INTRODUCTION
Perfluorocarbon liquids (PFCLs) are synthetic fluorinated hydrocarbon fluids that are odorless, colorless, heavier than water, and minimally viscous. Since first introduced into vitreoretinal surgery by Chang et al[1], they have been successfully used as intraoperative soft tools for manipulation and reposition of the retina. These liquids were thought to be inert; however, the latent intravitreal inflammation and damage caused by the residual PFCLs could not be ignored.Animal experiments also revealed that prolonged presence of PFCLs might induce cell damage and evoke an intraocular inflammatory reaction[2-3]. In former studies, pneumatic vitrectomy(in which gas-mediated vitreous compression was performed with 0.4 mL perfluoropropane to remove the vitreous) or 20G vitrectomy was used; however, with the development of a more modern technique, micro-invasive vitreoretinal surgeries(MIVS), such as 23G and 25G, are utilized more frequently.PFCL has also recently been used as a short-term vitreous substitute in primary rhegmatogenous retinal detachment cases[4]. Additionally, the manufacture of PFCLs has changed.Recently, there has developed a novel method (Chinese Patent No. 201610104987) of processing fluoride alkane, and this method can remove trace amounts of hydrogen-containing impurities, which could be harmful to the intraocular tissue.Therefore, to explore the potential toxicity of PFCLs as short-term tamponades in modern MIVS, we performed the following study.
MATERIALS AND METHODS
Ethical Approval All experiments were approved by the animal research review committee of the Zhejiang Institute of Medical Device Testing and performed in accordance to the resolution on care of animals in research from the Association for Research in Vision and Ophthalmology.
Animals and Grouping New Zealand rabbits were utilized,and 48 eyes were included and randomly, evenly assigned into four groups. Eyes underwent vitrectomy first and then 0.1 mL of perfluorooctane (PFO) for ophthalmic surgery (Jieshi Medical Technology Co. Ltd., Shanghai, China, use the novel purification method of Chinese Patent No.201610104987) was injected in group PFOa and 0.1 mL of F-Octane (FLUORON Gmbh, Neu-Ulm, Germany) in group PFOb. Eyes of the pars plana vitrectomy (PPV) control group were filled with balanced salt solution (BSS), and eyes with no surgical intervention were included as the blank control.
Surgical Procedure and Follow-up Examination All of the rabbits were operated upon under general anesthesia induced by intramuscular application of ketamine hydrochloride(30 mg/kg) and xylazine hydrochloride (5 mg/kg) combined with sodium pentobarbital. The pupils were dilated with tropicamide phenylephrine eye drops (0.5% tropicamide and 1% phenylephrine). All vitrectomies were carried out by one surgeon (Jiang CH) using a commercially available device(Constellation, Alcon) and a 23G system. After the vitreous was removed, 0.5 mL of PFOs were injected into the vitreous cavity and maintained for 20min to simulate a real surgical situation. Then, the PFOs were removed, and in the two PFO groups, another 0.1 mL of PFOs was injected before the end of surgery. For each PFO group, the same PFO was used intraoperatively and at the end of surgery, in PPV group F-Octane was used during the operation.
Follow-up observation was performed the first day after surgery and then weekly and included examination for corneal and lens opacities. Eyes were enucleated at 1 (16 eyes), 4 (16 eyes) and 12wk (16 eyes) after the surgery. At any time of the study, eyes would be excluded if any form of complication,such as cataracts or retinal detachments, was noticed, or when animal death occurred, in which case the rabbit would then be replaced by one of the same-sex and similar weight.
Hematoxylin and Eosin Staining Rabbits were euthanized by carbon dioxide inhalation, and eyes were fixed with 4%paraformaldehyde as soon as they were enucleated. Tissue was embedded in paraffin and then cut to a thickness of 5 µm.Sections from within 0.2 mm of the optic disc were chosen for processing. After deparaffinization and rehydration, sections were treated with sodium citrate buffer (pH 6.0, 10min) and blocking buffer [10% normal goat serum, 0.05% Triton X-100 in phosphate-buffered saline (PBS); 1h, room temperature].Then, the specimens were stained with hematoxylin and eosin(H&E). The slides were dehydrated and placed on a coverslip.
