INTRODUCTION
Herpetic eye disease caused by herpes simplex virus(HSV) is a condition that usually develops as an epithelial, stromal or endothelial keratitis, or uveitis. The disease is a major cause of corneal blindness. The incidence of HSV keratitis is approximately 1.5 million, worldwide,including 40 000 new cases of related blindness each year[1-3].In most cases, HSV keratitis/uveitis manifests as a unilateral disease[4]. After the first episode of infection during the first year, the disease recurs in 10% of patients, within 2y-23%, 5y-36% and 20y-60% of patients[5].
HSV keratitis/uveitis is a common cause of corneal hypoesthesia,which is associated with a reduced number of sensory nerves plexus at the corneal sub-epithelium layer, and tends to progress with HSV keratitis recurrences[6-9]. Recurrent episodes of the infection may lead to corneal scarring, opacities or irregular astigmatism[10]. The nerve loss in HSV-1 infected corneas includes the nerve endings in the epithelium, the nerve plexus at the subepithelium interface, and the large nerve stalks that enter the corneal stroma at the limbus[11]. Corneal hypoesthesia presents an increased risk for neurotrophic corneal damage[9].
Mouse models show that after loss of sensory nerves, the corneal stroma gradually reinervates. The innervating nerves sprout and hyperinnervate the corneal stroma, but do not form the nerve plexus at the subepithelium layer or extend neurites into the epithelium, remaining the cornea less sensitive[9,11].Hyperinervation of corneal stroma by sympathetic nerves determines HSV keratitis severity and disturbance of the sensory nerve plexus and epithelial nerve endings regeneration[9].
Laser scanning confocal microscopy (LSCM) is a noninvasive method of examining the cornea at the cellular level. This method is becoming as an ancillary for slit-lamp biomicroscopy, which has been the gold standard for detecting epithelial defects, stromal oedema and infiltration, keratic precipitates, and iritis due to HSV[12-13]. Corneal sub-basal nerves, which are reduced due to HSV keratitis/uveitis, can also be clearly visible by LSCM[14].
Recently, there has been studies analyzing the morphology changes of corneal cells and their associations with changes in corneal innervation and sensation and investigating alterations of sub-basal corneal nerves for patients with HSV keratitis[12,15-17]. To our knowledge, so far, longitudinal studies that have analyzed this data, and studies that have compared the results between HSV keratitis and uveitis patients have not been performed.
Our research is a longitudinal study with the main purpose to describe and correlate corneal sensation and sub-basal nerve fibres decrease in HSV epithelial, stromal, endothelial keratitis and uveitis compared with contralateral eye; after then, to compare the data of primary and repeated tests after 6mo.
SUBJECTS AND METHODS
Ethical Approval The study was approved by the Biomedical Research Ethics Committee 2015-07-09 No. BE-2-26 and 2017-01-26 No. P1-BE-2-26/2015. Written informed consent was obtained from all subjects who participated in the study.
Subjects A prospective clinical study included 30 HSV eyes and 30 contralateral eyes of 30 patients (16 men and 14 women, mean age 57.5±18.6y, range 33-85). Inclusion criterion was diagnosis of active unilateral HSV keratitis/uveitis,according to the clinical examination. Epithelial keratitis was diagnosed in patients with characterized multiple epithelial blisters filled with fluid, isolated or merged dendritic infiltrates.Herpes stromal keratitis was diagnosed in patients who had stromal opacities or destruction of corneal stroma, ulcerative infiltrates and/or neovascularization. Patients who presented with characterized swelling of the central part of stroma with the ring infiltrate and precipitate on corneal endothelium had endothelial keratitis. Patients with unilateral keratic precipitates, high intraocular pressure and patchy iris atrophy had anterior herpetic uveitis. For all patients, serology tests to detect anti-HSV antibodies were performed. Exclusion criteria were a previous history of other ocular infection, contact lens wearing, diabetes mellitus, glaucoma and previous trauma,intraocular or refractive surgery. Both eyes underwent an ophthalmological examination, Cochet-Bonnet aesthesiometry(Cochet-Bonnet; Luneau Ophthalmologie, Chartres, France)and LSCM (Heidelberg Retina Tomograph 3 with the Rostock Cornea Module, Germany) of the central cornea. The most representative image (typically at a depth of 50 to 70 μm) ofthe sub-basal nerve plexus was selected for analysis of each eye. The corneal nerve analysis was performed using the semiautomated tracing program NeuronJ[18]. The sub-basal nerves were categorized as main nerves; branches, which branched from the main nerves and the total nerves as main nerves with nerve branches. Nerve density was assessed by measuring the total length of the nerve fibres (main nerves and nerve branches) in mm/mm2. Main nerve trunks and nerve branching were defined as the total number in one image. The number of total nerves measured was defined as the number of all nerves,including main nerve trunks and branches in one image and were reported as number per image as described in previous studies[15,17].
