INTRODUCTION
Cataract surgery is the most common intraocular surgery in ophthalmology practice. The technological advancement of surgical machinery and intraocular lenses (IOLs) have made this maneuver easier, faster and safer. In addition, patients with cataract problems can gain better vision and recovery after the surgery. However, the most serious complication of cataract surgery is post-operative endophthalmitis which can deteriorate vision and cause blindness[1]. Diabetes mellitus is a very common metabolic illness that detrimentally affects the microvascular system. Many previous studies conducted in the various surgical specialties, including ophthalmology,have found that diabetes is associated with a significantly higher risk of post-operative or surgical site infection years[2-7].Nonetheless, in the field of ophthalmology, there has been no research conducted demonstrating the risk of developing postoperative endophthalmitis after cataract surgery in the diabetic group specifically. The Endophthalmitis Vitrectomy Study,the landmark study of post-cataract surgery endophthalmitis(PCE), investigated different responses between diabetic and non-diabetic patients and recommended early vitrectomy for diabetic patients. However, this study did not specifically examine the risk factors for developing PCE in diabetic patients[8].
This work focuses on diabetic patients and aims to identify risk factors including demographics, pre-operative characteristics,surgical settings and complications associated with PCE.
SUBJECTS AND METHODS
Ethical Approval A case-control study was initiated by retrospectively reviewing medical charts of patients developing PCE at the Department of Ophthalmology, Rajavithi Hospital,Bangkok, Thailand from January 2007 to December 2015. The study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Rajavithi Hospital.Informed consent was waived due to the retrospective nature of the study.
Study Design and Data Collection Individuals were considered to have endophthalmitis if hypopyon or vitritis was present or if the patient received an intravitreal injection of antibiotics for presumed endophthalmitis[9-10]. PCE was defined as an endophthalmitis occurring after uncombined cataract surgery. In the present study, exclusion criteria were any of the following: 1) PCE developing in non-diabetic patients; 2)missing laboratory or operative data (any patients); 3) records indicating non-compliance with drug treatment or less than 1mo of follow-up (any patients). The remaining type 2 diabetic patients who did not fulfill any exclusion criteria were included in the study. The case group consisted of diabetic patients who developed PCE post cataract surgery, whereas the control group consisted of type 2 diabetic patients undergoing cataract surgery who did not develop PCE. For each case, 10 control patients were randomly selected from the surgical logbook of patients who underwent cataract surgery within 14d before or after each case.Data collected included variables typically associated with PCE, which mostly involved diabetic control and operative profile. Diabetes was defined as a fasting plasma glucose level ≥126 mg/dL (or a glucose level ≥200 mg/dL for those not fasting), the use of insulin or oral hypoglycemic drugs,or a reliable self-reported history of diabetes. Preoperatively,patients had fasting plasma glucose measured as a part of routine pre-operative testing, which occurred on average 6wk(1-12) before the operation. Measurements performed more than three months before the operation were not accepted. If plasma glucose was measured multiple times before an operation,the most recent result was used. Glycated hemoglobin(HbA1c) was also gathered, although it was not included in our pre-operative laboratory routine. All diabetic treatments documented in the study were employed by primary care physicians or endocrinologists at the time surgery was appointed.In regards to operative profile, operative risk factors collected included antiseptic technique, eyelash removal, type of cataract surgery, type of IOL used, complications, the presence of stitches and different operating rooms occupied. In addition,microbiologic profiles, treatment modalities and visual outcomes of PCE cases were collected. Presenting acuity was defined as the acuity at the first visit for endophthalmitis.Snellen visual acuity of the affected eye was converted to the logarithm of the minimum angle of resolution (logMAR).The following scale was applied to logMAR values: counting fingers, 2.00; hand motion, 2.30; light perception, 2.60; and no light perception, 2.90[11-12].
The preoperative evaluation and preparation for endophthalmitis prophylaxis included an absence of periocular and ocular surface infection, and lacrimal drainage system obstruction.Antibiotic eyedrops were not routinely employed prior to the cataract surgery. Just before surgery, periorbital disinfection was performed using gauze soaked with 10% povidone iodine solution. This was followed by a thorough cleaning of the periorbital region with 2 cotton swabs soaked with 10% povidone-iodine solution. Afterwards, irrigation of the conjunctival sac with 10 mL of 5% povidone-iodine solution was performed. On those days, no peri-operative antibiotic prophylaxis, including intracameral and subconjunctival injections, were used by any surgeons in our hospital.
Postoperatively, the decisions using topical antibiotics(levofloxacin or tobramycin) were based on surgeon preferences. Steroid eyedrops were prescribed for 1-2wk. The postoperative check-ups were usually performed at day 1, 1wk,1, 3mo and then as needed afterwards. When endophthalmitis was diagnosed, the patient immediately underwent an aqueous and/or vitreous tap and/or vitrectomy to isolate any organisms followed by intravitreal injections of vancomycin and ceftazidime. A prescription of systemic antibiotics was dependent on surgeons’ preferences. Both culture-positive and culture-negative cases were included, and after sampling the causative microorganisms, antibiotic treatment was initiated.A pars plana vitrectomy was conducted on the endophthalmitis patients who presented with light perception or worse[13].