Immunohistochemical Staining of TNF-α, Caspase-3,IL-6 and IL-8 Endogenous peroxidases in paraffin slices were hydrated with hydrogen peroxide (3% in methanol) for 30min and then boiled in sodium citrate buffer for 10min to achieve antigenicity. After blockage with 3% bovine serum albumin, the sections were incubated overnight at 4℃with primary antibodies [mouse anti tumor necrosis factor(TNF)-α, 1:100; mouse anti Caspase-3, 1:100; mouse anti interleukins (IL)-6, 1:50; mouse anti IL-8, 1:50; Abcam,USA] in PBS supplemented with 0.5% Triton X-100 (Santa Cruz, USA), followed by incubation with appropriate HRP-coupled secondary antibodies (Goat anti-mouse IgG, 1:200;Arigo, Taiwan, China). Slides were counterstained using Mayer’s hematoxylin and dehydrated. Then, they were mounted and viewed with a confocal microscope (DM5500B,Leica, Germany). Finally, the staining densities of the target protein signals in the images were quantified by means of CaseViewer and Pannoramic Viewer software (3DHISTECH Ltd., Hungary). The ratio of integrated optical density of all the positive staining in the field of each photograph was calculated as the mean optical density of the target proteins.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Assay Paraffined tissue sections were pretreated with frozen methanol for 20min and then with frozen methanol/acetic acid (2:1) for another 20min. After washing with PBS, the sections were treated with reagents from the in situ Cell Death Detection Kit, Fluorescein (Roche, USA),as described by the manufacturer’s protocol. Briefly, sections were immersed in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) reaction mixture (50 μL enzyme solution added to 450 μL label solution) at 37℃ for 60min,and nuclei were counterstained with Hoechst (Sigma-Aldrich,USA). After the reaction was stopped by washing with PBS at room temperature, treated sections were viewed and photographed with a confocal microscope (DM5500B, Leica,Germany) with equilibrated parameters. In each area, the numbers of total cells and TUNEL-positive cells were counted and averaged.
Statistical Analysis All results were presented as the mean±standard deviation and analyzed using SPSS 24.0 software. Paired-comparisons were analyzed by one-way analysis of variance (ANOVA). A value of P<0.05 was used to determine the statistical significance.