Table 1 Demographic data of the patients with HSV keratitis/uveitis
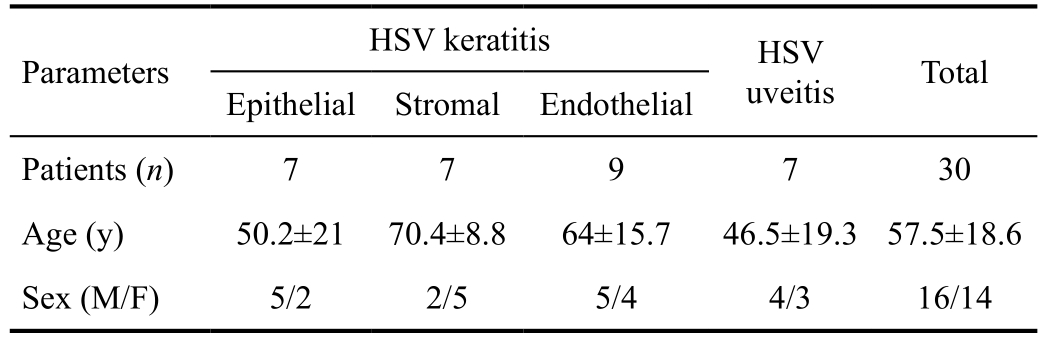
HSV: Herpes simplex virus.
?
Statistical Analysis The results were analyzed by Kruskal-Wallis and Mann-Whitney tests. The Kruskal-Wallis test was applied to compare the scores for more than 2 independent groups and the Mann-Whitney for the scores of two independent groups. Differences on dependent variables were analyzed by the Wilcoxon signed-rank test. The Pearson correlation coefficient was calculated to determine relationships between nerve density parameters and aesthesiometry results in HSV affected eyes. Differences were considered statistically significant, when P values<0.05. After 6mo from the first clinical examination, the same investigation was repeated and the data were compared with the primary test results.
RESULTS
Thirty HSV keratitis/uveitis eyes, including epithelial (n=7),stromal (n=7), endothelial keratitis (n=9) and uveitis (n=7),were analyzed and compared with contralateral, clinically unaffected eyes. Demographic data of all subgroups are presented in Table 1.
Although the patients in the subgroup of the stromal keratitis were older, we did not find a correlation between the age and corneal sensation or sub-basal nerve fibres parameters(P>0.05). HSV affected eyes showed a significant reduction in the mean corneal sensation and sub-basal nerve plexus parameters when compared with contralateral eyes. In particular, the mean corneal sensation (3.1±1.6 vs 5.3±0.8 cm),mean nerve density (7.5±5.6 vs 18.1±5.3 mm/mm2), the total number of nerve fibres (5.7±4.4 vs 15.1±5.4), the number of main nerve trunks (2.3±1.6 vs 5.8±2.2), and the number ofbranches (3.4±3.0 vs 8.4±4.7) were all found to be significantly lower (P<0.05).
