INTRODUCTION
Vascular structural and functional impairments can lead to numerous common human diseases. Hypertension,which affects almost one-third of adults in the US, is characterized by the structural and functional macroand micro-vascular alterations[1]. These changes result in disturbances of perfusion in brain, heart, kidney, and other organs. Elevated blood pressure (BP) also affects ocular vasculature to cause hypertensive retinopathy, which is a series of extensive or focal retinal micro-vascular impairments. It comprise of retinal microaneurysms, arteriolar thickening,arteriovenous nicking and, in severe cases, Elschnig’s spots,flame hemorrhage, cotton wool spots, hard exudates, optic nerve oedema and, serous retinal detachment[2-3]. These types of lesions indicate the reduction or loss of blood flow in retinal respective area linked to alterations of vessel structure and function[2].
The wall of small artery can be divided into 3 layers: intima,media and adventitia. There are several indicators that describe the structural properties of small arteries, such as lumen diameter, wall or media thickness, wall- or mediato-lumen ratios. The wall- or media-to-lumen ratio is more physiologically important[4]. It has been shown that essential hypertension is accompanied by an increase in media-to-lumen ratio and a decrease in luminal diameter in small arteries[4].Thus, the structure of small arteries is remodeled in patients with essential hypertension.
Elevated sympathetic neural tone is considered as a contributor to the development and maintenance of hypertension[5-7].However, the sympathetic nerve overactivation in hypertension is not a global response, it does not occur in certain hypertensive patients and animal models[8-10]. Sympathetic nervous release norepinephrine, which in turn stimulate postsynaptic α1-adrenergic receptor (α1-AR) expressed in vascular smooth muscle cells, and evoke vasoconstriction[11]. At present, the characteristics of vascular contractile responsiveness in hypertension is poorly understood. The aim of this study was to determine whether the structure and contractile responsiveness of ocular ciliary arteries (OCAs) were altered in spontaneously hypertensive rat (SHR) compared with non-hypertensive Wistar Kyoto rat (WKY). Vascular morphometry and isometric tension measurement were used in this study.
MATERIALS AND METHODS
Ethical Approval This study was approved by the Animal Experiment Committee of Akita University. All rats were treated according to the principles of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cardiovascular Variables In this experiment, 20-week-old male SHR and WKY were studied. All rats were housed in specific pathogen-free (SPF) at +20℃ and under a controlled 12-hour light-dark cycle and fed standard feed and water on demand. We measured the heart rate (HR), the systolic BP(SBP) and the diastolic BP (DBP) of rats with an electronic sphygmomanometer (BP-98A; Tokyo, Japan).
Isolation of the Ocular Ciliary Arteries After measuring the cardiovascular variables, rats were euthanized with ether (Abbott, IL, USA). Then, their eyes were immediately removed to ensure that the optic nerve attached to the eye is as long as possible (Figure 1A). The eyes were placed in a Krebs solution bubbled with 95% O2 and 5% CO2. Our previous article has described in detail the composition of Krebs solution[12]. Under a dissecting microscope, the distal section of OCA (length: 3-4 mm) and surrounding connective tissue were separated and cut off from the optic nerve (Figure 1B). Only one vascular segment was taken from an eyeball.
Vascular Morphometry In order to study the characteristics of the vascular morphology, the OCA segments were treated with several steps, such as 10% formalin fixation (30min),paraffin-embedding, cross-sections (5 μm) cutting, and hematoxylin-eosin (H&E) staining. Then we used an Olympus microscope (×10 magnification) to collected pictures. The lumen diameter and media thickness of OCAs were measured,and the ratios of media-to-lumen diameter were calculated.Four sectors (from four animals) of each group were analyzed.

Figure 1 Ocular ciliary artery A: Rat eyeball; B: The vascular segment was cut from the distal section of the ciliary artery.
Isometric Tension Recording Isolated vascular segments(2 mm in length) were mounted in the chamber of Myograph System® (JP Trading, Aarhus, Denmark). Our previous reports have described in detail the procedure for mounting the OCAs[13-14]. After the equilibration period, contractions of the OCA were evoked by a high-K solution and lasted for 20min.Then, 1 µmol/L carbachol was added to induce relaxation of OCA. Carbachol was a cholinergic agonist acting on vascular endothelial cell receptors[15]. If high-K-induced contraction was less than 2 mN or carbachol-induced relaxation was less than 0.5 mN, such vascular segments were excluded from this study. After verifying the arterial responsiveness to high-K and carbachol, the medium in the chamber was replaced by Krebs solution.
