INTRODUCTION
The pathogenetic correlation between central retinal vein occlusion (CRVO) and open angle glaucoma(OAG) is complex. CRVO has a cardiovascular risk profile that is stronger for the ischemic disease type[1]. Diffuse senile arteriosclerosis and the senescent involution of collagen(lesions within the trabecular meshwork, lamina cribrosa, and common adventitial sheath of retinal vessels) are the main causes in most patients with retinal vein occlusions (RVO),but these phenomena are also involved in the pathogenesis of primary open angle glaucoma (POAG), representing a common ground for the both diseases. However, most CRVO cases are not associated with POAG, and the vast majority of POAG patients do not simultaneously develop RVO. Therefore, a common insult to the vascular system and collagen[2] may be presumed in only a subset of these patients. However, clinical case-control CRVO studies[1,3-4] have highlighted that POAG is a predictive, independent risk factor that is essential for the appearance of CRVO. POAG precedes and promotes the occurrence of CRVO by deforming the lamina cribrosa and secondarily distorting the vein as it passes through the optic nerve head. Hayreh et al[5] consider ocular hypertension/glaucoma to contribute to CRVO by producing stasis within the central retinal vein. By contrast, 2 studies[6-7] found that OAG was not a significant risk factor for developing CRVO.
The aim of this study is to prospectively evaluate the management and cumulative prevalence of OAG, including POAG and pseudoexfoliative glaucoma (PEXG), in patients with central/hemicentral RVOs over a 3-year follow-up period.
SUBJECTS AND METHODS
Ethical Approval The study was conducted in accordance with the Declaration of Helsinki and the protocol was reviewed and approved by the Institutional Review Board/Ethics Committee of the University. Patients provided written informed consent before entering the study.
Subjects The study included 57 patients with unilateral acute central/hemicentral RVOs who attended the Eye Clinic of the University of Medicine, Cluj-Napoca, Romania. The patients encompassed in the study had a unilateral CRVO or hemicentral RVO with a duration of symptoms of a venous occlusive event of ≤1mo[8-10]. Exclusion criteria consisted of prior ocular surgery, aphakia and pseudophakia, presence of any diabetic retinopathy in either eye, other retinal vascular diseases in the study eye or age-related macular degeneration in either eye.
Patient Examination Determination of the best-corrected visual acuity, perimetry, biomicroscopy, gonioscopy, ocular fundus examination and measurement of the intraocular pressure (IOP) were undertaken every 2mo during the first year and every 6mo thereafter for the next 2y. Optical coherence tomography, fluorescein angiography and stereoscopic photography of the optic disc were conducted every 6mo throughout the entire follow-up period.
Central/hemicentral Retinal Vein Occlusion Classification Central/hemicentral RVOs were divided into two groups:nonischemic and ischemic forms. The eligibility criteria for acute nonischemic and ischemic central/hemicentral RVOs have been comprehensively detailed in our previous publications[8-11].
Treatment OAGs were treated with the current ocular hypotensive medications used worldwide and/or surgery. We aimed to reduce the IOP by 30% from baseline values for the 3 OAG forms existing in our series.
Diagnostic Criteria
POAG associated with central/hemicentral RVOs[12](examination of the uninvolved fellow eye) 1) Structural and/or functional glaucomatous lesion; structural lesion includes acquired characteristic progressive optic neuropathy(cupping/saucerization of the optic disc, diffuse or localized thinning of the neuroretinal rim area, and/or retinal nerve fiber layer changes with diffuse or localized defects); functional damage encompasses characteristic reproducible changes in the visual field [retinal nerve fiber bundle defects (paracentral defects)], corresponding to optic disc lesions; 2) IOP within statistically normal limits (10-21 mm Hg; normal pressure glaucoma) or with increased values (>21 mm Hg; highpressure glaucoma); 3) Open anterior chamber angle (not occludable, no neovascularization, no goniodysgenesis); 4)No obvious evidence of an ocular or systemic possible cause of IOP increase (pseudoexfoliation, ocular trauma, pigment dispersion, use of steroids); 5) No ocular fundus or neurologic lesion other than glaucomatous cupping that could explain the visual field defect; 6) Clear ocular media.
PEXG associated with central/hemicentral RVOs[12]Changes include those referred to in the POAG diagnosis, to which the pseudoexfoliative syndrome adds: 1) Dandruff-like exfoliation material on the pupil border and on the surface of the anterior lens capsule, except the central zone; 2) Irregular and typically moth-eaten appearance of the pupillary collarette;3) Pigmentary loss from the central or mid-iris; 4) Pigment granules in the angle; 5) Loose zonule with occasional phacodonesis.
Perimetric progression of the OAG[13-15] The amount of glaucomatous visual field deterioration needed for reaching the perimetric progression defined by the Early Manifest Glaucoma Study (EMGS) was measured in conventional units and expressed as an average loss of -1.93 decibels (dB) of the mean deviation (MD)[16] in comparison with the baseline values. Progression was considered to have occurred if a preexisting visual field defect had indented (deepened) or expanded or if there was a new defect.
Main Outcome Measurements The primary outcome measurement was the cumulative prevalence of OAG in patients with acute central/hemicentral RVOs. The secondary outcome measurement was the efficacy of treatment as assessed by the cumulative prevalence of OAG progression.
Statistical Analysis Data analysis were conducted with the SPSS statistical software (version 16) and Epiinfo 2000.All of the patients’ conditions were described by descriptive analysis. Differences in means and medians between central/hemicentral RVOs with and without OAG were assessed using the Student’s t-test for normally distributed data, and both the Mann-Whitney U and Wilcoxon tests for nonparametric distributions. Categorical data were analyzed using Pearson’s Chi-square test and Fisher’s exact test. To account for deaths and losses to follow-up we used the Kaplan-Meier method to estimate cumulative incidence. Statistical significance was defined as P<0.05.