Table 2 Quantitative analysis of the corneal sensation and sub-basal nerve fibres parameters in HSV keratitis/uveitis subgroups and contralateral eyes mean±SD
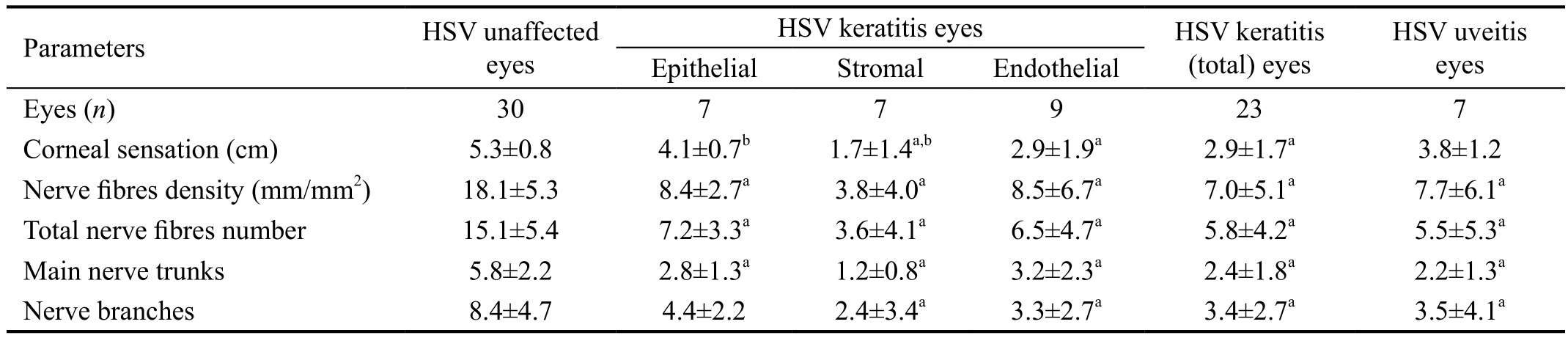
HSV: Herpes simplex virus. aDifference between HSV keratitis and contralateral eyes (P<0.05); bDifference of corneal sensation between epithelial and stromal keratitis (P<0.05).
Parameters HSV unaffected eyes HSV uveitis eyes Epithelial Stromal Endothelial HSV keratitis eyes HSV keratitis(total) eyes Eyes (n) 30 7 7 9 23 7 Corneal sensation (cm) 5.3±0.8 2.9±1.9a 2.9±1.7a 3.8±1.2 Nerve fibres density (mm/mm2) 18.1±5.3 8.4±2.7a 3.8±4.0a 8.5±6.7a 7.0±5.1a 7.7±6.1a Total nerve fibres number 15.1±5.4 7.2±3.3a 3.6±4.1a 6.5±4.7a 5.8±4.2a 5.5±5.3a Main nerve trunks 5.8±2.2 2.8±1.3a 1.2±0.8a 3.2±2.3a 2.4±1.8a 2.2±1.3a Nerve branches 8.4±4.7 4.4±2.2 2.4±3.4a 3.3±2.7a 3.4±2.7a 3.5±4.1a
Subgroup analysis of the HSV keratitis/uveitis eyes demonstrated that the eyes with epithelial, stromal, endothelial keratitis and uveitis had a reduced corneal sensation and sub-basal nerve plexus parameters, as compared with the contralateral eye parameters, including the mean corneal sensation, nerve fibres density, the total number of nerves, the number of main nerve trunks, and the number of branches, some of which reached statistical significance (Table 2).
A comparison between keratitis subgroups demonstrated that the stromal keratitis group showed a more profound decrease in sensation and sub-basal nerve parameters as compared with the epithelial and endothelial subgroups, however only the corneal sensation results between stromal and epithelial keratitis reached statistical significance (P<0.05; Table 2).HSV keratitis eyes, as compared with HSV uveitis eyes,showed a decrease of corneal sensation (2.9±1.7 vs 3.8±1.2 cm),sub-basal nerve fibres density (7.0±5.1 vs 7.7±6.1 mm/mm2)and nerve branches (3.4±2.7 vs 3.5±4.1) parameters, but did not reach statistical significance (P>0.05). The decrease in corneal sensation significantly correlated to the diminishment of the density of sub-basal corneal nerve fibres in patients with HSV keratitis/uveitis (r=0.6; P<0.05).