Statistical Analysis Statistical analysis was performed using the SPSS statistical software version 16.0 (SPSS Inc,Chicago, IL, USA). Descriptive analyses were used to describe demographics, pre-operative characteristic, surgical settings and complications. The Mann-Whitney U test was used to compare data between two unrelated groups in which normal distributions were not verified. A univariate analysis by Chisquare test or Fisher’s exact test was performed to demonstrate significance of associated factors with a P value <0.1.Variables that were significant in the univariate analysis were examined in a multivariate logistic regression model by which differences were considered statistically significant with a P value <0.05.
RESULTS
Demographic Data of the Case Group Of the 24 090 patients undergoing cataract surgery from 2007 to 2015, 1476 cases (6.1%) had diabetes prior to surgery. The incidence rate of PCE in diabetic patients was 1.0% (15/1476) which was greater than the incidence of PCE in non-diabetic patients 0.11% (25/22 614). Fifteen diabetic cases with PCE and 179 diabetic controls without PCE were included in the study.In the case group, 8 (53%) were females. The median age at time of cataract surgery was 68 (range, 36-80)y. Two patients(14%) had non-ophthalmic complications including diabetic nephropathy and diabetic neuropathy. None of the patients were immunocompromised or under immunosuppressive treatments. At the time of surgery, 4 (27%) experienced any degree of diabetic retinopathy (DR) while 2 (13%) of these had severe non-proliferative or proliferative DR.
Table 1 Demographics and pre-operative characteristics comparison between diabetic patients with PCE (case) and without PCE (control) groups n (%)
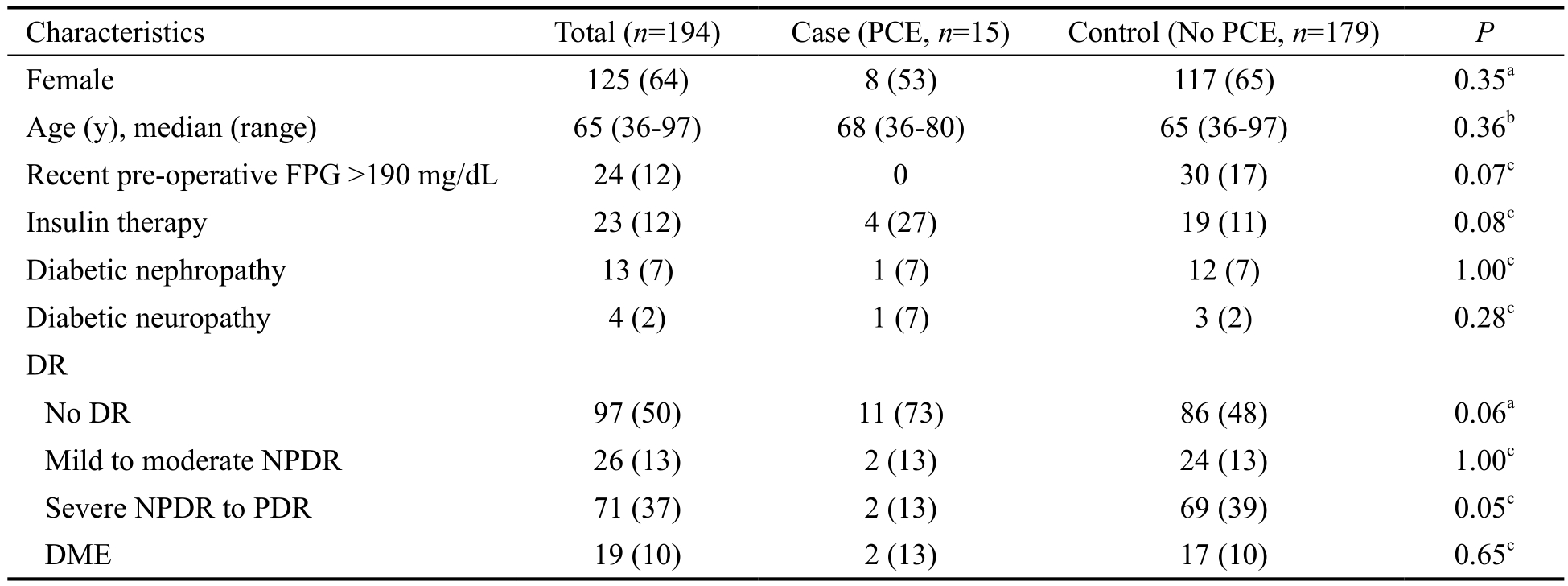
PCE: Post-cataract surgery endophthalmitis; FPG: Fasting plasma glucose; DR: Diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy; DME: Diabetic macular edema. aChi-square test; bMann-Whitney U test; cFisher’s exact test.
