueous humor out fl ow[28], and this may explain the lack of correlation between postoperative intraocular Inflammation rise and IOP reduction observed in our study group.
Additionally, intraocular inflammation is considered a major risk factor for developing post-surgical cystoid macular edema[29], a possible complication of various ocular surgeries,including cyclodestructive procedures[11,13,30]. This matter is relevant since UCP is addressed also to eyes with good visual potential and in less advanced stage of disease, being an early option in glaucoma management. The lower increase of intraocular inflammation after UCP compared to traditional cyclodestructive techniques may provide an additional advantage of the former technique. In this study, a shallow anterior chamber was associated with a higher intraocular inflammation rise after UCP. A similar association was observed in a previous study evaluating the postoperative aqueous fl are after trabeculectomy[31]. Two main explanations could be proposed: fifirstly, leaked proteins may be diluted in a smaller aqueous volume in eyes with shallow anterior chamber; secondly, a better targeting of the ciliary body may occur in these eyes. Previous studies reported a significant higher flare in eyes with pseudoexfoliation syndrome[32], as well as a higher increase in fl are after trabeculectomy in eyes with exfoliative glaucoma compared to those with primary open angle glaucoma[22]. However, in the present study flare values were similar both before and after surgery among the different types of glaucoma.
Repeated treatment with UCP have been proposed in case of partial or insufficient IOP reduction[33], but the proper timeframe has yet to be defined. Our study showed that inflammation returned to baseline values three months after surgery, because of the gradual recovery of blood-aqueous barrier. This timeframe may be considered reasonable for repeating UCP treatment, when required.
Conflicts of Interest:Pellegrini M, None; Sebastiani S,None; Giannaccare G, None; Campos EC, None.
1 Floriani I, Quaranta L, Rulli E, Katsanos A, Varano L, Frezzotti P,Rossi GC, Carmassi L, Rolle T, Ratiglia R, Gandolfi S, Fossarello M,Uva M, Hollander L, Poli D, Grignolo F; Italian Study Group on QoL in glaucoma. Health-related quality of life in patients with primary open-angle glaucoma. An Italian multicentre observational study. Acta Ophthalmol 2016;94(5):e278-e286.
2 European Glaucoma Society: Terminology and Guidelines for Glaucoma, 4th ed. Savona, PubliComm, 2014.
3 Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol 2007;91(12):1631-1635.
4 Shields MB, Shields SE. Noncontact transscleral Nd: YAG cyclophotocoagulation: a long-term follow-up of 500 patients. Trans Am Ophthalmol Soc 1994;92:271-283; discussion 283-287.
5 De Roetth A Jr. Cryosurgery for the treatment of glaucoma. Trans Am Ophthalmol Soc 1965;63:189-204.
6 Aptel F, Charrel T, Palazzi X, Chapelon JY, Denis P, Lafon C.Histologic effects of a new device for high-intensity focused ultrasound cyclocoagulation. Invest Ophthalmol Vis Sci 2010;51(10):5092-5098.
7 Giannaccare G, Sebastiani S, Campos EC. Ultrasound cyclo plasty in eyes with glaucoma. J Vis Exp 2018(131).
8 Mastropasqua R, Agnifili L, Fasanella V, Toto L, Brescia L, Di Staso S,Doronzo E, Marchini G. Uveo-scleral out fl ow pathways after ultrasonic cyclocoagulation in refractory glaucoma: an anterior segment optical coherence tomography and in vivo confocal study. Br J Ophthalmol 2016;100(12):1668-1675.
9 Aptel F, Charrel T, Lafon C, Romano F, Chapelon JY, Blumen-Ohana E, Nordmann JP, Denis P. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci 2011;52(12):8747-8753.
10 Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case series. Curr Med Res Opin 2014;30(8):1599-1605.
11 Denis P, Aptel F, Rouland JF, Nordmann JP, Lachkar Y, Renard JP,Sellem E, Baudouin C, Bron A. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci 2015;56(2):1089-1096.
12 Melamed S, Goldenfeld M, Cotlear D, Skaat A, Moroz I. Highintensity focused ultrasound treatment in refractory glaucoma patients:results at 1 year of prospective clinical study. Eur J Ophthalmol 2015;25(6):483-489.
13 Aptel F, Denis P, Rouland JF, Renard JP, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol 2016;94(5): e268-e277.