RESULTS
Clinical Observations During the study, 4 eyes were excluded due to complications, including intra-operative cataract formation and postoperative retinal detachment.Another 4 eyes were then recruited. In the first week, the eyes that received vitrectomy showed corneal edema, exudation in the anterior chamber, which gradually went away and disappeared totally after 4wk. No other apparent abnormality was found after 4wk. In some eyes belonging to the PFO groups, small bubbles of PFOs were visible in the vitreous cavity.
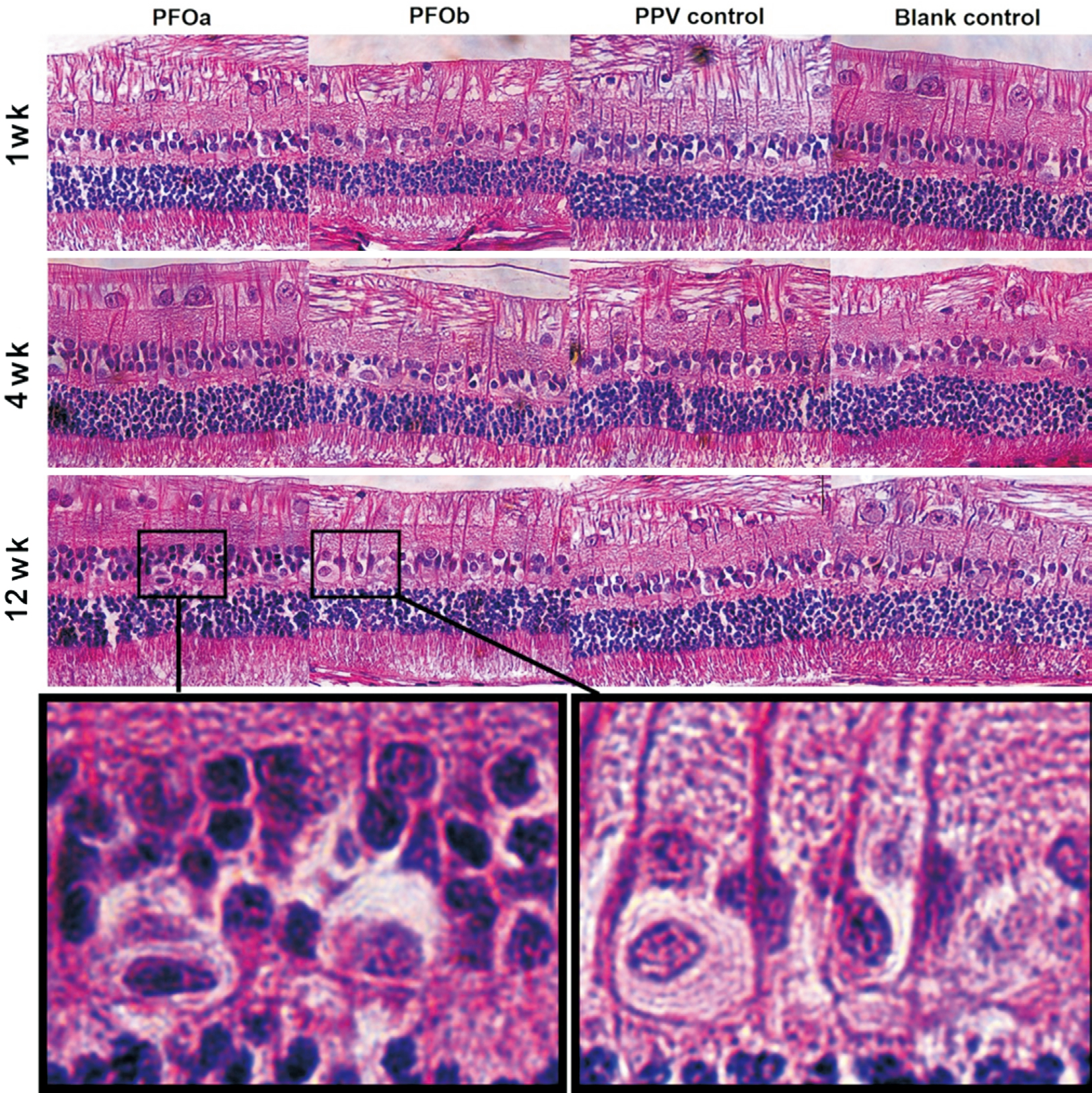
Figure 1 Histological section of the inferior retina at 1, 4 and 12wk after surgery Hematoxylin and eosin, 200×; The frames show the vacuolization in the inner nuclear layer of PFOa group and PFOb group.
Hematoxylin and Eosin Staining Light-microscopic examination revealed an unbroken appearance and distinct layers in the retina at 1 and 4wk after surgery in all four groups (Figure 1). However, at 12wk, slight disarrangement of the outer nuclear layer (ONL) and occasional vacuolization in the inner nuclear layer (INL) of the inferior retina were found similarly in the two PFOs groups (Figure 1), while the structures of the superior retina were relatively uninfluenced(Figure 2). Nuclear counts of the INL and ONL did not differ among the four groups at 1, 4 or 12wk follow-ups (all P>0.05).
Immunohistochemical Findings The H-Score of neither Caspase-3 nor TNF-α was different among the four groups at any time points (all P>0.05). However, compared to the blank control group, the level of IL-6 was found elevated in PFOb group at 4wk after surgery (P<0.05). Additionally, IL-8 was elevated in the PFOa and PPV control groups at 4wk, as well as in all three surgical groups at 12wk (all P<0.05; Figure 3).
TUNEL Assay After 1wk, the apoptotic index (%) was not different among the four groups (P>0.05); however, at 4 and 12wk, all three surgical groups demonstrated a significantly higher apoptotic index (%) than the blank controls (P<0.05;Figures 4 and 5), although the indices were similar among three surgical groups (P>0.05). The TUNEL positive cells were mainly found at the inferior retina in all groups (Figure 2).