Characteristics Total (n=194) Case (PCE, n=15) Control (No PCE, n=179) P Female 125 (64) 8 (53) 117 (65) 0.35a Age (y), median (range) 65 (36-97) 68 (36-80) 65 (36-97) 0.36b Recent pre-operative FPG >190 mg/dL 24 (12) 0 30 (17) 0.07c Insulin therapy 23 (12) 4 (27) 19 (11) 0.08c Diabetic nephropathy 13 (7) 1 (7) 12 (7) 1.00c Diabetic neuropathy 4 (2) 1 (7) 3 (2) 0.28c DR No DR 97 (50) 11 (73) 86 (48) 0.06a Mild to moderate NPDR 26 (13) 2 (13) 24 (13) 1.00c Severe NPDR to PDR 71 (37) 2 (13) 69 (39) 0.05c DME 19 (10) 2 (13) 17 (10) 0.65c
Table 2 Comparison of surgical settings and complications between diabetic patients with PCE (case) and without PCE(control) groups n (%)
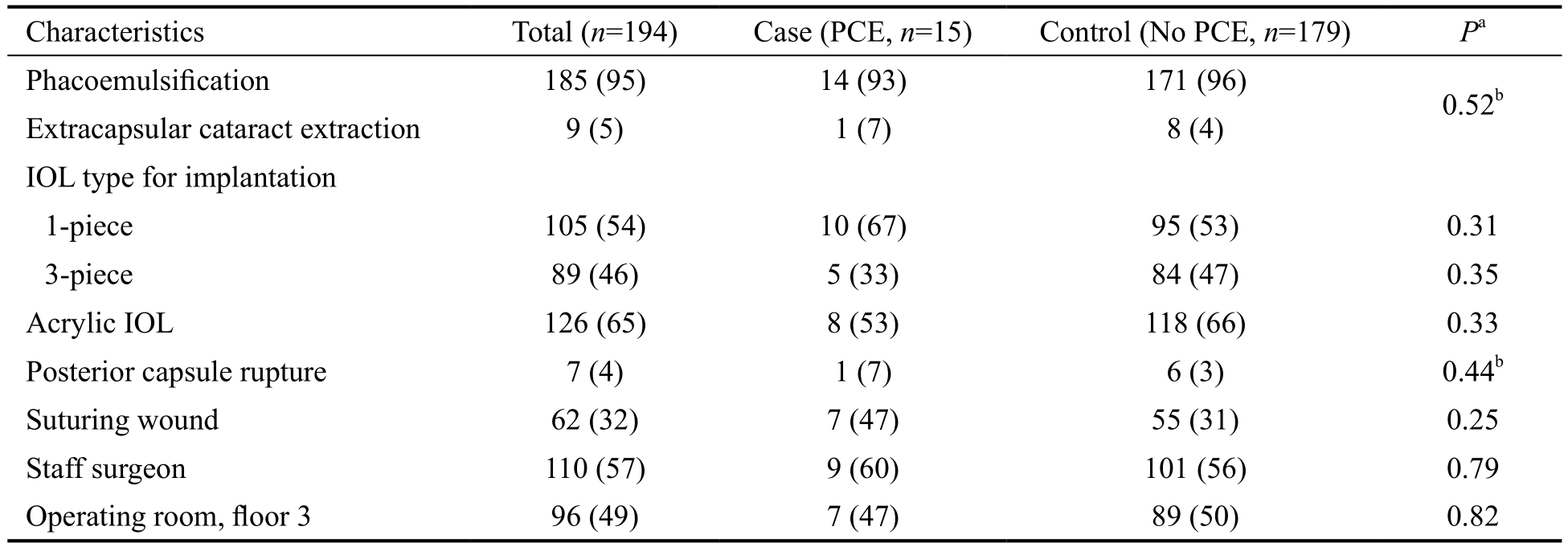
PCE: Post-cataract surgery endophthalmitis; IOL: Intraocular lens. aChi-square test; bFisher’s exact test.
Characteristics Total (n=194) Case (PCE, n=15) Control (No PCE, n=179) Pa Phacoemulsification 185 (95) 14 (93) 171 (96) 0.52b Extracapsular cataract extraction 9 (5) 1 (7) 8 (4)IOL type for implantation 1-piece 105 (54) 10 (67) 95 (53) 0.31 3-piece 89 (46) 5 (33) 84 (47) 0.35 Acrylic IOL 126 (65) 8 (53) 118 (66) 0.33 Posterior capsule rupture 7 (4) 1 (7) 6 (3) 0.44b Suturing wound 62 (32) 7 (47) 55 (31) 0.25 Staff surgeon 110 (57) 9 (60) 101 (56) 0.79 Operating room, floor 3 96 (49) 7 (47) 89 (50) 0.82
Demographic Comparison of Case and Control Group Regarding pre-operative fasting plasma glucose and HbA1c levels, no patients with PCE had pre-operative fasting plasma glucose levels of more than 190 mg/dL. HbA1c levels were recorded for 33% (5/15) of the case group and 21% (37/179) of the control group. Of these, the median HbA1c level of case and control groups was 8.5% (range, 5.4%-9.6%) and 7.5% (range,5.1%-12.4%) respectively. The comparison of median HbA1c levels between case and control groups was not significantly different by Mann-Whitney U Test (P=0.57). Table 1 demonstrates demographics and pre-operative characteristic comparisons between case and control groups.
