INTRODUCTION
Ocular graft-versus-host disease (GVHD) affects about 60%of the allogeneic hematopoietic stem cell transplantation(HSCT) recipients and is associated with ocular discomfort,vision loss, and quality of life[1-3]. Patients commonly complain of irritation, foreign body sensation, photophobia, conjunctival hyperemia, excessive tearing secondary to reflex secretion,and mucoid discharge, and they often require complex therapies including tear supplementations, topical or systemic immunosuppressant, and surgical treatment[3-4]. There are several types of ocular GVHD such as chronic, acute, lateonset acute, and overlap syndromes. The most common type is chronic ocular graft-versus-host disease (coGVHD).
Clinical manifestations of coGVHD are diverse, but the most frequent one is dry eye disease (DED), observed in 69%-77% of patients with coGVHD[5-6]. The pathological effects of coGVHD on the tear film are known to be distinct and more deleterious than other biological causes of DED[5-8]. All of lacrimal functional unit and tear film layers can be affected by coGVHD. Both the major and accessory lacrimal glands can be influenced by mononuclear cell infiltration, and resulted in decrease in tear production. The conjunctival disease contains a wide variety from mild hyperemia to pseudomembranous and cicatrizing conjunctivitis and loss of goblet cells. Changes in the lid margin with blepharitis and meibomian glands loss lead to defective lipid layer, tear film instability, and increased evaporation[7-8].
Meibomian gland dysfunction (MGD) is found to be the second most frequent complication and the prevalence of MGD in coGVHD is reaching to 47.8%[9]. Previous studies reported functional impairment of the meibomian gland (decreased secretion quality and quantity) and chronic blepharitis with obstruction of the meibomian gland opening[6-8]. Recently,several studies have reported the morphological damage of the meibomian gland in coGVHD. Ban et al[9] demonstrated excessive fibrosis, atrophy, and loss of gland area using in vivo confocal microscopy and Engel et al[10] reported the loss of the meibomian gland area using infrared images of upper eyelid.Similarly, recent studies have also demonstrated that MGD and Sjögren’s syndrome (SS) are related to each other[11-13]. Sullivan et al[11] reported that patients with primary and secondary SS had meibomian gland orifice metaplasia, an increased number of occluded meibomian gland orifices, and a reduced quality of meibomian gland secretions. Chen et al[12] also described that primary SS patients showed higher meibomian gland loss,as well as higher lid abnormality score. In addition, we also previously demonstrated that patients with SS had more severe MGD with lower mean meiboscore and meibomian gland expressibility than patients with non-SS[13].
Although previous studies have reported impaired of meibomian gland structure and function[9-10], little knowledge is available on the amount of meibomian gland damage in coGVHD and no studies have directly compared it to other biologic causes of DED such as SS and MGD.
Therefore, we investigated the damage in meibomian gland in patient with DED associated with coGVHD and compared it with SS, a major form of aqueous deficient DED and MGD, a common cause of evaporative DED. In addition, we analyzed the correlation of the morphological and functional features of meibomian gland with parameters of ocular surface and disease severity in coGVHD.
SUBJECTS AND METHODS
Ethical Approval This study followed the tenets of the Declaration of Helsinki and local ethics committee approval was also obtained. Informed consent was obtained from all participants.
A retrospective analysis of data was performed on the records of 135 subjects who visited the Department of Ophthalmology,Chonnam National University Hospital between April 2012 and March 2016.
Subjects Thirty eyes from 30 patients with a clinical diagnosis of DED associated with coGVHD were included in this study. The diagnostic criteria of DED was as follows: the Schirmer test without anesthesia (Schirmer I test) showed less than 5 mm/5min; tear film breakup time (TFBUT) indicated less than 5s; moderate-to-severe superficial punctate keratitis,defined as a corneal staining score of more than 2; and OcularSurface Disease Index (OSDI) score of more than 30 (scale of 0-59). The diagnosis and evaluation of severity of coGVHD was based on modified International Chronic Ocular Criteria Ocular GVHD (ICCGVHD) criteria (Table 1)[14-15]. In brief,this criterion uses the Schirmer score without anesthesia,conjunctival injection, corneal staining, and the Ocular Surface Disease Index (OSDI) to obtain an overall score and categorize the disease as none (scores 0), mild (scores 1-4), moderate(scores 5-8), or severe (scores 9-11). The patients with coGVHD were compared with randomly selected age-matched(within ±3y) patients with SS, age- and sex-matched patients with MGD or normal controls. Patients with SS could not be matched with sex as there were not sufficient male SS patients.SS was diagnosed according to the proposed criteria by the American College of Rheumatology/European League Against Rheumatism and confirmed by both an ophthalmologist and a rheumatologist[16]. MGD was diagnosed by an experienced single ophthalmologist (Yoon KC) based on the presence of ocular surface-related symptoms, one or more lid margin abnormalities (plugged MG orifices, vascular engorgement,irregular lid margin, and anterior or posterior malposition of the mucocutaneous junction), and decreased meibum quality and expression[17]. All control participants had no anterior segment abnormality by slit-lamp examination with ocular surface tests and no complaint of ocular surface irritation.