DISCUSSION
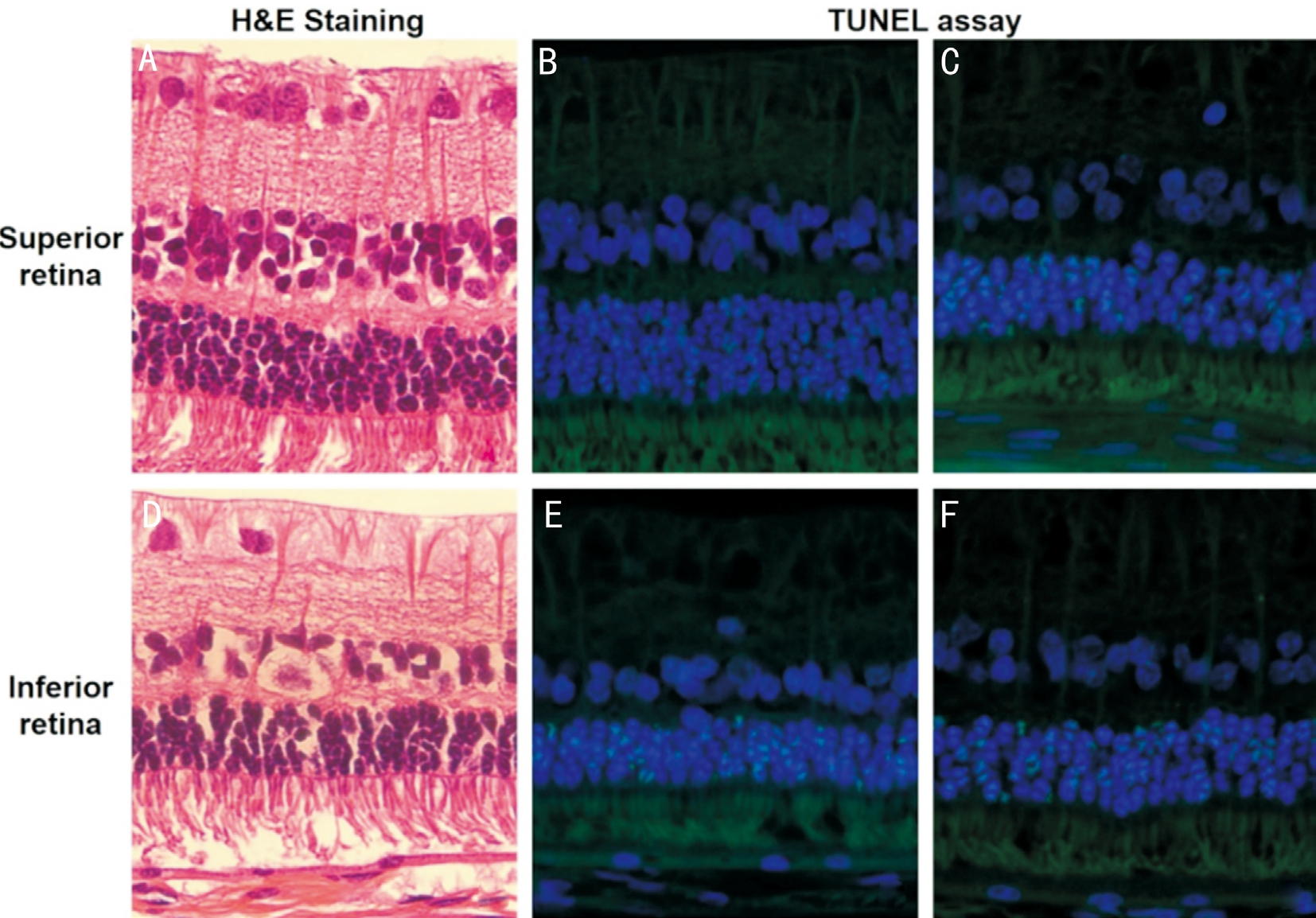
Figure 2 Change of different parts of the retina at 12wk A, B, D and E are samples from the PFOa group, and C and F are samples from PPV group. In the PFOa group: H&E staining showing that the superior retina had normal structure (A), while a slight disarrangement in the inferior retina (D); immunohistochemical staining intensity (TUNEL) was stronger in the inferior area (E) than the superior (B); in the PPV group,immunohistochemical staining intensity (TUNEL) was also stronger in the inferior area (F) than the superior (C).