None of the cases and controls underwent immediate sequential bilateral cataract surgery. Most procedures performed in the case group were phacoemulsification (93%). The most common types of IOL used were 1-piece, acrylic and foldable IOL. Intraoperative complications included one case of posterior capsule rupture and vitreous loss. Most surgeons performing surgery were staff surgeons. All patients developed endophthalmitis within 4wk and the median onset of endophthalmitis after cataract surgery was 5d (range,1-23d). Table 2 shows comparisons of surgical settings and complications between case and control groups.
Univariate and Multivariate Analysis To evaluate associated factors influencing the incidence of PCE in diabetic patients,relevant characteristics of the case and the control groups were initially analyzed using a univariate method. From univariate analysis, 4 factors including recent pre-operative fasting plasma glucose, insulin therapy, presence of DR, and severe non-proliferative or proliferative DR were significantlyassociated with PCE (Tables 1 and 2). Multivariate logistic regression analysis, adjusting for blood glucose level, revealed that insulin treatment was significantly associated with an increased risk of endophthalmitis (OR 3.9, 95%CI 1.0-15.0,P=0.04) compared to patients without insulin treatment.
Table 3 Characteristics of 15 cases with PCE n (%)
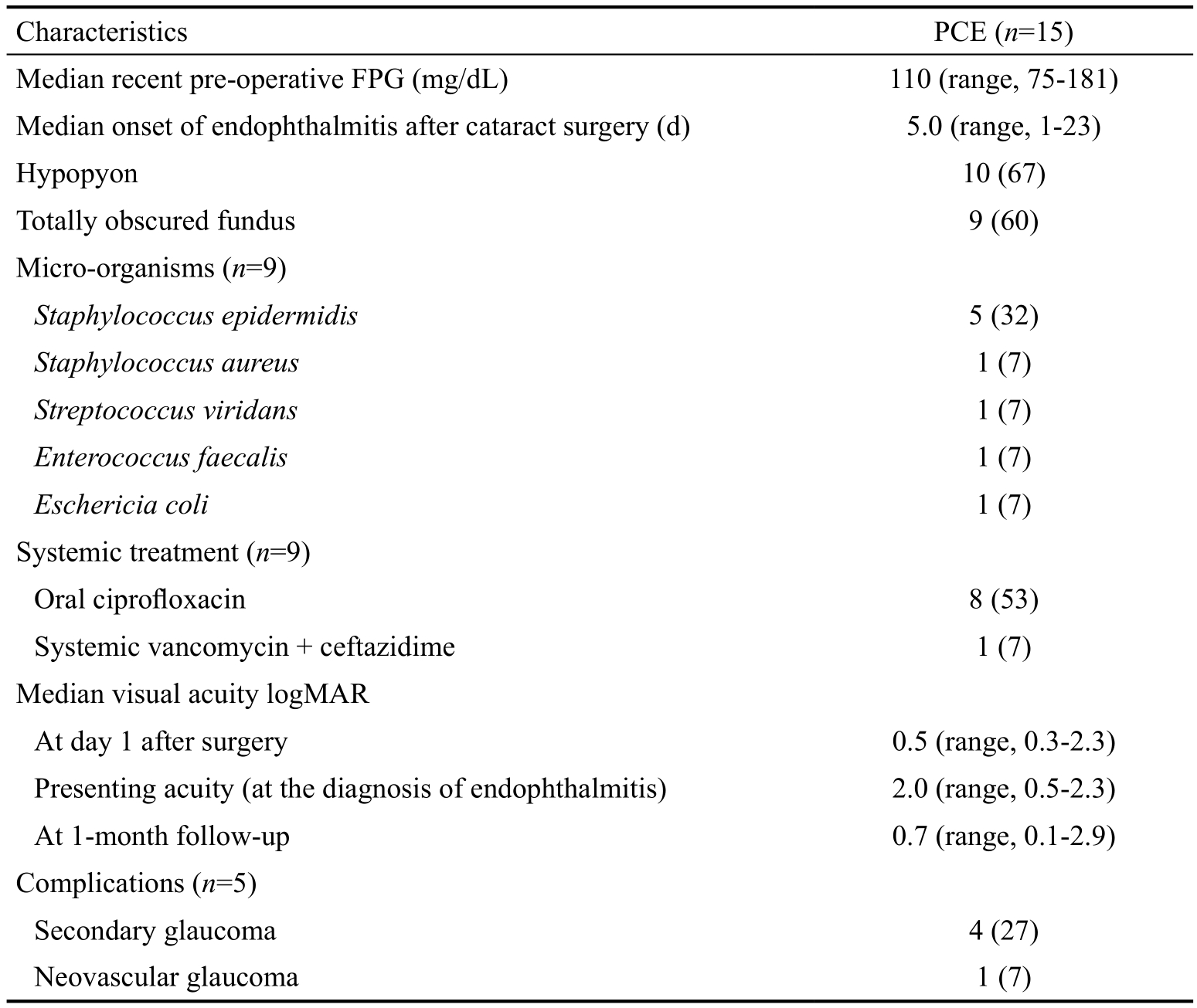
PCE: Post-cataract surgery endophthalmitis; FPG: Fasting plasma glucose.