Table 1 Modified International Chronic Ocular GVHD Consensus Group criteria
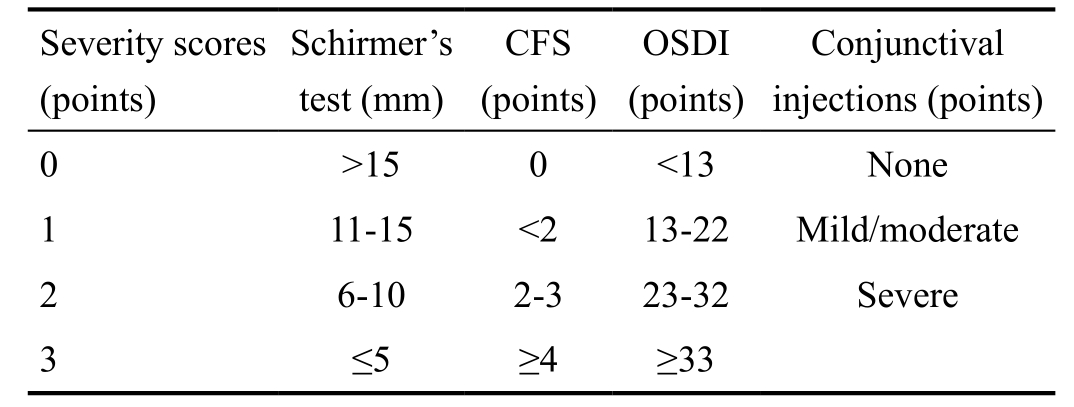
CFS: Corneal fluorescein staining; OSDI: Ocular Surface Disease Index;Diagnosis and severity classification: total score (points), none: 0;mild: 1-4; moderate: 5-8; severe: 9-11.
?
Exclusion criteria of all the subjects was age less than 18y,presence of ocular or systemic disease, subjects unable to complete the questionnaire, ocular surgery within the last 3mo before eye examination, use of topical or systemic medications that may affect the ocular surface, or contact lens wear.
Clinical Evaluation All subjects underwent a clinical evaluation, which is divided into two parts: ocular surface examination and meibomian gland evaluation. Ocular surface examination was followed by meibomian gland evaluation.Before clinical evaluations, patients were required to complete OSDI questionnaire. This questionnaire gives a range of 0 (no symptoms) till 100 (severe symptoms)[18]. All evaluations were conducted by the same examiner.
Ocular Surface Examination Ocular surface examination consisted of Schirmer test without anesthesia, TFBUT test,and ocular surface vital staining. Schirmer test was performed with the patient’s eyes closed for 5min without anesthesia. The strips of Schirmer test were positioned between the lateral and middle third of the lower eyelid. The length of wetting strip was determined using the millimeter scale. For measurement of TFBUT, the interval between the appearance of the first dry spot and a complete blink was recorded after the instillation of fluorescein. TFBUT was evaluated three times, and the mean value was recorded. The double vital staining method was adopted for the ocular surface staining scores[19]. After the instillation of mixed 1% unpreserved fluorescein (Alcon,Fort Worth, TX, USA) with 1% unpreserved lissamine green(Leiter’s Pharmacy, San Jose, CA, USA) solution into the culde-sac, ocular surface staining was evaluated through a white light and cobalt blue filter. Ocular surface was classified into 3 zones: nasal conjunctiva, cornea, and temporal conjunctival areas. The score of ocular surface staining ranged from 0 to 3 for each zone. The presence of sparse staining in a zone was rated as 1 point; not covering the entire zone with frequent puncta as 2 points, and punctuate staining throughout the entire zone as 3 points. A score of 0-3 points for corneal fluorescein staining and a score of 0-6 for conjunctival lissamine green staining.
Meibomian Gland Evaluation Meibomian gland evaluation consisted of gland morphology (gland dropout) and function (meibum expressibility and quality) and lid margin assessment[20]. For evaluating the gland dropout, infrared images of the upper and lower lid meibomian glands were documented using the Oculus Keratograph 5M (Oculus GmbH,Wetzlar, Germany) and meibomian gland dropout (meiboscore)was classified using a four-grade scale (0-3): degree 0, no loss of meibomian gland; degree 1, <33% of dropout zone;degree 2, 33%-66% of dropout zone; and degree 3, >66% of dropout zone. Upper and lower eyelids scores were added, to calculate a total meiboscore (0-6) for each eye[21]. The meibum expressibility score (MES) was graded by counting the central eight expressed meibomian gland orifices of the lower lid as follows: Grade 0 means all glands are expressible; Grade 1 means 3-4 glands are expressible; Grade 2 means 1-2 glands expressible; Grade 3 means no gland is expressible. The meibum quality score (MQS) was graded using a 0-3 grading scale as follows: Grade 0, normal, clear oil expressed; Grade 1, opaque, diffusely turbid, normal viscosity; Grade 2, opaque,increased viscosity; Grade 3, inspissated (thick, toothpaste-like appearance) meibum or non-expressible glands. Lid margin abnormality scores were documented in accordance with the existence of the following four signs: vascular engorgement,glandular orifices obstruction, lid margin irregularity, and anterior or posterior malposition of the mucocutaneous junction. The score was ranged from 0 to 4.
Statistical Analysis Statistical analysis was performed using a PASW for Windows (version 18.0; SPSS, Chicago, IL, USA).For each subject, 1 eye was chosen randomly for statistical analysis. Comparisons of parametric values among groups were performed using one-way analysis of variance (ANOVA).Comparisons of non-parametric values among groups were performed with the Kruskal-Wallis test. Tukey honest significant difference tests (for parametric variables) and Bonferroni adjustment after Mann-Whitney U tests (for nonparametric variables) were used as post hoc tests for multiple comparisons between the groups. The correlations between meibomian gland parameters with ocular surface parameters and severity score by ICCGVHD criteria in the coGVHD group were calculated using Spearman correlation analysis.P<0.05 was considered statistically significant.
RESULTS
A total of 135 subjects were included for the study. The coGVHD group (14 males and 16 females, average age 48.25±11.74y), the SS group (5 males and 30 females, average age 48.27±10.97y), the MGD group (16 males and 19 females,average age 48.31±12.12y), and control group (16 males and 19 females, average age 48.45±11.48y). The demographic information of the coGVHD group is summarized in Table 2.Totally 24 (80.00%) patients had both systemic and ocular GVHD, 6 (20.00%) had only ocular GVHD. According to ICCGVHD criteria, 6 (20.00%) patients was mild grade, 20(66.67%) was moderate grade, and 4 (13.33%) was severe grade.