After 6mo, the same examination of HSV, affected and contralateral eyes was performed, and compared with the primary examination data. Corneal sensation and the parameters of sub-basal nerve fibres were increased: corneal sensation (4.6±1.3 vs 3.1±1.6 cm; P<0.05), nerve density(12.2±4.7 vs 7.5±5.6 mm/mm2; P<0.05), the total number of nerves (8.6±3.5 vs 5.7±4.4; P<0.05), the number of main nerve trunks (3.2±1.3 vs 2.3±1.6; P<0.05), and the number of branches (5.6±3.1 vs 3.4±3.0; P<0.05), however the mean nerve density, the total number of nerves, the number of main nerve trunks and the number of branches still did not reach the contralateral eyes parameters (P<0.05).
DISCUSSION
Cornea is the most densely innervated tissue in the human body, supplied by the terminal branches of the ophthalmic division of the trigeminal nerve. Small nerve branches from the anterior stroma penetrate Bowman’s layer and run between Bowman’s layer and the basal epithelium, configuring the subbasal nerve plexus. Corneal innervation provides not only corneal sensation and a protective function, but also plays an important role on the regulation of epithelial integrity,proliferation, and wound healing[19-21]. During our study we compared the parameters of sub-basal nerve fibres in HSV keratitis and uveitis patients and presented a decrease of subbasal nerve fibre parameters by LSCM in HSV affected eyes,and correlated LSCM findings with corneal sensation.
The diminishment of the corneal sensation and sub-basal nerve plexus was noted in all subgroups, this was more severe in the stromal keratitis group, however only the corneal sensation decrease between stromal and epithelial keratitis reached statistical significance. Nagasato et al[17] found that sub-basal nerve changes were more severe in epithelial and stromal types of HSV keratitis than in the endothelial type compared with controls. The study of Hamrah et al[15] showed a significant correlation between corneal sensation and sub-basal nerve fibre parameters.
In addition to the examination of the eyes with HSV, we compared the results of diseased and contralateral eyes. We analyzed the results of each HSV keratitis/uveitis subgroups(epithelial, stromal, endothelial keratitis and uveitis) and found a significant decrease of corneal sensation and sub-basal nerve fibre parameters as compared to contralateral eyes. The subbasal nerve density in normal eyes is about 21.7 mm/mm2[22].In our study, we found that the sub-basal nerve density of contralateral eyes was 18.1±5.3 mm/mm2. These findings suggest that HSV corneal disease could potentially lead to a loss of corneal innervation not only in HSV affected, but also in contralateral, clinically unaffected eyes. Similar results were presented in Moein et al[23] study.
Corneal nerves may be damaged due to many ocular and systemic pathological conditions, such as acute ocular infection, herpetic eye disease, diabetes, etc. Corneal nerve dysfunction causes a partial or complete neurotrophic keratopathy, which can result in persistent epithelial defects,stromal thinning and perforation, vision loss or blindness[24].HSV keratitis is usually unilateral (the incidence of bilateral is 3%-11.9%), even though bilateral corneal nerve changes have been found in our and previous studies[15,17,25]. The exact mechanisms of contralateral eye changes are still unclear. It could be explained by the central nervous system, mediated contralateral effects, where central nervous system pathways are responsible for affecting of contralateral undamaged neurons[26-27].
For the first time entering in the human body HSV virus through the retrograde axonal path enters the sensory ganglia,where remains lifelong in a latent state. HSV might travel from trigeminal ganglia between nerve anastomosis to the contralateral mesencephalic trigeminal nucleus. It allows primary trigeminal fibres to cross the pontine tegmentum to reach the contralateral principal nucleus and to cause contralateral damage to the distal nerve plexus without contralateral clinical manifestations[25,28]. These anatomic variations make it possible that unilateral nerve damage may result in bilateral changes, although corneal sensory innervation is mediated by unilateral ophthalmic nerve pathway. In addition, biochemical mechanisms may also play a role by releasing inflammatory mediators from neurons[26-27,29].We found that after six months corneal sensation and subbasal nerve fibre parameters were significantly increased, as compared with the data of the previous examination; however the mean nerve density, the total number of nerves, the number of main nerve trunks and the number of branches still did not reach the parameters of the clinically unaffected eyes.The similar results were presented in Moein et al[23] study,where corneal sensation an sub-basal nerve parameters were measured after 37mo of the disease manifestation.