PCE (n=15)Median recent pre-operative FPG (mg/dL) 110 (range, 75-181)Median onset of endophthalmitis after cataract surgery (d) 5.0 (range, 1-23)Hypopyon 10 (67)Totally obscured fundus 9 (60)Micro-organisms (n=9)Staphylococcus epidermidis 5 (32)Staphylococcus aureus 1 (7)Streptococcus viridans 1 (7)Enterococcus faecalis 1 (7)Eschericia coli 1 (7)Systemic treatment (n=9)Oral ciprofloxacin 8 (53)Systemic vancomycin + ceftazidime 1 (7)Median visual acuity logMAR At day 1 after surgery 0.5 (range, 0.3-2.3)Presenting acuity (at the diagnosis of endophthalmitis) 2.0 (range, 0.5-2.3)At 1-month follow-up 0.7 (range, 0.1-2.9)Complications (n=5)Secondary glaucoma 4 (27)Neovascular glaucoma 1 (7)Characteristics
Causative Pathogens and Visual Prognosis The organism causing PCE was identified by a positive culture from a vitreous specimen in 9 (60%) of the 15 patients (Table 3).Among the positive cultured specimens, the most common causative agents identified were gram-positive organisms(89%). Staphylococcus species represented the most common group, and was found in 6 (67%) patients. Fungus was not detected in any of the samples. All cases received intravitreal injections of vancomycin and ceftazidime. Seventy-three percent underwent immediate pars plana vitrectomy. None were eviscerated or enucleated. However, two cases (13%)experienced phthisis bulbi at a median follow-up period of 20 (range, 3-112)mo. At 1-month and 3-months follow-up,median best corrected visual acuity (BCVA) was equal at 0.7 logMAR (20/100) compared with 0.5 logMAR (20/60) at day 1 after surgery. Sixty-seven percent had BCVA of 20/200 or better. Table 4 shows the clinical summary of all cases in detail.
DISCUSSION
Previous studies reported various risk factors associated with PCE in the normal population, for example, pre-operative medical conditions, type of cataract surgery, intraoperative complications, no intracameral antibiotic usage[14-18]. Among these, diabetes has been the most common metabolic illness associated with PCE[2,9,14,19-21]. However, there have been no studies performed to specifically investigate PCE in diabetic patients. The present study pioneered investigation of the risk factors for endophthalmitis after cataract surgery in patients with diabetes and demonstrated insulin treatment as the only significant factor associated with PCE.
On the contrary to unanimously agreed upon perioperative glycemic control, insulin treatment, though rarely reported,has been controversial as an associated risk factor with nonsurgical[4] and surgically related infection[22-23]. Contradictory results regarding wound infection after caesarean section in insulin-dependent diabetic women were reported by the two previous studies. Though insulin was not included in an analysis of the research, given that it was the designated treatment of the study population, it could be a confounding variable influencing the occurrence of infection[22-23].
The only available literature related to insulin treatment and the eye is accelerating corneal epithelial healing by insulin treatment which may represent a preventive effect of insulin on PCE[24-25]. However, there has been no evidence proving the efficacy of insulin treatment in corneal wound healing after cataract surgery. Recently, Donnelly et al[4] performed a large prospective cohort study examining the association of diabetesand insulin therapy with hospitalization for infection and found that participants receiving insulin therapy experienced a more pronounced infection risk. They hypothesized that insulin use may function as a marker for advanced diabetic disease and could affect risk of infection. In addition, an unexplained mechanism directly related to insulin and inoculation of pathogenic organisms into soft-tissue by regular insulin injections could pose a higher risk of infection to diabetic participants receiving insulin therapy compared to those not receiving insulin[4,26].