The contractile responsiveness of the isolated OCAs to norepinephrine was determined. Generally, the segment on the resting tone was maintained for 30min. Then 0.1-100 µmol/L norepinephrine was added in a cumulative manner every ten minutes. To investigate whether prostaglandins, nitric oxide(NO), and endothelium-derived hyperpolarizing factor (EDHF)were implicated in norepinephrine-induced contraction,10 µmol/L indomethacin, a cyclooxygenase inhibitor[16];100 µmol/L NG-nitro-L-arginine methylester (L-NAME), a NO synthase inhibitor[17]; or 0.1 µmol/L iberiotoxin, a largeconductance calcium-activated K+ (BKCa) channel blocker[18]was applied. Our previous study has demonstrated that the BKCa channels are utilized in EDHF signaling pathway in OCA[19]. Indomethacin, L-NAME or iberiotoxin was added 30min prior to norepinephrine-induced contractions.
Drugs The following drugs were used: norepinephrine,L-NAME, indomethacin, iberiotoxin, and carbachol hydrochloride(Sigma, St. Louis, MO, USA). The concentrations of these drugs were referred to the molarity in the myograph chambers.
Statistical Analysis Means±standard deviations were used to express the measured values and n represented the number of studied vessel segments unless specifically indicated.Unpaired two-tailed t-test was performed to analyze the statistical differences between the values. One-way analysis of variance was used to determine the differences between these concentration-response curves. Statistical significant was set at probability values less than 0.05.

Figure 2 Cross sections of ocular ciliary artery A: SHR; B: WKY. The vessels were stained with hematoxylin-eosin (H&E).
RESULTS
Cardiovascular Variables We measured the cardiovascular variables of SHR and WKY to confirm SHR have developed sustaining hypertension. At 20wk of age, although the HR didn’t differ between SHR and WKY, both SBP and DBP were higher in SHR compared with WKY (P<0.05, Table 1).
However, these BP values appear overestimated (in WKY,SBP/DBP: 146.2±8.2/104.8±12.7 mm Hg). It may be that we have measured the tail artery BP and restraining the animals that tends to make them stressed. Thus, before measuring the BP, we have let the rats move freely on the table for several minutes to calm them down. The first measure was more likely higher, and subsequent measurements would be stable. So, we measured BP four times per rat.
Structure of Ocular Ciliary Artery In SHR, common pathological changes of OCAs caused by hypertension were observed. These changes include lumen diameter and mediato-lumen ratios and they are summarized in Table 2. An overall narrowing of vessels was observed in SHR compared to WYK.The media of vessels became thicker, the lumen became smaller, and the media-to-lumen ratio became larger in SHR compared with WKY (P<0.05; Figure 2).
Norepinephrine-Induced Contraction The norepinephrineinduced concentration-response curve of OCAs were weaker in SHR (n=7) compared with WKY (n=6, P<0.05; Figure 3). The contractions at 100 µmol/L norepinephrine were 3.83±0.78 mN for SHR and 6.30±1.10 mN for WKY.
OCAs were pretreated with iberiotoxin, L-NAME, or indomethacin 30min before norepinephrine-induced contraction. Iberiotoxin (0.1 µmol/L) has not changed the norepinephrine-induced contraction in OCAs from SHR (n=7)or WKY (n=6, P>0.05; Figure 4A, 4B). L-NAME (100 µmol/L)increased the norepinephrine-induced contraction in vessels from both SHR (n=7) and WKY (n=6, P<0.05; Figure 5A, 5B).However, the increased extents were similar in SHR and WKY(P>0.05; Figure 5C). Indomethacin (10 µmol/L) decreased the contraction curves of OCAs from WKY (n=6, P<0.05;Figure 6A), but did not change those contractions of OCAs from SHR (n=7, P>0.05; Figure 6B). The contraction curves of WKY incubated with indomethacin were equal to that of SHR without indomethacin (P>0.05; Figure 6C).
Table 1 Comparison of cardiovascular variables between SHR and WKY

SHR: Spontaneously hypertensive rats; WKY: Wistar Kyoto rats;HR: Heart rate; BPM: Beats per min. SBP: Systolic blood pressure;DBP: Diastolic blood pressure.
?
Table 2 Morphological analyses of OCAs

SHR: Spontaneously hypertensive rats; WKY: Wistar Kyoto rats.
?
DISCUSSION
In this study, we compared the structure and contractile responsiveness of OCAs between SHR and WKY. Vessels from SHR demonstrated the significant remodeling with an increase of media-to-lumen ratio and a reduction of lumen diameter. On the other hands, vascular contractility was uniformly decreased in SHR, in conjunction with sustained elevated BP.