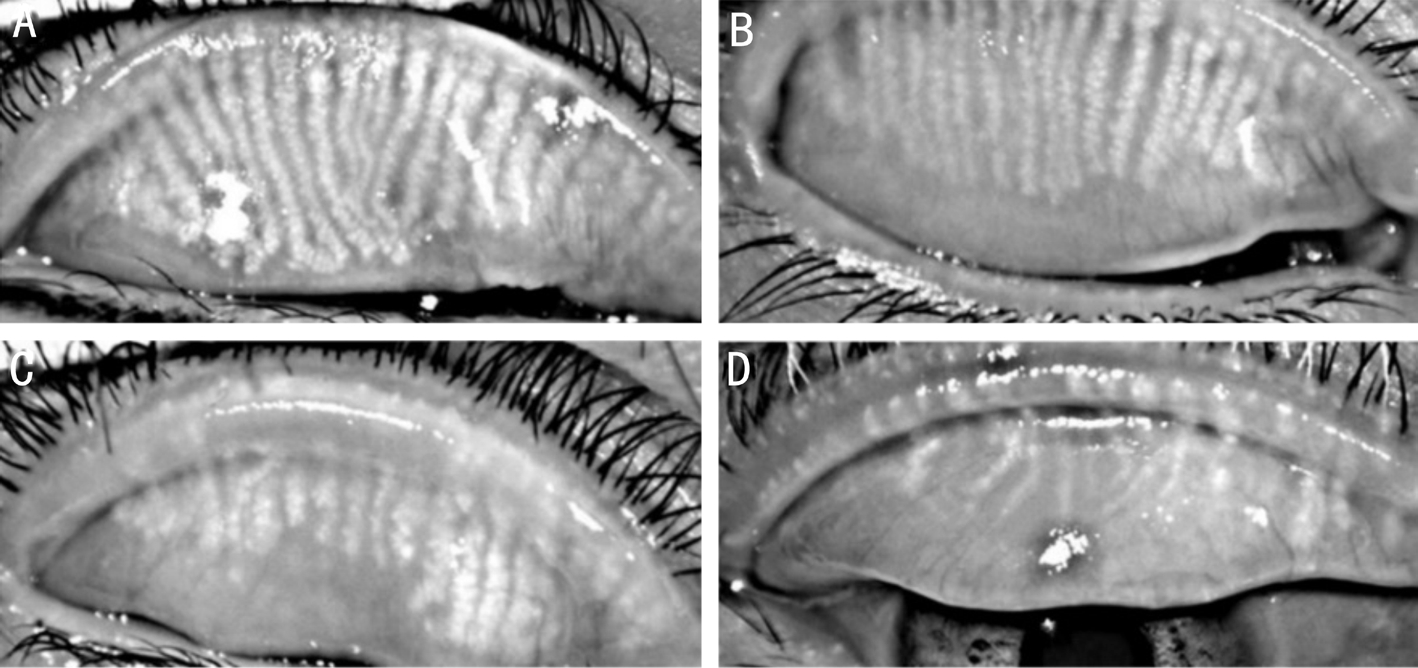
Figure 1 Representative meibographic images of the 4 groups A: Control group; B: SS group; C: MGD group; D: Chronic ocular GVHD group.
Table 2 Demographic information of patient with dry eye associated with chronic ocular GVHD
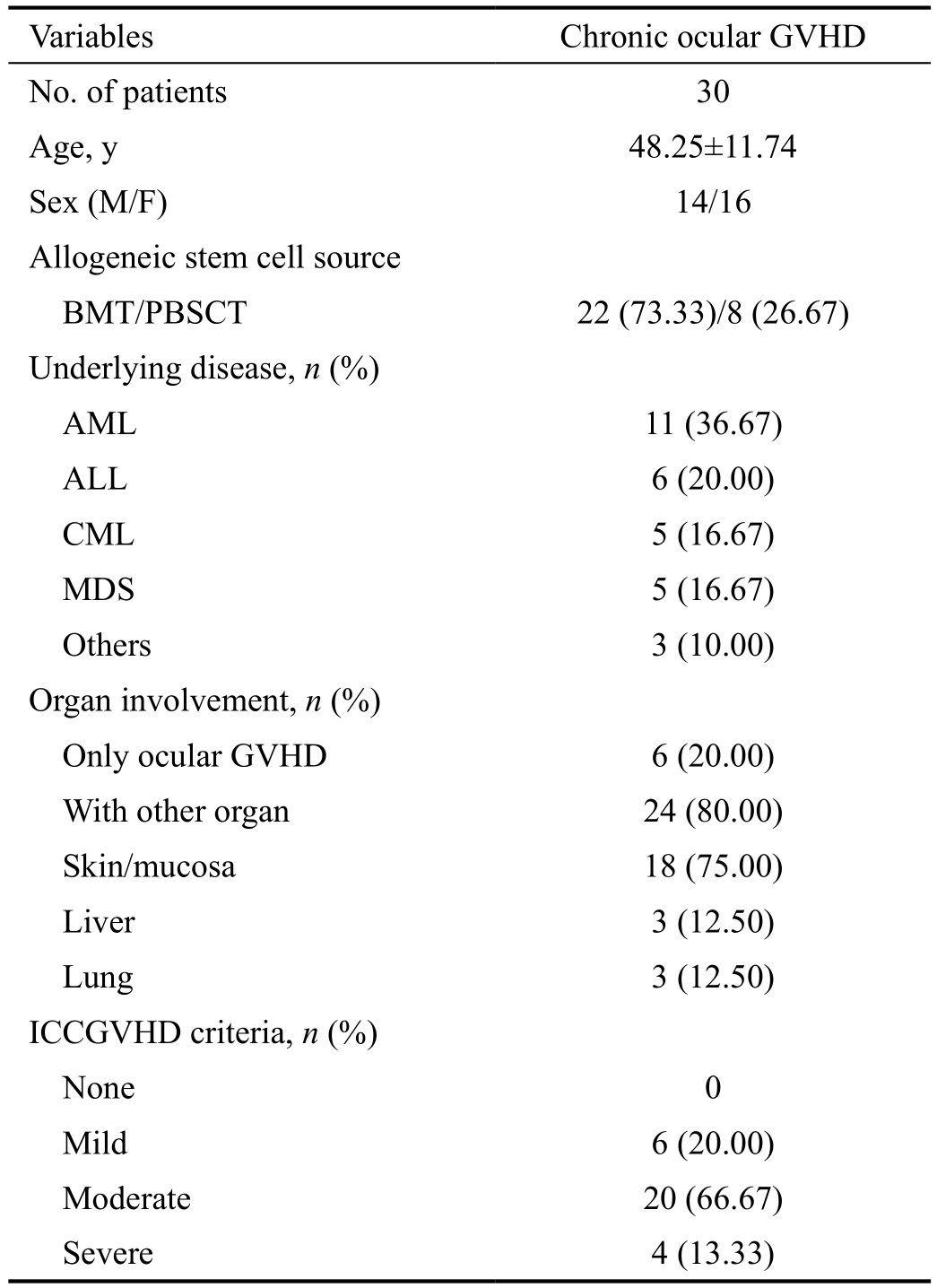
Values are presented as mean±standard deviation or n (%). GVHD:Graft-versus-host disease; BMT: Bone marrow transplantation;PBSCT: Peripheral blood stem cell transplantation; AML: Acute myeloid leukemia; ALL: Acute lymphoblastic leukemia; CML:Chronic myeloid leukemia; MDS: Myelodysplastic syndrome;ICCGVHD: International Chronic Ocular GVHD Consensus Group.