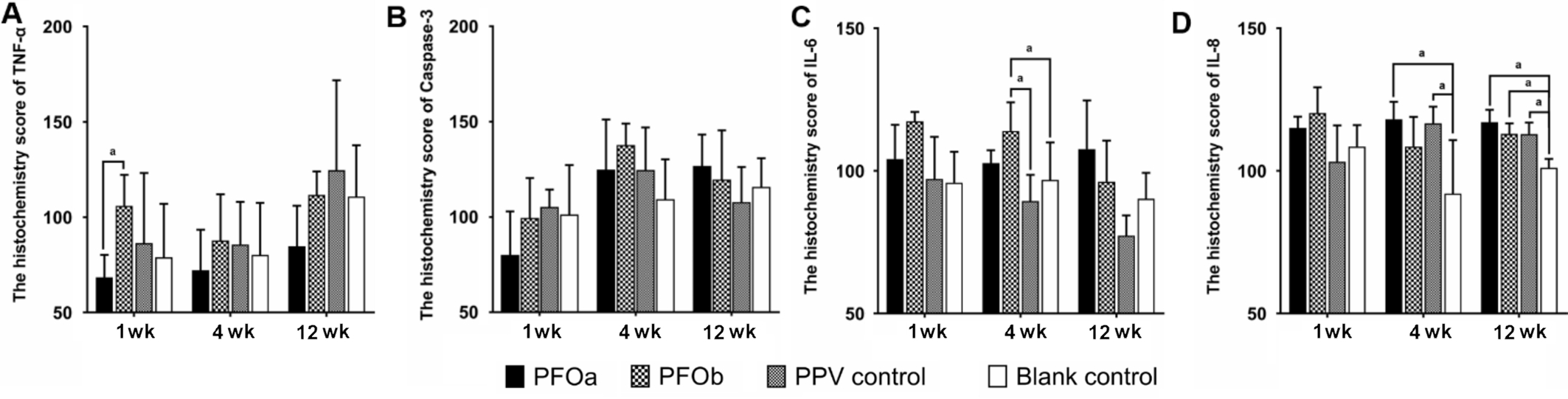
Figure 3 Immunohistochemical staining intensity (H-Score) of TNF-α (A), Caspase-3 (B), IL-6 (C) and IL-8 (D) at different time point The H-Score of neither Caspase-3 nor TNF-α was different among the four groups at any time points. The H-Score of IL-6 was elevated in PFOb group at 4wk after surgery, and the H-Score of IL-8 was elevated in the PFOa and PPV groups at 4wk and in three surgical groups at 12wk.ANOVA test was used to compare measurements between the four groups. aP<0.05.
Among a variety of PFCLs used clinically, PFO[1-3] and perfluorodecalin (PFD, C10F18)[3,5] were the most commonly used. In the present study, the change of the retina over 1-12wk after vitrectomy and residual PFOs was studied in rabbit eyes.The counting of cell nuclei with a standard magnification field had long been used to reflect cell densities of the INL and ONL of the retina[5]. Our results indicate that the three vitrectomy groups had similar cell densities in the INL and ONL layers to those of the controls at all time points, and only slight changes were occasionally found in the two PFO groups at 12wk. This was in accordance with the report of Mackiewicz et al[5] and seemed to support that PFO was well-tolerated for short-term residue.TNF-α is an important pro-inflammatory and pro-apoptotic cytokine[6-8], and IL-6 and IL-8 are pro-inflammatory cytokines.These factors were reported to be elevated in post-vitrectomy human eyes[9-11]. In the present study, TNF-α and IL-6 did not change significantly in the PFO groups, but IL-8 was elevated in all three surgical groups. Interestingly, IL-8 was similarly elevated in all three vitrectomy groups, including the group with simple vitrectomy and BSS tamponade. Additionally, the TUNEL test demonstrated that the apoptotic index (%) was similarly increased in all three surgical groups at 4 and 12wk,with no difference among them. Formerly in Mackiewicz et al’s[5] study, sparse TUNEL-positive cells in the ONL was found similarly in eyes with PFD or BSS filling. Our results were in accordance with theirs. It was reported that PPV could cause damage to the ocular tissues by means of fluctuating intraocular pressure or by effects of infusion or mechanical injury[12-15]. Additionally, our findings further indicate that vitrectomy alone could trigger inflammation and lead to retinal damage; a small amount of PFO residue might not cause further damage.