Table 4 Clinical summary of diabetic patients developing PCE
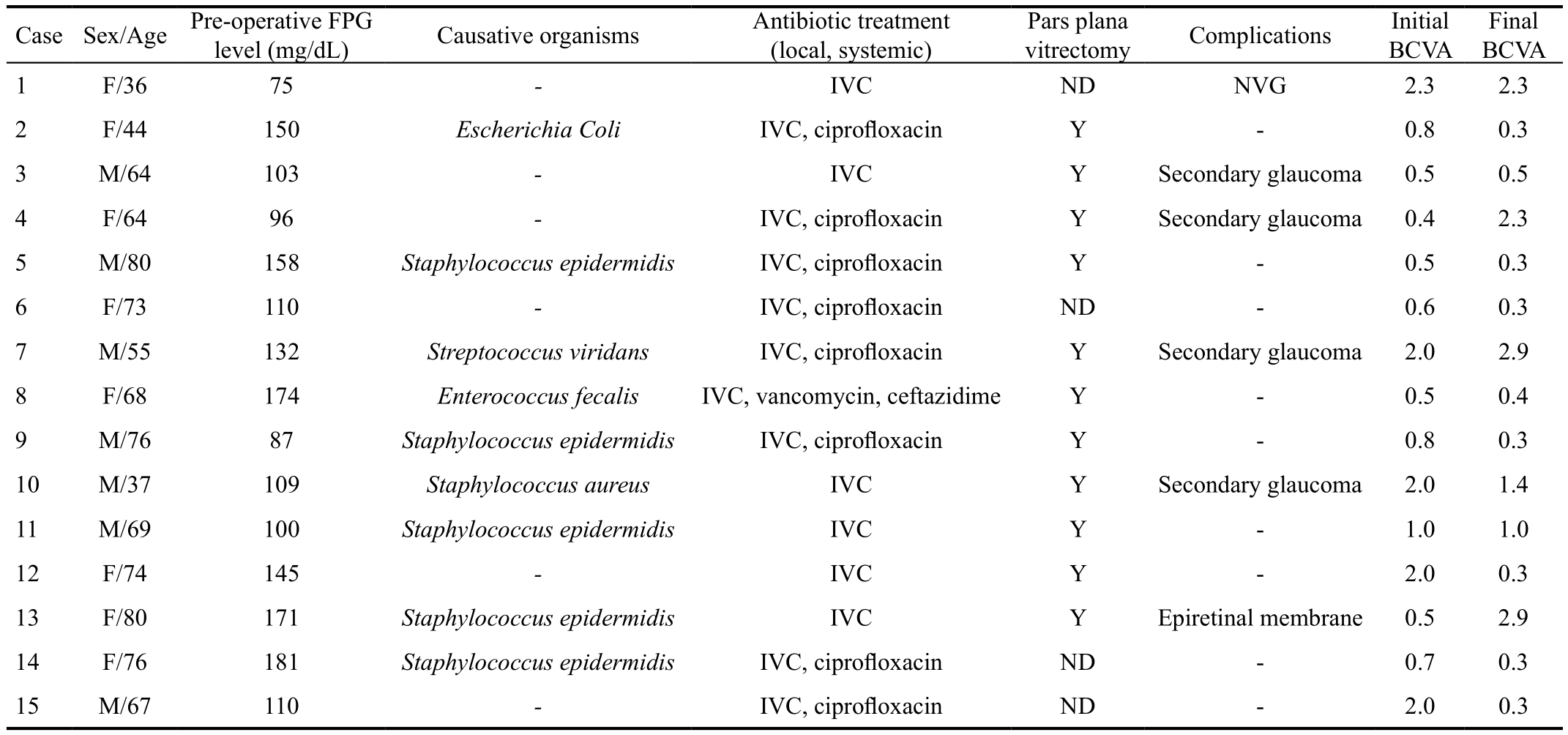
PCE: Post-cataract surgery endophthalmitis; FPG: Fasting plasma glucose; BCVA: Best corrected visual acuity; F: Female; M: Male; IVC:Intravitreal injection of vancomycin and ceftazidime; Y: Yes; ND: Not done; NVG: Neovascular glaucoma.
Final BCVA 1 F/36 75 - IVC ND NVG 2.3 2.3 2 F/44 150 Escherichia Coli IVC, ciprofloxacin Y - 0.8 0.3 3 M/64 103 - IVC Y Secondary glaucoma 0.5 0.5 4 F/64 96 - IVC, ciprofloxacin Y Secondary glaucoma 0.4 2.3 5 M/80 158 Staphylococcus epidermidis IVC, ciprofloxacin Y - 0.5 0.3 6 F/73 110 - IVC, ciprofloxacin ND - 0.6 0.3 7 M/55 132 Streptococcus viridans IVC, ciprofloxacin Y Secondary glaucoma 2.0 2.9 8 F/68 174 Enterococcus fecalis IVC, vancomycin, ceftazidime Y - 0.5 0.4 9 M/76 87 Staphylococcus epidermidis IVC, ciprofloxacin Y - 0.8 0.3 10 M/37 109 Staphylococcus aureus IVC Y Secondary glaucoma 2.0 1.4 11 M/69 100 Staphylococcus epidermidis IVC Y - 1.0 1.0 12 F/74 145 - IVC Y - 2.0 0.3 13 F/80 171 Staphylococcus epidermidis IVC Y Epiretinal membrane 0.5 2.9 14 F/76 181 Staphylococcus epidermidis IVC, ciprofloxacin ND - 0.7 0.3 15 M/67 110 - IVC, ciprofloxacin ND - 2.0 0.3 CaseSex/Age Pre-operative FPG Pars plana level (mg/dL) Causative organisms Antibiotic treatment(local, systemic)vitrectomy Complications Initial BCVA
Poor peri-operative control of glycemic markers can influence surgical site infection as presented in various subspecialties[27-33]. This includes optimizing control of fasting plasma glucose and HbA1c level. HbA1c is vital and has been thought to represent average glycemia roughly over the last 6-8wk. It is also associated with microvascular complications and macrovascular diseases[34-35]. Unfortunately, our HbA1c data gathered was insufficient to rule out HbA1c as a significant factor associated with endophthalmitis after cataract surgery in type 2 diabetic patients, because it was not routinely performed in pre-operative screening for diabetic patients. Further study has been established including HbA1c profile within preoperative screening for diabetic patients in our setting.