Although regeneration of corneal nerves is still not fully explained process, it showed that during corneal damage increased immune response correlates with decreased innervations[30]. Cruzat et al[30] demonstrated a strong and significant correlation between the increase in dendritiform cell density and the decrease in sub-basal corneal nerves in patients with infection keratitis, suggesting a connection between corneal immune response and nerve alterations.
Chucair-Elliott et al[31] in mouse model showed that HSV infected corneas significantly retain both structure and sensitivity of the corneal nerve network after levels of pro-inflammatory cytokines and influx of macrophages and CD8+ T cells into the cornea are reduced. The same researcher demonstrated that semaphorins, which are axonal growth cone guidance molecules, also play a role in corneal reinervation[11].
Injured corneal stromal fibroblasts release semaphorins,neurotrophins, immune regulatory factors and matricellular proteins and play a role in nerve regeneration growth,differentiation and wound healing[32]. Nerve regeneration and inflammation processes might share common pathways in tissue repair. Inflammation management could be a strategy for improving corneal nerves regeneration.
A limitation of the present study may be related to the small sample of tested patients, the evaluation of only central part of the cornea, Cochet-Bonnet aesthesiometer, which only measures mechanical nociceptors, and LSCM testing method.LSCM is a contact diagnostic tool, thus it may cause an ocular discomfort to a patient leading to increasing eye movements that may blur the images. In this study, we compared the results between HSV keratitis/uveitis and contralateral, clinically unaffected eyes, but we did not have a healthy control group.This is the main flaw of this study. Therefore, a larger clinical research must be performed to provide more detailed results.
The results of our study could be informative for clinical practice as a part of diagnostic procedures, which help to diagnose HSV keratitis and uveitis, to determine exactly the depth of keratitis, to value the severity of infection, and corneal cells damage. Furthermore, the obtained results could aid clinical practitioners in defining of prognostic factors for the disease outcomes.
LSCM (HRTII-RCM) is a relatively new, non-invasive,painless technique that does not carry any risk of complications[33].LSCM and corneal Cochet-Bonnet aesthesiometry allows to observe corneal sub-basal nerves morphology, density and function. The study of corneal sensory innervation and sensation in HSV affected corneas demonstrates a significant decrease of analyzed parameters and a positive correlation between corneal sensation and sub-basal nerve plexus density.The examination of the same patients after 6mo reveals the significant increase of corneal sensation and sub-basal nerve parameters; however the parameters of corneal sub-basal nerves still do not reach the clinically unaffected eyes.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zemaitiene R, None; Rakauskiene M,None; Danileviciene V, None; Use V, None; Kriauciuniene L,None; Zaliuniene D, None.
1 Zagaria MAE. HSV keratitis: an important infectious cause of blindness.US Pharm 2015;40(4):16-18.
2 Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis:an epidemiologic update. Surv Ophthalmol 2012;57(5):448-462.
3 Pepose JS, Keadle TL, Morrison LA. Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol 2006;141(3):547-557.
4 Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea 2001;20(1):1-13.
5 Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol 1989;107(8):1160-1165.
6 Norn MS. Dendritic (herpetic) keratitis. IV. Follow-up examination of corneal sensitivity. Acta Ophthalmol 1970;48(3):383-395.
7 Gallar J, Tervo TM, Neira W, Holopainen JM, Lamberg ME, Miñana F,Acosta MC, Belmonte C. Selective changes in human corneal sensation associated with herpes simplex virus keratitis. Invest Ophthalmol Vis Sci 2010;51(9):4516-4522.
8 Rosenberg ME, Tervo TM, Müller LJ, Moilanen JA, Vesaluoma MH. In vivo confocal microscopy after herpes keratitis. Cornea 2002;21(3):265-269.