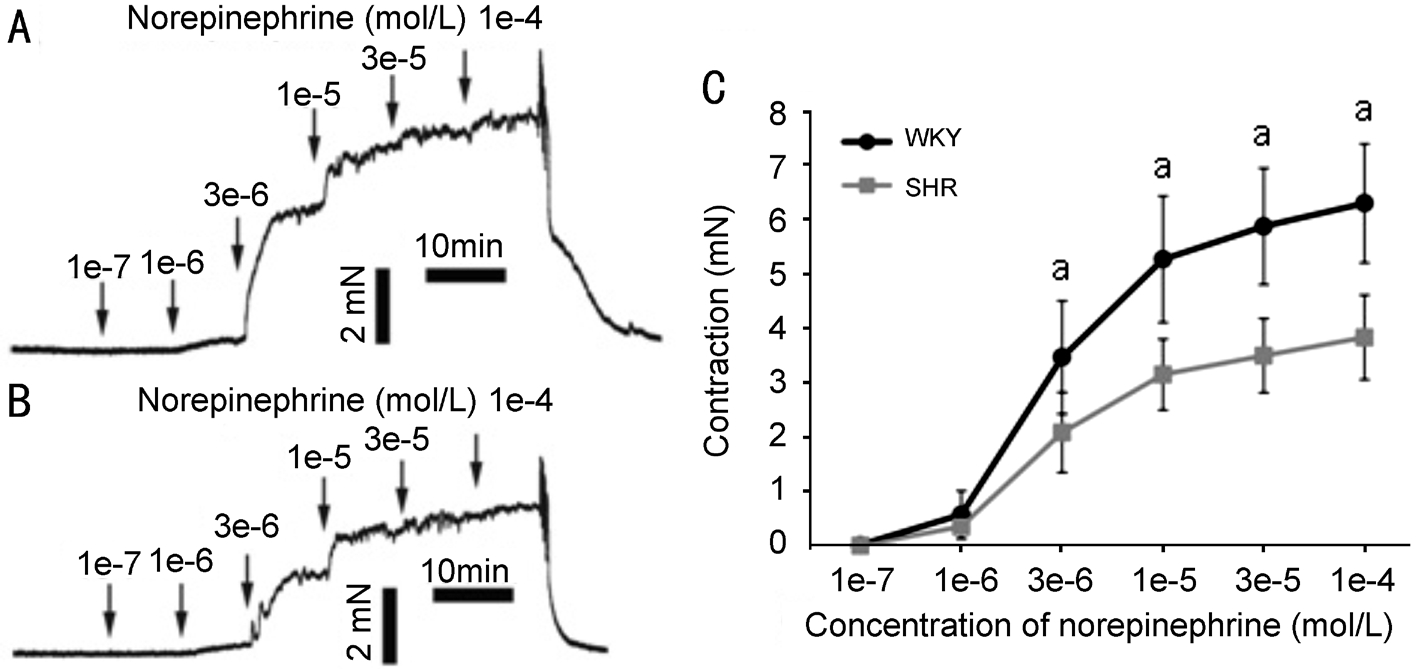
Figure 3 Norepinephrine-induced contractions A representative contraction curve of OCAs from WKY (A) or SHR (B) induced by norepinephrine in a cumulative manner. Horizontal axis is time (min) and vertical axis is isometric tension in millinewtons (mN). C: Greater contraction occurred in OCAs of WKY compared with those of SHR. aP<0.05.

Figure 4 Effect of iberiotoxin on norepinephrine-induced contraction of OCAs Iberiotoxin (0.1 µmol/L) has not changed the contraction curves of OCAs from WKY (A) or SHR (B).

Figure 5 Effect of L-NAME on norepinephrine-induced contraction of OCAs L-NAME (100 µmol/L) increased the contraction curves of OCAs from both WKY (A) and SHR (B). C: The increased extents of vasoconstrictions were similar in WKY and SHR. aP<0.05.
Remodeling of small arteries is a crucial factor in the pathological process of essential hypertension[20]. It has been strongly suggested that small arteries in patients with essential hypertension had the structural alterations: an increase of media-to-lumen ratio and a decrease of lumen volume[21]. The present study has shown that OCAs from SHR are remodeled in the same way as human essential hypertensions. Similarly,corrosion cast/scanning electron microscopy showed that the choroidal arteries of SHR were tortuosity, caliber irregularity and generalized narrowing[22]. Micro-vascular alterations can lead to the tissue perfusion disorder and increased susceptibility to ischemia[1]. A remodeled OCA reduces blood and oxygen supply to the eye, causes ocular circulatory insufficiency and eventually leads to hypertensive retinopathy. Notably, patients suffering from hypertension develop deteriorated tissue blood perfusion that cause visual dysfunction and damage.For example, hypertension is a frequent risk for nonarteritic anterior ischemic optic neuropathy (NAION) characterized by sudden visual loss in the patients older than 50y[23]. The pathogenesis of NAION is loss of the ciliary circulation perfusion at the optic disc, causing ischemia and hypoxia,resulting in axonal swelling and, capillary dilatation and fluid leakage.