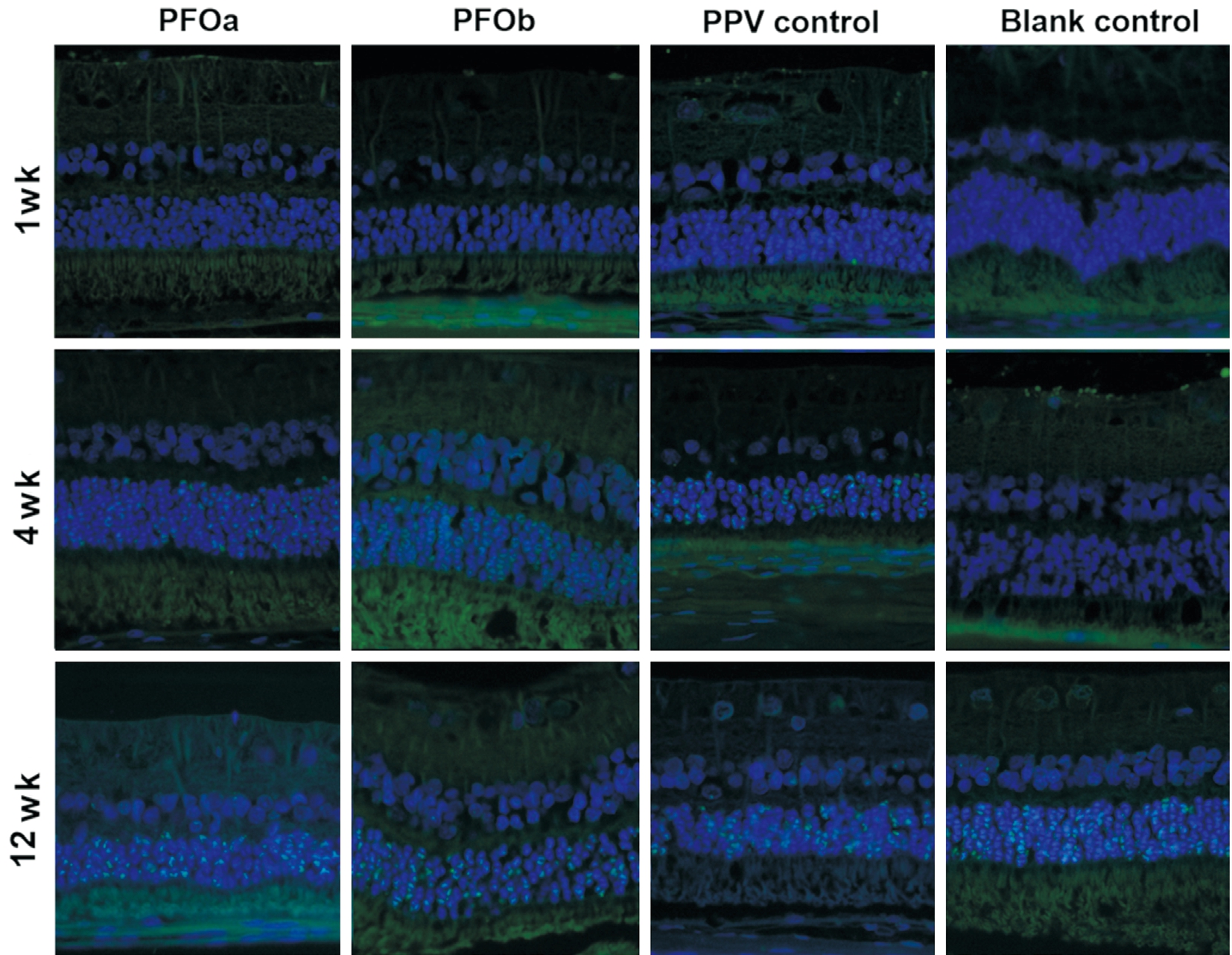
Figure 4 TUNEL positive nuclei of the inferior retina at 1, 4 and 12wk (TUNEL, 200×, merged) The apoptotic index (%) was not different among the four groups (P>0.05) at 1wk, but at 4 and 12wk, all surgical groups demonstrated a higher apoptotic index (%) than in the blank control (P<0.05).