Regarding pre-operative fasting plasma glucose level, most ophthalmologists in Thailand have followed guidelines of other specialties indicating that fasting plasma glucose levels before intraocular surgery should be kept less than 200 mg/dL in order to prevent the risk of post-operative infection[31-32]. Interestingly,none of the endophthalmitis cases in our series had fasting plasma glucose levels greater than 190 mg/dL. Though this may suggest that strict glycemic control may not be mandatory prior to cataract surgery, caution should be exercised in the interpretation of the results. Besides, surgery can cause patients metabolic stress and create a state of functional insulin insufficiency, resulting in transient hyperglycemia during and after an operation[36]. Post-operative glucose level was also reported as a risk of surgical site infection[32-33].
The present study is the first reporting an incidence of PCE in diabetic patients, which is significantly greater than one in non-diabetic patients. This is not surprising given that patients with diabetes have been known to have an impaired immune response which poses a higher risk for developing postoperative infection[37-38]. However, the incidence of PCE could have been reduced by using intracameral antibiotic prophylaxis which is a standard practice of the present day[39].Regarding isolated micro-organisms, the most common organism isolated from diabetic patients with PCE in our study was coagulase-negative which is also consistent with the results of previous works reported on PCE in diabetic patients and the general population[1,40-41].
Every case received immediate intravitreal antibiotic injections as standard treatment. Though we agreed and followed the recommendation by EVS for early vitrectomy in PCE developing in diabetic patients, improvement of vision at 1-month follow-up compared with pre-operative vision was demonstrated in only 47% (7/15) of cases. The cases experiencing postoperative BCVA of better or equal than 20/40 in our study was only 6% (1/15) compared with previous studies reporting 32%-39% in diabetic patients developing PCE[8,41]. Besides impaired immune function and microvascular complications in diabetic patients as aforementioned, the difference could be due to previous studies’ potential bias of using final BCVA with variable follow-up instead of BCVA at the same follow-up[42].
This study is limited by its retrospective fashion and by the fact that results are from a single hospital database. In addition,the profile of HbA1c and duration of diabetes was lacking for analysis. Ideally to achieve a more updated and precise glycemic level, pre-operative and post-operative fasting plasma glucose monitoring is necessary. Pre-operative fasting plasma glucose should be more recent, for example, within one month prior to surgery. The reproducibility of the study is also limited since, nowadays, intracameral antibiotic prophylaxis is routinely used in cataract surgery. In conclusion, this retrospective case-control study reports associated risk factors of PCE in a cohort of diabetic patients to include only insulin treatment. We believe that insulin treatment is a proxy marker for advanced diabetic disease and could affect risk of infection.Further studies investigating the impact of HbA1c and other diabetes-related factors should be performed to elucidate profiles in pre-operative diabetic status as risk factors for PCE in diabetic patients.
ACKNOWLEDGEMENTS
in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999;67(2):352-360; discussion 360-362.
7 Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infectionrelated mortality in the US. Diabetes Care 2001;24(6):1044-1049.
8 Doft BH, Wisniewski SR, Kelsey SF, Fitzgerald SG; Endophthalmitis Vitrectomy Study Group. Diabetes and postoperative endophthalmitis in the endophthalmitis vitrectomy study. Arch Ophthalmol 2001;119(5):650-656.
9 Montan PG, Koranyi G, Setterquist HE, Stridh A, Philipson BT,Wiklund K. Endophthalmitis after cataract surgery: risk factors relating to technique and events of the operation and patient history: a retrospective case-control study. Ophthalmology 1998;105(12):2171-2177.
10 Gower EW, Keay LJ, Stare DE, Arora P, Cassard SD, Behrens A,Tielsch JM, Schein OD. Characteristics of endophthalmitis after cataract surgery in the United States medicare population. Ophthalmology 2015;122(8):1625-1632.
11 The ischemic optic neuropathy decompression trial (IONDT): design and methods. Control Clin Trials 1998;19(3):276-296.
12 Deramo VA, Cox TA, Syed AB, Lee PP, Fekrat S. Vision-related quality of life in people with central retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2003;121(9):1297-1302.
13 Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113(12):1479-1496.
14 Apisarnthanarak A, Jirajariyavej S, Thongphubeth K, Yuekyen C,Warren DK, Fraser VJ. Outbreak of postoperative endophthalmitis in a Thai tertiary care center. Infect Control Hosp Epidemiol 2008;29(6):564-566.
15 Wejde G, Samolov B, Seregard S, Koranyi G, Montan PG. Risk factors for endophthalmitis following cataract surgery: a retrospective casecontrol study. J Hosp Infect 2005;61(3):251-256.
16 Li JH, Morlet N, Ng JQ, Semmens JB, Knuiman MW; Team EPSWA. Significant nonsurgical risk factors for endophthalmitis after cataract surgery: EPSWA fourth report. Invest Ophthalmol Vis Sci 2004;45(5):1321-1328.