Variables Chronic ocular GVHD No. of patients 30 Age, y 48.25±11.74 Sex (M/F) 14/16 Allogeneic stem cell source BMT/PBSCT 22 (73.33)/8 (26.67)Underlying disease, n (%)AML 11 (36.67)ALL 6 (20.00)CML 5 (16.67)MDS 5 (16.67)Others 3 (10.00)Organ involvement, n (%)Only ocular GVHD 6 (20.00)With other organ 24 (80.00)Skin/mucosa 18 (75.00)Liver 3 (12.50)Lung 3 (12.50)ICCGVHD criteria, n (%)None 0 Mild 6 (20.00)Moderate 20 (66.67)Severe 4 (13.33)
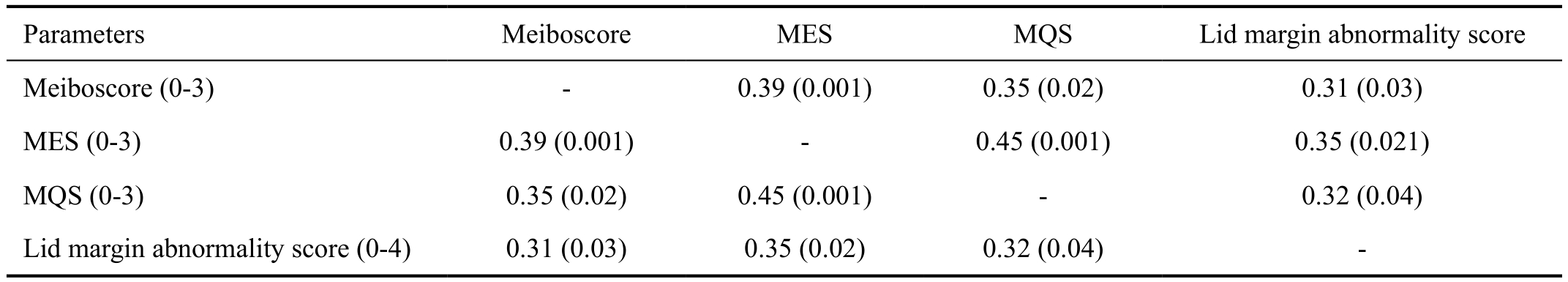
The results of clinical evaluation of 4 groups are summarized in Table 3. Compared with the other 3 groups, the control group had a significantly lower OSDI score, higher Schirmer test results, TFBUT, and lower corneal and conjunctival staining score (all P<0.05). Also, the control group had a lower meiboscore, MES, MQS, and lid margin abnormality score(all P<0.05). In ocular surface examination, the coGVHD group had a significantly higher OSDI, lower TFBUT, and higher conjunctival staining score than the SS group and MGD groups (all P<0.05). Although Schirmer test value was lower and corneal staining scores was higher in the coGVHD group compared to the MGD group (P=0.03 and P=0.002), there was no significant difference with those of the SS group (P=0.57 and P=0.10). In meibomian gland evaluation, the coGVHD group had a higher meiboscore, MES, and MQS than the SS group and MGD groups (all P<0.05). The lid margin abnormality score in the coGVHD group was higher than that of the SS group (P=0.002), but did not differ from the MGD group (P=0.27).Representative images of meibography in 4 groups are shown in Figure 1. The patient with coGVHD showed not only the most severe loss of gland area but also the most pathologic changes of gland structure such as shortening, distortion, and dilation.To analyze the associations between morphological and functional damage of the meibomian gland in the coGVHD group, the correlations among the results of meibomian gland evaluation were investigated (Table 4). Meiboscore was positively correlated with MES (r=0.39, P=0.001) and MQS (r=0.35, P=0.02). Also, there was a positive correlation between meiboscore and lid margin abnormality score (r=0.31,P=0.03). The correlations between the meibomian gland parameters and ocular surface parameters in the coGVHD group are shown in Table 5. Meiboscore was correlated with OSDI (r=0.35, P=0.02) and TFBUT (r=-0.43, P=0.001).MES and MQS were significantly correlated with the corneal staining score (r=0.31, P=0.03; r=0.33, P=0.03). Lid margin abnormality score was also correlated with conjunctival staining score (r=0.32, P=0.04).