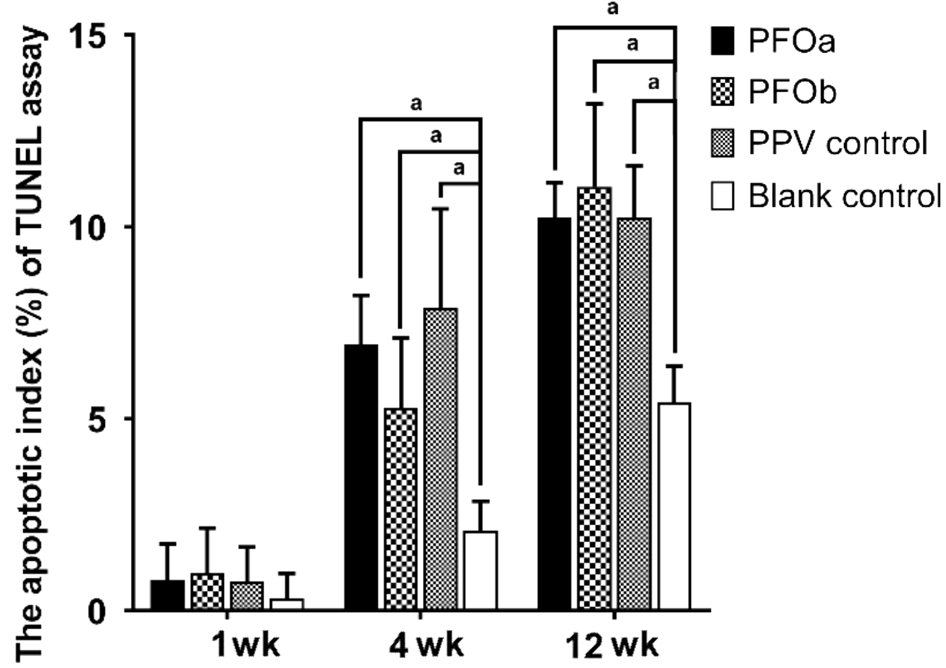
Figure 5 The apoptotic index of the inferior retina (results of the TUNEL assay) The apoptotic index (%) was not different among the four groups at 1wk, while at 4 and 12wk, all three surgical groups demonstrated a significantly higher apoptotic index (%) than in the blank control (P<0.05), but the indices were similar among them(P>0.05). The ANOVA test was used to compare measurements between the four groups. aP<0.05.
It was interesting to notice that the Caspase-3 results were similar among the four groups at all time points, while the apoptotic index (%) was increased in all three surgical groups at 4 and 12wk. Caspase-3, which is regarded as a marker of apoptosis that is based on cytoplasmic localization in cells containing activated caspase, plays a most important role in the process of apoptosis, while TUNEL is a method widely used to identify and quantify for detecting apoptotic DNA fragmentation or excessive DNA breakage in individual cells[16]. It may also label cells having DNA damaged by means other than apoptosis; for example, necroptosis, which was proved to be a way of caspase-independent cell death.Necroptosis cells were stained positively by TUNEL but not Caspase-3[17]. In addition, apoptosis could be mediated via not only a caspase-dependent but also a caspase-independent pathway. The uncorrelated level between the Caspase-3 and TUNEL assays might reveal that the cell death induced by PFCLs tamponade or vitrectomy is mediated via a caspaseindependent pathway.
As PFOs are heavier than water, it was not surprising that disarrangement and vacuolization of retinal layers were found in the inferior retina. However, in the simple vitrectomy group,positive TUNEL cells were also found predominantly in the inferior retina. The PFO used during the surgery might be responsible. On the other hand, the results of other studies and those of our study[9-10] indicate that inflammatory factors and other toxicity substances were increased postoperatively.Proteins are slightly soluble but disperse in the form of colloids in water[18-19]. As proteins, which are heavier than water, could precipitate at the inferior retina and lead to the damage prominently found at the inferior. On the other hand,most TUNEL positive cells were found in the ONL of the retina. Stolba et al[20] and Mackiewicz et al[5] also found that perfluorocarbon could lead to cell loss and nuclear drop-downs of the ONL, or sparse TUNEL-positive cells in the ONL.Although our results are similar, the reason why the ONL is more vulnerable than other layers of the retina is still unclear.In this study, we used only histopathologic and molecular methods but no functional measurement, the disarrangement of the ONL and some vacuolization of the INL might be correlated with some functional change. Future study included electroretinogram or other functional evaluation might further improve our understanding.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Key Research &Development Plan (No.2017YFC0108200); the Shanghai Committee of Science and Technology (No.16140901000;No.13430710500; No.15DZ1942204).
Conflicts of Interest: Li QC, None; Yu J, None; Jiang CH,None; Zhu HH, None; Liu K, None; Zhao JC, None.