17 Wong TY, Chee SP. Risk factors of acute endophthalmitis after cataract extraction: a case-control study in Asian eyes. Br J Ophthalmol 2004;88(1):29-31.
18 Cao H, Zhang L, Li LP, Lo S. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One 2013;8(8):e71731.
19 Scott IU, Flynn HW Jr, Feuer W. Endophthalmitis after secondary intraocular lens implantation. A case-report study. Ophthalmology 1995;102(12):1925-1931.
20 Nagaki Y, Hayasaka S, Kadoi C, Matsumoto M, Yanagisawa S,Watanabe K, Watanabe K, Hayasaka Y, Ikeda N, Sato S, Kataoka Y,Togashi M, Abe T. Bacterial endophthalmitis after small-incision cataract surgery. effect of incision placement and intraocular lens type. J Cataract Refract Surg 2003;29(1):20-26.
Conflicts of Interest: Silpa-archa S, None; Papirachnart A,None; Singhanetr P, None; Preble JM, None.
1 Nentwich MM, Ta CN, Kreutzer TC, Li B, Schwarzbach F, Yactayo-Miranda YM, Kampik A, Miño de Kaspar H. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J Cataract Refract Surg 2015;41(1):58-66.
2 Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology 2016;123(2):295-301.
3 de Vries FE, Gans SL, Solomkin JS, Allegranzi B, Egger M, Dellinger EP, Boermeester MA. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg 2017;104(2):e95-e105.
4 Donnelly JP, Nair S, Griffin R, Baddley JW, Safford MM, Wang HE,Shapiro NI. Association of diabetes and insulin therapy with risk of hospitalization for infection and 28-day mortality risk. Clin Infect Dis 2017;64(4):435-442.
5 Frisch A, Chandra P, Smiley D, Peng LM, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010;33(8):1783-1788.
6 Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection
21 Wong TY, Chee SP. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology 2004;111(4):699-705.
22 Diamond MP, Entman SS, Salyer SL, Vaughn WK, Boehm FH.Increased risk of endometritis and wound infection after cesarean section in insulin-dependent diabetic women. Am J Obstet Gynecol 1986;155(2):297-300.
23 Riley LE, Tuomala RE, Heeren T, Greene MF. Low risk of postcesarean section infection in insulin-requiring diabetic women. Diabetes Care 1996;19(6):597-600.
24 Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type I diabetic rats. Exp Eye Res 2009;89(5):686-692.
25 Zagon IS, Sassani JW, McLaughlin PJ. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes 2006;55(4):1141-1147.
26 Finucane K, Ambrey P, Narayan S, Archer CB, Dayan C. Insulin injection abscesses caused by Mycobacterium chelonae. Diabetes Care 2003;26(8):2483-2484.
27 Bock M, Johansson T, Fritsch G, Flamm M, Hansbauer B, Mann E, Sönnichsen A. The impact of preoperative testing for blood glucose concentration and haemoglobin A1c on mortality, changes in management and complications in noncardiac elective surgery: a systematic review.Eur J Anaesthesiol 2015;32(3):152-159.
28 Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults.Cochrane Database Syst Rev 2009(3):CD006806.
29 Blankush JM, Leitman IM, Soleiman A, Tran T. Association between elevated pre-operative glycosylated hemoglobin and post-operative infections after non-emergent surgery. Ann Med Surg (Lond) 2016;10:77-82.30 Rollins KE, Varadhan KK, Dhatariya K, Lobo DN. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr 2016;35(2):308-316.
31 Hwang JS, Kim SJ, Bamne AB, Na YG, Kim TK. Do glycemic markers predict occurrence of complications after total knee arthroplasty in patients with diabetes? Clin Orthop Relat Res 2015;473(5):1726-1731.
32 Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care 1999;22(9):1408-1414.
33 Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol 2011;5(2):412-418.
34 Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM, National Academy of Clinical Biochemistry. Position statement executive summary:guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2011;34(6):1419-1423.
35 Kilpatrick ES. Haemoglobin A1c in the diagnosis and monitoring of diabetes mellitus. J Clin Pathol 2008;61(9):977-982.
36 Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85(1):109-117.
37 Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved.Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab 1992;18(3):187-201.
38 Mowat A, Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med 1971;284(12):621-627.
39 Herrinton LJ, Shorstein NH, Paschal JF, Liu LY, Contreras R,Winthrop KL, Chang WJ, Melles RB, Fong DS. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016;123(2):287-294.
40 Dev S, Pulido JS, Tessler HH, Mittra RA, Han DP, Mieler WF,Connor TB Jr. Progression of diabetic retinopathy after endophthalmitis.Ophthalmology 1999;106(4):774-781.
41 Zhu YN, Chen XY, Chen PQ, Wu JJ, Hua HX, Yao K. The occurrence rate of acute-onset postoperative endophthalmitis after cataract surgery in Chinese small- and medium-scale departments of ophthalmology. Sci Rep 2017;7:40776.
42 DiLoreto DA Jr, Bressler NM, Bressler SB, Schachat AP. Use of best and final visual acuity outcomes in ophthalmological research. Arch Ophthalmol 2003;121(11):1586-1590.