To investigate the relationship between meibomian gland damage and disease severity of coGVHD, correlation analysis between each of the meibomian gland assessments and disease severity score by ICCGVHD criteria was performed. There was a significant correlation between all of the meibomian gland parameters and ICCGVHD score (meiboscore, r=0.62;MES, r=0.47; MQS, r=0.47; lid margin abnormality score,r=0.55; all P<0.05) and meiboscore was the most highly correlated with disease severity score (Figure 2).
DISCUSSION
In this study, we observed more severe ocular discomfort(OSDI) and worse ocular surface parameters (TFBUT and ocular surface staining) in the coGVHD group than the SS and MGD groups. Schirmer test value in the coGVHD group was lower than in the MGD group, but there was no difference to the SS group, suggesting the compromised tear production in the coGVHD group. We also demonstrated that patients with DED associated with coGVHD tend to show more severe morphological (gland dropout) and functional (poor meibum quality and quantity) meibomian gland damage than that of patients with other types of DED, e.g. SS and MGD. In addition, meibomian gland damage was associated with oculardiscomfort, unstable tear film, and ocular surface staining.Finally, meibomian gland parameters (meiboscore, MES,MQS, and lid margin abnormality score) had a significant relationship to disease severity of coGVHD.
Table 3 Comparisons of ocular surface examination and meibomian gland assessment among the control, SS, MGD, and chronic ocular GVHD group
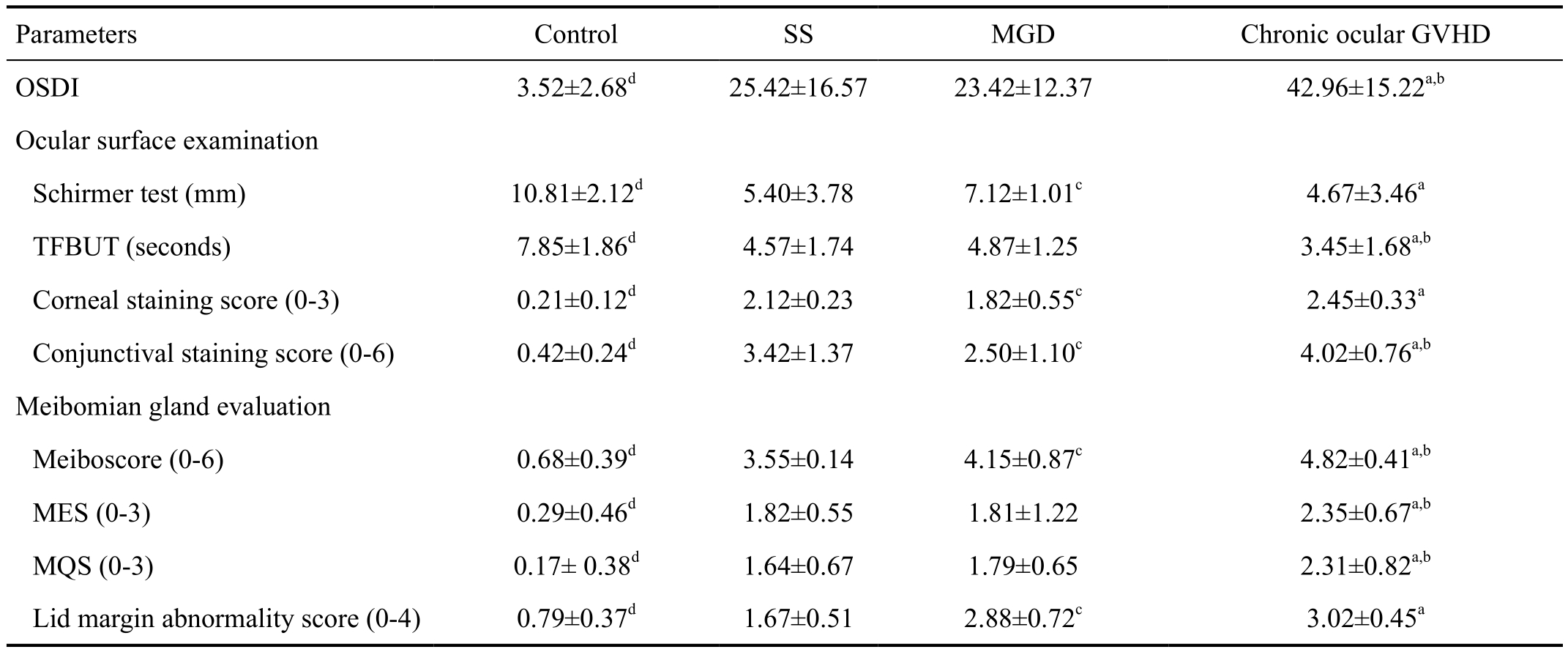
Values are presented as mean±standard deviation. OSDI: Ocular Surface Disease Index; TFBUT: Tear film breakup time; MES: Meibum expressibility score; MQS: Meibum quality score. aP<0.05 in chronic ocular GVHD group vs MGD group; bP<0.05 in chronic ocular GVHD group vs SS group; cP<0.05 in SS group vs MGD group; dP<0.05 in control group vs other three groups.
Parameters Control SS MGD Chronic ocular GVHD OSDI 3.52±2.68d 25.42±16.57 23.42±12.37 42.96±15.22a,b Ocular surface examination Schirmer test (mm) 10.81±2.12d 5.40±3.78 7.12±1.01c 4.67±3.46a TFBUT (seconds) 7.85±1.86d 4.57±1.74 4.87±1.25 3.45±1.68a,b Corneal staining score (0-3) 0.21±0.12d 2.12±0.23 1.82±0.55c 2.45±0.33a Conjunctival staining score (0-6) 0.42±0.24d 3.42±1.37 2.50±1.10c 4.02±0.76a,b Meibomian gland evaluation Meiboscore (0-6) 0.68±0.39d 3.55±0.14 4.15±0.87c 4.82±0.41a,b MES (0-3) 0.29±0.46d 1.82±0.55 1.81±1.22 2.35±0.67a,b MQS (0-3) 0.17± 0.38d 1.64±0.67 1.79±0.65 2.31±0.82a,b Lid margin abnormality score (0-4) 0.79±0.37d 1.67±0.51 2.88±0.72c 3.02±0.45a
Table 4 Correlations between meibomian gland parameters in chronic GVHD patients r (P)
MES: Meibum expressibility score; MQS: Meibum quality score.