1 Chang S, Sparrow JR, Iwamoto T, Gershbein A, Ross R, Ortiz R.Experimental studies of tolerance to intravitreal perfluoro-n-octane liquid.Retina 1991;11(4):367-374.
2 Eckardt C, Nicolai U, Winter M, Knop E. Experimental intraocular tolerance to liquid perfluorooctane and perfluoropolyether. Retina 1991;11(4):375-384.
3 Augustin AJ, Spitznas M, Koch FH, Böker T, Lutz J. Local effects of different perfluorochemical agents. Graefes Arch Clin Exp Ophthalmol 1995;233(1):45-47.
4 Reza AT. Postoperative Perfluro-N-Octane tamponade for complex retinal detachment surgery. Bangladesh Med Res Counc Bull 2014;40(2):63-69.
5 Mackiewicz J, Maaijwee K, Lüke C, Kociok N, Hiebl W, Meinert H,Joussen AM. Effect of gravity in long-term vitreous tamponade: in vivo investigation using perfluorocarbon liquids and semi-fluorinated alkanes.Graefes Arch Clin Exp Ophthalmol 2007;245(5):665-675.
6 Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AH, Ugel S, Sasso MS, Qualls JE, Kratochvill F, Zanovello P, Molon B, Ries CH, Runza V,Hoves S, Bilocq AM, Bindea G, Mazza EM, Bicciato S, Galon J, Murray PJ, Bronte V. T cell cancer therapy requires CD40-CD40L activation of tumor necrosis factor and inducible nitric-oxide-synthase-producing dendritic cells. Cancer Cell 2016;30(4):651.
7 Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA 2017;318(17):1679.
8 West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factorneutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017;23(5):579-589.
9 Gu RP, Zhou M, Jiang CH, Yu J, Xu GZ. Elevated concentration of cytokines in aqueous in post-vitrectomy eyes. Clin Exp Ophthalmol 2016;44(2):128-134.
10 Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS,Abrams GW, Kumar A. Assessment of neurotrophins and inflammatory mediators in vitreous of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 2017;58(12):5594-5603.
11 Takeuchi M, Sato T, Sakurai Y, Taguchi M, Harimoto K, Karasawa Y, Ito M. Association between aqueous humor and vitreous fluid levels of Th17 cell-related cytokines in patients with proliferative diabetic retinopathy. PLoS One 2017;12(5):e0178230.
12 Hirata A, Yonemura N, Hasumura T, Murata Y, Negi A. Effect of infusion air pressure on visual field defects after macular hole surgery. Am J Ophthalmol 2000;130(5):611-616.
13 Oz O, Gürelik G, Akyürek N, Cinel L, Hondur A. A short duration transient ischemia induces apoptosis in retinal layers: an experimental study in rabbits. Eur J Ophthalmol 2005;15(2):233-238.
14 Yang SS, McDonald HR, Everett AI, Johnson RN, Jumper JM, Fu AD. Retinal damage caused by air-fluid exchange during pars plana vitrectomy. Retina 2006;26(3):334-338.
15 Minami M, Oku H, Okuno T, Fukuhara M, Ikeda T. High infusion pressure in conjunction with vitreous surgery alters the morphology and function of the retina of rabbits. Acta Ophthalmol Scand 2007;85(6):633-639.
16 Lozano GM, Bejarano I, Espino J, González D, Ortiz A, García JF,Rodríguez AB, Pariente JA. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev 2009;55(6):615-621.
17 Zhang T, Zhang Y, Cui MY, Jin L, Wang YM, Lv F, Liu YL, Zheng W, Shang HB, Zhang J, Zhang M, Wu HK, Guo JJ, Zhang XQ, Hu XL,Cao CM, Xiao RP. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 2016;22(2):175-182.
18 Belloni L. Yes, pair correlations alone do determine sedimentation profiles of highly charged colloids. J Chem Phys 2005;123(20):204705.
19 Dubois M, Schönhoff M, Meister A, Belloni L, Zemb T, Möhwald H.Equation of state of colloids coated by polyelectrolyte multilayers. Phys Rev E Stat Nonlin Soft Matter Phys 2006;74(5 Pt 1):051402.
20 Stolba U, Krepler K, Velikay-Parel M, Binder S. The effect of specific gravity of perfluorocarbon liquid on the retina after experimental vitreous substitution. Graefes Arch Clin Exp Ophthalmol 2004;242(11):931-936.