14 Giannaccare G, Vagge A, Gizzi C, Bagnis A, Sebastiani S, Del Noce C,Fresina M, Traverso CE, Campos EC. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol 2017;255(3):599-605.
15 Giannaccare G, Vagge A, Sebastiani S, Urbini LE, Corazza P,Pellegrini M, Carmassi L, Bergamini F, Traverso CE, Campos EC.Ultrasound Cyclo-Plasty in patients with glaucoma: 1-year results from a multicentre prospective study. Ophthalmic Res 2018:1-6.
16 Giannaccare G, Finzi A, Sebastiani S, Greco F, Versura P, Campos EC. The comparative efficacy and tolerability of diclofenac 0.1% and bromfenac 0.09% ophthalmic solutions after cataract surgery. Curr Eye Res 2018;43(12):1445-1453.
17 Weber M, Kodjikian L, Kruse FE, Zagorski Z, Allaire CM. efficacy and safety of indomethacin 0.1% eye drops compared with ketorolac 0.5% eye drops in the management of ocular Inflammation after cataract surgery. Acta Ophthalmol 2013;91(1):e15-e21.
18 Laurell CG, Zettefirström C, Philipson B, Syrén-Nordqvist S. Randomized study of the blood-aqueous barrier reaction after phacoemulsi fication and extracapsular cataract extraction. Acta Ophthalmol Scand 1998;76(5):573-578.
19 Küchle M, Nguyen NX, Naumann GO. Aqueous flare following penetrating keratoplasty and in corneal graft rejection. Arch Ophthalmol 1994;112(3):354-358.
20 Pisella PJ, Albou-Ganem C, Bourges JL, Debbasch C, Limon S.Evaluation of anterior chamber inflammation after corneal refractive surgery. Cornea 1999;18(3):302-305.
21 Nguyen NX, Küchle M, Martus P, Naumann GO. Quantification of blood: aqueous barrier breakdown after trabeculectomy: pseudoexfoliation versus primary open-angle glaucoma. J Glaucoma 1999;8(1):18-23.
22 Chiou AG, Mermoud A, Jewelewicz DA. Post-operative Inflammation following deep sclerectomy with collagen implant versus standard trabeculectomy. Graefes Arch Clin Exp Ophthalmol 1998;236(8):593-596.
23 Heinz C, Zurek-Imhoff B, Koch J, Rösel M, Heiligenhaus A.Long-term reduction of laser flare values after trabeculectomy but not after cyclodestructive procedures in uveitis patients. Int Ophthalmol 2011;31(3):205-210.
24 Coca-Prados M. The blood-aqueous barrier in health and disease. J Glaucoma 2014;23(8 Suppl 1):S36-S38.
25 Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT.Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol 2010;38(3):266-272.
26 Aquino MC, Barton K, Tan AM, Sng C, Li X, Loon SC,Chew PT. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol 2015;43(1):40-46.
27 Liu GJ, Mizukawa A, Okisaka S. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd: YAG laser cyclophotocoagulation. Ophthalmic Res 1994;26(2):65-79.
28 Ersoy L, Caramoy A, Ristau T, Kirchhof B, Fauser S. Aqueous fl are is increased in patients with clinically significant cystoid macular oedema after cataract surgery. Br J Ophthalmol 2013;97(7):862-865.
29 Pohlmann D, Schlickeiser S, Metzner S, Lenglinger M, Winterhalter S, Pleyer U. Different composition of intraocular immune mediators in Posner-Schlossman-Syndrome and Fuchs' Uveitis. PLoS One 2018;13(6):e0199301.
30 Kosoko O, Gaasterland DE, Pollack IP, Enger CL. Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 1996;103(8):1294-1302.
31 Inatani M, Ogata-Iwao M, Takihara Y, Iwao K, Inoue T, Kasaoka N, Tanihara H. A prospective study of postoperative aqueous flare in trabeculectomy alone versus phacotrabeculectomy. Nippon Ganka Gakkai Zasshi 2012;116(9):856-861.
32 Küchle M, Nguyen NX, Horn F, Naumann GO. Quantitative assessment of aqueous flare and aqueous ‘cells' in pseudoexfoliation syndrome. Acta Ophthalmol (Copenh) 1992;70(2):201-208.
33 De Gregorio A, Pedrotti E, Stevan G, Montali M, Morselli S. Safety and efficacy of multiple cyclocoagulation of ciliary bodies by highintensity focused ultrasound in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol 2017;255(12):2429-2435.