Parameters Meiboscore MES MQS Lid margin abnormality score Meiboscore (0-3) - 0.39 (0.001) 0.35 (0.02) 0.31 (0.03)MES (0-3) 0.39 (0.001) - 0.45 (0.001) 0.35 (0.021)MQS (0-3) 0.35 (0.02) 0.45 (0.001) - 0.32 (0.04)Lid margin abnormality score (0-4) 0.31 (0.03) 0.35 (0.02) 0.32 (0.04) -
Table 5 Correlations between ocular surface and meibomian gland parameters in chronic ocular GVHD r (P)
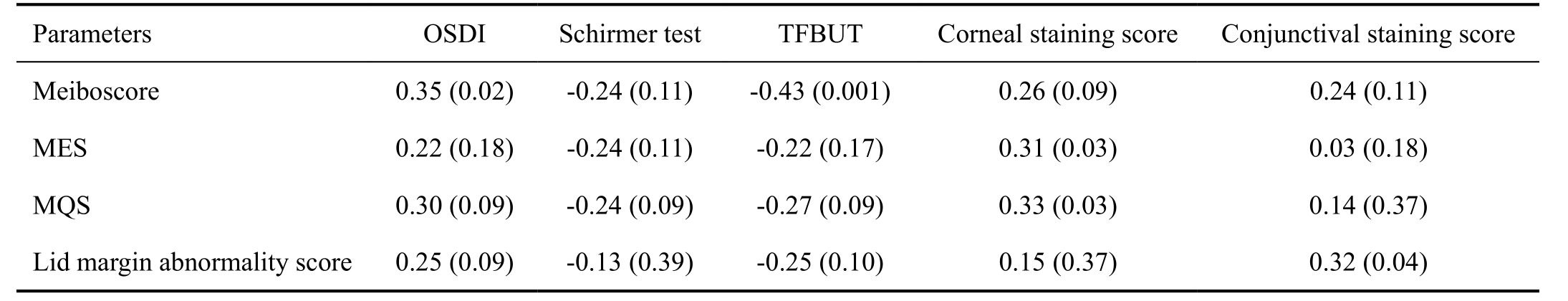
OSDI: Ocular surface disease index; TFBUT: Tear film breakup time; MES: Meibum expressibility score; MQS: Meibum quality score.
?
Prior studies revealed that excessive fibrosis and tissue atrophy are remarkable histological features of lacrimal gland in coGVHD[22]. Although both coGVHD and SS are autoimmune disorder with chronic inflammation of lacrimal gland and display aqueous deficient DED, pathogenesis is different from each other. Lymphocytes, predominantly T cells, were observed mostly in the periductal lesions of the lacrimal gland from coGVHD patients, whereas B cells were the predominantly infiltrated cells in the acinar lesions of the lacrimal gland from SS patients[23]. Further studies with quantitatively assessment of tear production may be needed to more objectively evaluate the impaired tear production in patients with coGVHD.
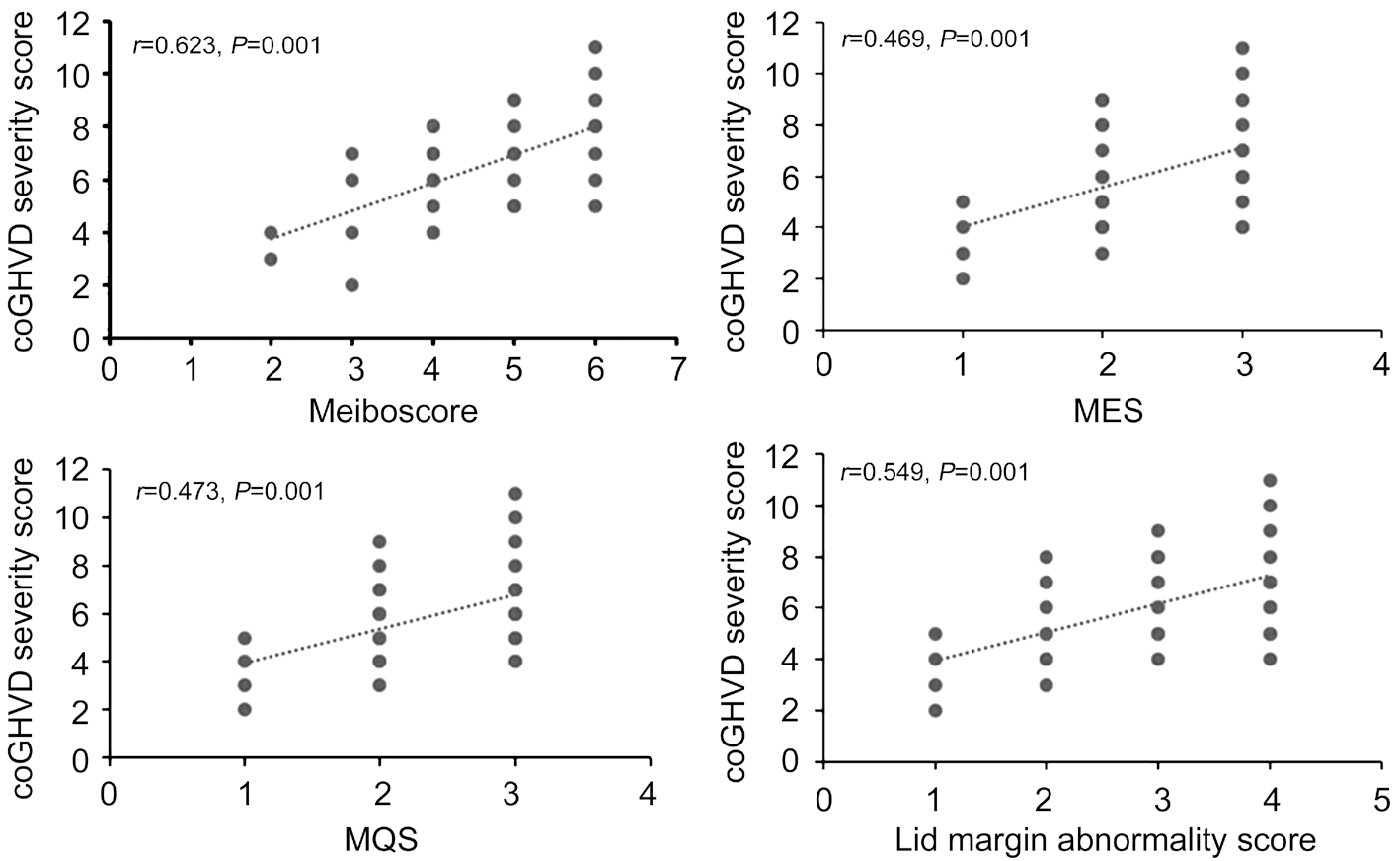
Figure 2 Correlations between meibomian gland parameters and chronic ocular GVHD severity scores There was a significant correlation between all of the meibomian gland parameters and ICCGVHD score and meiboscore was the most highly correlated with disease severity score.