On the other hands, our study has shown that concentrationdependent contraction curves to norepinephrine were uniformly diminished in OCA segments from SHR compared with WKY. Regulation of vasomotor activity is achieved through the complex interactions of two types of vascular regulatory factors, vasoconstrictors and vasodilators, and the combined effect of these factors determines the vascular tone[24].Norepinephrine stimulates postsynaptic α1-AR expressed in vascular smooth muscle cells, and regulates arterial contraction by acting as a vasoconstrictor[11]. Vascular endothelial cells can release both vasoconstrictor prostaglandins and vasodilators,such as EDHF, NO, and prostacyclin[25].

Figure 6 Effect of indomethacin on norepinephrine-induced contraction of OCAs A: Indomethacin (10 µmol/L) decreased the contraction curves of OCAs from WKY; B: Indomethacin has not changed the contraction of OCAs from SHR; C: The contraction curves of WKY incubated with indomethacin were equal to that of SHR without indomethacin. aP<0.05.
In present study, the involvement of EDHF, NO, or prostaglandins in norepinephrine-induced vasoconstriction was separately analyzed by incubation with iberiotoxin,L-NAME, or indomethacin, respectively. In both experimental groups, iberiotoxin has not changed norepinephrine-induced vasoconstrictions, suggesting that there is not participation of EDHF in the vasoconstrictions. However, the norepinephrineinduced contractions were increased by L-NAME, and the extents of increases were similar in SHR and WKY. This result indicated that NO participates in the regulation of vasoconstriction and is not affected by hypertension. Unlike iberiotoxin and L-NAME, the effects of indomethacin on vasoconstrictions were different between WKY and SHR.Indomethacin decreased the contraction curves of WKY,but did not change those of SHR. It means that the effect of prostaglandins on norepinephrine-induced contraction was destroyed in SHR. Indomethacin could block the production of both vasodilator and vasoconstrictor prostaglandins. It has been reported that the integrated vascular regulatory effect of both kinds of prostaglandins is vasoconstriction in rats[26]. The present study also proved this finding. A recent study by Blixt et al[27] showed an increase of endothelin-1 mediated vasoconstriction occurring in the ophthalmic artery at 48h after ischemia. In their study, the endothelium of the ophthalmic artery was shown to be intact, and did not have an influence on the contractile response. Our results demonstrated that hypertension disrupted the vasoconstrictive effect of endothelium-derived prostaglandins.
The decreased contractile responsiveness of OCAs in SHR may actually be a protective mechanism for ocular disease caused by hypertension. Unchecked decline in the vascular lumen can lead to tissue ischemia in hypertension. Disruption of vasoconstrictive effect of prostaglandins results in diminished vascular contraction and, in turn, prevents continual decline in the vascular lumen and further tissue ischemia.
Taken together, our study demonstrated the fact that the structure and function of OCAs were altered in hypertension.OCAs from SHR were remodeled with decreased lumen diameter and increased media-to-lumen ratio. Moreover,the contractile responsiveness of OCAs from SHR was diminished due to the disruption of vasoconstrictive effect of prostaglandins. Although a remodeled OCAs might cause decreased ocular blood supply, the reduced contractile responsiveness of these vessels suggest that it could inhibit further diminishment of the vascular lumen and prevent extensive tissue ischemia.
ACKNOWLEDGEMENTS
Foundations: Supported by the Natural Science Foundation of China (No.81100695); the Japan Society for the Promotion of Science (JSPS) ([C] 25462750).
Conflicts of Interest: Dong YR, None; Gustafson CE, None;Wang J, None; Cui JZ, None; Yoshitomi T, None.
1 Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens 2014;32(2):216-224.
2 Bhargava M, Ikram MK, Wong TY. How does hypertension affect your eyes? J Hum Hypertens 2012;26(2):71-83.
3 Steinegger K, Bergin C, Guex-Crosier Y. Malignant hypertension:clinical manifestations of 7 cases. Klin Monbl Augenheilkd 2015;232(4):590-592.