9 Yun HM, Lathrop KL, Hendricks RL. A central role for sympathetic nerves in herpes stromal keratitis in mice. Invest Ophthalmol Vis Sci 2016;57(4):1749-1756.
10 Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res 2006;25(4):355-380.
11 Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci 2015;56(2):1097-1107.
12 Hamrah P, Sahin A, Dastjerdi MH, Shahatit BM, Bayhan HA, Dana RZ, Pavan-Langston D. Cellular changes of the corneal epithelium and stroma in herpes simplex keratitis: an in vivo confocal microscopy study.Ophthalmology 2012;119(9):1791-1797.
13 Patel DV, McGhee CN. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques:a major review. Clin Exp Ophthalmol 2007;35(1):71-88.
14 Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol 2009;93(7):853-860.
15 Hamrah P, Cruzat A, Dastjerdi MH, Zheng LX, Shahatit BM, Bayhan HA, Dana RZ, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology 2010;117(10):1930-1936.
16 Müller RT, Abedi F, Cruzat A, Witkin D, Baniasadi N, Cavalcanti BM, Jamali A, Chodosh J, Dana RZ, Pavan-Langston D, Hamrah P. Degeneration and regeneration of subbasal corneal nerves after infectious keratitis: a longitudinal in vivo confocal microscopy study.Ophthalmology 2015;122(11):2200-2209.
17 Nagasato D, Araki-Sasaki K, Kojima T, Ideta R, Dogru M.Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Jpn J Ophthalmol 2011;55(5):444-450.
18 Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 2004;58(2):167-176.
19 Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea 2001;20(4):374-384.
20 Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea 2005;24(5):608-613.
21 Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol 1980;69(1):196-201.
22 Patel DV, McGhee CN. Mapping of the normal human corneal sub-Basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci 2005;46(12):4485-4488.
23 Moein HR, Kheirkhah A, Muller RT, Cruzat AC, Pavan-Langston D, Hamrah P. Corneal nerve regeneration after herpes simplex keratitis: A longitudinal in vivo confocal microscopy study. Ocul Surf 2018;16(2):218-225.
24 Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye(Lond) 2003;17(8):989-995.
25 Müller RT, Pourmirzaie R, Pavan-Langston D, Cavalcanti BM,Aggarwal S, Colón C, Jamali A, Cruzat A, Hamrah P. In vivo confocal microscopy demonstrates bilateral loss of endothelial cells in unilateral herpes simplex keratitis. Invest Ophthalmol Vis Sci 2015;56(8):4899-4906.
26 Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci 1999;22(3):122-127.
27 Yamaguchi T, Turhan A, Harris DL, Hu K, Prüss H, von Andrian U, Hamrah P. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One 2013;8(8):e70908.
28 Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol 1988;268(1):91-108.
29 Cruzat A, Schrems WA, Schrems-Hoesl LM, Cavalcanti BM, Baniasadi N, Witkin D, Pavan-Langston D, Dana RZ, Hamrah P. Contralateral clinically unaffected eyes of patients with unilateral infectious keratitis demonstrate a sympathetic immune response. Invest Ophthalmol Vis Sci 2015;56(11):6612-6620.
30 Cruzat A, Hamrah P, Cavalcanti BM, Zheng LX, Colby K, Pavan-Langston D. Corneal reinnervation and sensation recovery in patients with herpes zoster ophthalmicus: an in vivo and ex vivo study of corneal nerves. Cornea 2016;35(5):619-625.
31 Chucair-Elliott AJ, Carr MM, Carr DJJ. Long-term consequences of topical dexamethasone treatment during acute corneal HSV-1 infection on the immune system. J Leukoc Biol 2017;101(5):1253-1261.
32 Yam GH, Williams GP, Setiawan M, Yusoff NZ, Lee XW, Htoon HM, Zhou L, Fuest M, Mehta JS. Nerve regeneration by human corneal stromal keratocytes and stromal fibroblasts. Sci Rep 2017;7:45396.
33 Labbé A, Dupas B, Hamard P, Baudouin C. In vivo confocal microscopy study of blebs after filtering surgery. Ophthalmology 2005;112(11):1979.