In addition, recent studies reported compromised meibomian gland functions and increased tear evaporation in coGVHD.Khanal and Tomlinson[24] reported the increased tear evaporation rate and osmolality and Engel et al[10] found a loss of the meibomian gland area in coGVHD patients. In this study,coGVHD patients showed more severe morphologic (gland loss) and functional (meibum expressibility and quality)meibomian gland damage compared to age- and sex-matched patients with MGD or age-matched patients with SS.Furthermore, more severe individual gland structural alteration(shortening, distortion, and dilation) as well as entire gland loss and loss of gland area were observed in coGVHD than other types of DED. These results can be explained by different pathomechanism of meibomian gland loss damage among the DED groups. Chronic ocular GVHD is a multifactorial and immunological process and may affect the meibomian gland structure more severely than other types of DED. In histologic study, ductal epithelial hyperkeratinization, shedding of keratinized material into the glandular ducts leading to obstructions of the orifice, and ultimate cystic dilatation with atrophy were found in MGD[25]. On the other hand,ductal epithelia destruction due to lymphocyte aggregation,sloughing of epithelial cells due to lymphocyte infiltration or pseudomembrane formation, and eventual extensive fibrosis around the orifice, ductules, ducts, and acini of the meibomian gland were observed in coGVHD[9]. These results suggesting the coGVHD group may have combined features of aqueous deficient and evaporative DED, and consequently, showed more severe ocular discomfort and poorer ocular surface condition than the SS or the DED group.
In our results, anatomical changes are closely associated with impaired function of the meibomian gland in coGVHD. Loss of the meibomian gland leads to deterioration in the quantity and quality of meibum, which consequently resulted in increased tear film instability and decreased TFBUT. Moreover, eyelid margin abnormalities were accompanied by meibomian gland damage in the course of coGVHD in this study. In addition to atrophy and irregularity of the eyelid margins, loss of lashes,stenosis of the lacrimal puncti, and entropion may aggravate the ocular surface damage and worsen the ocular discomforts in coGVHD.
To assess whether there is a relationship between meibomian gland damage and coGVHD severity, we evaluated the association between meibomian gland parameters (meibosocore,MES, MQS, and lid margin abnormality score) and coGHVD severity score. Interestingly, our results showed a strong positive correlation between the each of meibomian gland parameters and coGVHD severity score according to the ICCGVHD criteria. In other words, a progressive worsening occurred in all these parameters as the severity of the disease increased. Recently, Engel et al[10] showed a decreasing trend in meibomian gland area in the eyes with more severe affected by coGVHD. However, they failed to show statistically significant correlation between meibomian gland loss and disease severity evaluated by National Institutes of Health(NIH) consensus criteria. The NIH scoring system was originally developed to simplify the diagnosis of coGVHD for non-ophthalmologists, such as oncologists. It is easy to understand but may not be able to exactly evaluate the severity of the disease. There is an innate limitation in a scale that depends on behavior rather than objective findings. On the other hand, newly developed ICCGVHD criteria included patient symptom scores, ocular surface staining, and tear film dynamic and based on more objective findings, which are closely related to meibomian gland characteristics in patients with coGVHD.
In addition, there is a possibility of meibomian gland damage even before development of coGHVD[10]. Meibomian gland loss can occur prior to autologous stem cell transplantation,due to infiltration of the glands by tumor cells, chemotherapy or irradiation. Generally, meibomian gland loss occurs in early stage of disease and rapidly progresses and coGVHD is regarded as a precursor of systemic chronic GVHD[6]. In this context, our present data suggest that meibomian gland loss can be used as a marker of disease progression and therefore,evaluation of meibomian gland may enable us to early detect the disease progression and timely manage ocular and systemic GVHD.
There are several limitations of this study. First, we did not evaluate the possible confounding impact of various systemic conditions such as the presence of systemic GVHD, underlying diseases for allogeneic stem cell transplantation, source of stem cell, or conditioning regimen on the meibomian gland in coGVHD. Second, the SS group included more female subject than the other group in this study. Generally, lipid synthesis of meibomian gland was affected by hormone and especially,androgens induce a substantial influence on the function and structure of the meibomian gland[26]. Even though the coGVHD group had more male subjects than SS group in this study,meibomian gland damage in the coGVHD group was more severe than that of the SS group. There is a high prevalence of endocrine dysfunction including androgen deficiency in allogeneic HSCT recipients[27] and this could impact on impaired morphology and function of the meibomian gland in the coGVHD group. Finally, this is a retrospective study with the relatively small samples from one Asian institute, so further studies with a larger, multicenter, prospective design are necessary to validate this study.