4 Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci 2002;17:105-109.
5 Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S,Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010;285(23):17271-17276.
6 Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116(6):976-990.
7 Hurr C, Young CN. Neural control of non-vasomotor organs in hypertension. Curr Hypertens Rep 2016;18(4):30.
8 Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 2010;55(3):644-651.
9 Stocker SD, Lang SM, Simmonds SS, Wenner MM, Farquhar WB.Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 2015;66(6):1184-1190.
10 Marinos A, Gamboa A, Celedonio JE, Preheim BA, Okamoto LE, Ramirez CE, Arnold AC, Diedrich A, Biaggioni I, Shibao CA.Hypertension in obese black women is not caused by increased sympathetic vascular tone. J Am Heart Assoc 2017;6(11):e006971.
11 Michelotti GA, Price DT, Schwinn DA. Alpha 1-adrenergic receptor regulation: basic science and clinical implications. Pharmacol Ther 2000;88(3):281-309.
12 Dong YR, Ishikawa H, Wu YZ, Yoshitomi T. Vasodilatory mechanism of levobunolol on vascular smooth muscle cells. Exp Eye Res 2007;84(6):1039-1046.
13 Dong YR, Watabe H, Su GF, Ishikawa H, Sato N, Yoshitomi T.Relaxing effect and mechanism of tafluprost on isolated rabbit ciliary arteries. Exp Eye Res 2008;87(3):251-256.
14 Dong YR, Sawada Y, Cui JZ, Hayakawa M, Ogino D, Ishikawa M,Yoshitomi T. Dorzolamide-induced relaxation of isolated rabbit ciliary arteries mediated by inhibition of extracellular calcium influx. Jpn J Ophthalmol 2016;60(2):103-110.
15 Garaliene V, Barsys V, Jakuška P, Benetis R. Action of calcium antagonists and agonists on isolated human thoracic arteries used for coronary artery bypass grafting. Pharmacol Rep 2012;64(3):733-738.
16 Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endotheliumderived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther 2006;318(1):276-281.
17 Kalsi JS, Rees RW, Hobbs AJ, Royle M, Kell PD, Ralph DJ, Moncada S, Cellek S. BAY41-2272, a novel nitric oxide independent soluble guanylate cyclase activator, relaxes human and rabbit corpus cavernosum in vitro. J Urol 2003;169(2):761-766.
18 Quignard JF, Félétou M, Edwards G, Duhault J, Weston AH,Vanhoutte PM. Role of endothelial cell hyperpolarization in EDHF-mediated responses in the guinea-pig carotid artery. Br J Pharmacol 2000;129(6):1103-1112.
19 Dong YR, Watabe H, Cui JZ, Abe S, Sato N, Ishikawa H, Yoshitomi T. Reduced effects of endothelium-derived hyperpolarizing factor in ocular ciliary arteries from spontaneous hypertensive rats. Exp Eye Res 2010;90(2):324-329.
20 Zhou ZH, Liao YH, Li LD, Wei F, Wang B, Wei YM, Wang M, Cheng X. Vascular damages in rats immunized by alpha1-adrenoceptor peptides.Cell Mol Immunol 2008;5(5):349-356.
21 Mulvany MJ. Structure and function of small arteries in hypertension.J Hypertens Suppl 1990;8(7):S225-S232.
22 Bhutto IA, Amemiya T. Choroidal vasculature changes in spontaneously hypertensive rats - transmission electron microscopy and scanning electron microscopy with casts. Ophthalmic Res 2002;34(2):54-62.
23 Turkoglu EB, Ilhan HD, Cetinkaya A, Bilgin AB, Apaydin KC.Nonarteritic anterior ischemic optic neuropathy in young patients. J Fr Ophtalmol 2015;38(5):421-426.
24 Pintérová M, Kuneš J, Zicha J. Altered neural and vascular mechanisms in hypertension. Physiol Res 2011;60(3):381-402.
25 Kang KT. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol Res 2014;30(3):141-148.
26 Travaglia TC, Berger RC, Luz MB, Furieri LB, Ribeiro JR, Vassallo DV, Mill JG, Stefanon I, Vassallo PF. Low-salt diet increases NO bioavailability and COX-2 vasoconstrictor prostanoid production in spontaneously hypertensive rats. Life Sci 2016;145:66-73.
27 Blixt FW, Johansson SE, Johnson L, Haanes KA, Warfvinge K,Edvinsson L. Enhanced endothelin-1 mediated vasoconstriction of the ophthalmic artery may exacerbate retinal damage after transient global cerebral ischemia in rat. PLoS One 2016;11(6):e0157669.