The prevalence of coGVHD continues to increase with improved survival rates after allogeneic HSCT. However,vision-related quality of life remains problematic due to underdiagnosis and -treatment of DED associated with coGVHD,and most patients with advanced DED continue to have residual symptoms despite the interventions. Our results demonstrate that patients with coGVHD showed more severe morphological and functional loss of the meibomian gland than SS-DED and these changes closely related to abnormalities in ocular surface and tear film. In addition, meibomian gland loss was found to be associated with coGVHD severity. Therefore,ophthalmic evaluation including morphologic and functional assessment of the meibomian gland is recommended in allogeneic HSCT recipients for early detection and adequate management of ocular and systemic GVHD manifestations.
ACKNOWLEDGEMENTS
Foundations: Supported by the Chonnam National University Hospital Biomedical Research Institute (CRI 18093-1); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science,ICT & Future Planning (No.2017R1A2B4003367).
Conflicts of Interest: Choi W, None; Ha JY, None; Li Y,None; Choi JH, None; Ji YS, None; Yoon KC, None.
1 Calissendorff B, el Azazi M, Lönnqvist B. Dry eye syndrome in longterm follow-up of bone marrow transplanted patients. Bone Marrow Transplant 1989;4(6):675-678.
2 Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood 2009;114(1):7-19.
3 Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea 2012;31(3):299-310.
4 Shikari H, Antin JH, Dana RZ. Ocular graft-versus-host disease: a review. Surv Ophthalmol 2013;58(3):233-251.
5 Hessen M, Akpek EK. Ocular graft-versus-host disease. Curr Opin Allergy Clin Immunol 2012;12(5):540-547.
6 Ogawa Y, Okamoto S, Wakui M, Watanabe R, Yamada M, Yoshino M, Ono M, Yang HY, Mashima Y, Oguchi Y, Ikeda Y, Tsubota K. Dry eye after haematopoietic stem cell transplantation. Br J Ophthalmol 1999;83(10):1125-1130.
7 Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea 2003;22(7 Suppl):S19-S27.
8 Westekemper H, Meller S, Citak S, Schulte C, Steuhl KP, Homey B,Meller D. Differential chemokine expression in chronic GVHD of the conjunctiva. Bone Marrow Transplant 2010;45(8):1340-1346.
9 Ban Y, Ogawa Y, Ibrahim OM, Tatematsu Y, Kamoi M, Uchino M,Yaguchi S, Dogru M, Tsubota K. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis 2011;17:2533-2543.
10 Engel LA, Wittig S, Bock F, Sauerbier L, Scheid C, Holtick U,Chemnitz JM, Hallek M, Cursiefen C, Steven P. Meibography and meibomian gland measurements in ocular graft-versus-host disease. Bone Marrow Transplant 2015;50(7):961-967.
11 Sullivan DA, Dana RZ, Sullivan RM, Krenzer KL, Sahin A,Arica B, Liu Y, Kam WR, Papas AS, Cermak JM. Meibomian gland dysfunction in primary and secondary sjögren syndrome. Ophthalmic Res 2018;59(4):193-205.
12 Chen XJ, Utheim ØA, Xiao JX, Adil MY, Stojanovic A, Tashbayev B,Jensen JL, Utheim TP. Meibomian gland features in a Norwegian cohort of patients with primary Sjögren´s syndrome. PLoS One 2017;12(9):e0184284.13 Kang YS, Lee HS, Li Y, Choi W, Yoon KC. Manifestation of meibomian gland dysfunction in patients with Sjögren’s syndrome, non-Sjögren’s dry eye, and non-dry eye controls. Int Ophthalmol 2018;38(3):1161-1167.
14 Ogawa Y, Kim SK, Dana RZ, Clayton J, Jain S, Rosenblatt MI, Perez VL, Shikari H, Riemens A, Tsubota K. International chronic ocular graftvs-host-disease (GVHD) consensus group: proposed diagnostic criteria for chronic GVHD (part I). Sci Rep 2013;3:3419.
15 Rapoport Y, Freeman T, Koyama T, Engelhardt BG, Jagasia M, Savani BN, Tran U, Kassim AA. Validation of international chronic ocular graftversus-host disease (GVHD) group diagnostic criteria as a chronic ocular GVHD-specific metric. Cornea 2017;36(2):258-263.
16 Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, Rasmussen A, Scofield H, Vitali C, Bowman SJ,Mariette X, International Sjögren’s Syndrome Criteria Working Group.2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76(1):9-16.
17 Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, Tomidokoro A, Amano S. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology 2009;116(11):2058-2063.e1.
18 Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol 2000;118(5):615-621.
19 Yoon KC, Im SK, Kim HG, You IC. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea 2011;30(9):972-976.
20 Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol 1999;117(6):723-729.
21 Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008;115(5):911-915.
22 Ogawa Y, Kuwana M, Yamazaki K, Mashima Y, Yamada M, Mori T,Okamoto S, Oguchi Y, Kawakami Y. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease.Invest Ophthalmol Vis Sci 2003;44(5):1888-1896.
23 Ogawa Y, Yamazaki K, Kuwana M, Mashima Y, Nakamura Y, Ishida S, Toda I, Oguchi Y, Tsubota K, Okamoto S, Kawakami Y. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci 2001;42(1):111-119.
24 Khanal S, Tomlinson A. Tear physiology in dry eye associated with chronic GVHD. Bone Marrow Transplant 2012;47(1):115-119.
25 Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. Statistical analysis with special reference to aging. Ophthalmology 1995;102(4):678-686.
26 Khandelwal P, Liu SH, Sullivan DA. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells.Mol Vis 2012;18:1055-1067.
27 Tauchmanovà L, Selleri C, Rosa GD, Pagano L, Orio F, Lombardi G,Rotoli B, Colao A. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer 2002;95(5):1076-1084.