RESULTS
Clinical and demographic characteristics of the central/hemicentral RVOs, with and without associated OAG, are shown in Table 1.
There were no significant differences between the venous occlusions with and without OAG with regard to age, sex,occlusion forms (ischemic/nonischemic), or occlusion types(CRVO/hemicentral RVO). Central corneal thickness (CCT)was significantly lower (P=0.001) in OAG patients associated with venous occlusions than in those without this association.OAG was observed in 3 clinical forms, namely, POAG with increased IOP (high-pressure glaucoma) in 4 patients (patients 1-4); POAG with normal IOP (normal-pressure glaucoma)in 3 patients (patients 5-7); and PEXG in 3 patients (patients 8-10; Table 2). The cumulative prevalence of OAG in patients with central/hemicentral RVOs was 19.6% (95%CI: 8.7-30.5;Figure 1).
Table 1 Baseline characteristics in the 57 central/hemicentral RVO patients with and without associated OAG
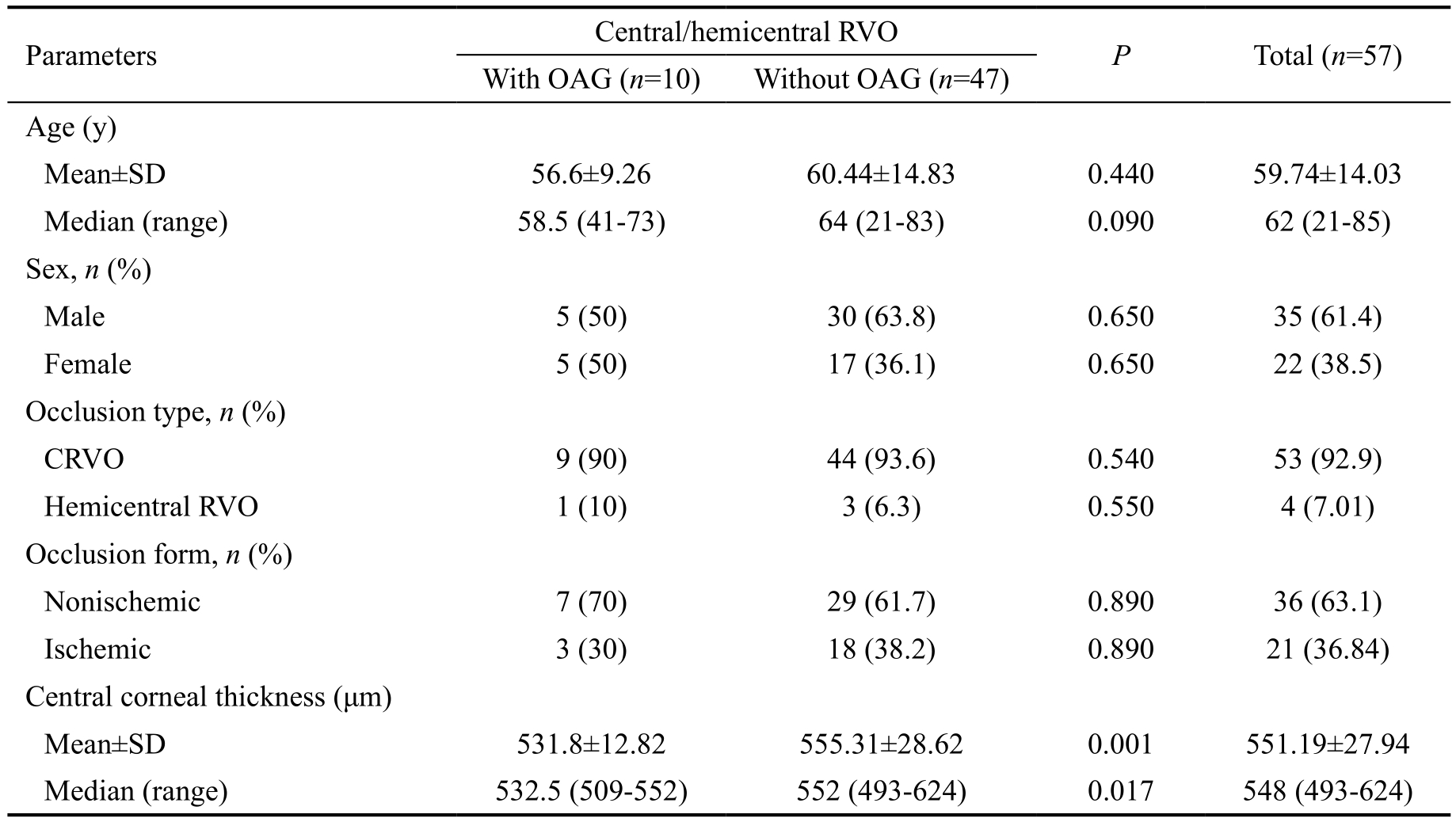
OAG: Open angle glaucoma; CRVO: Central retinal vein occlusion; RVO: Retinal vein occlusion.
Parameters Central/hemicentral RVO P Total (n=57)With OAG (n=10) Without OAG (n=47)Age (y)Mean±SD 56.6±9.26 60.44±14.83 0.440 59.74±14.03 Median (range) 58.5 (41-73) 64 (21-83) 0.090 62 (21-85)Sex, n (%)Male 5 (50) 30 (63.8) 0.650 35 (61.4)Female 5 (50) 17 (36.1) 0.650 22 (38.5)Occlusion type, n (%)CRVO 9 (90) 44 (93.6) 0.540 53 (92.9)Hemicentral RVO 1 (10) 3 (6.3) 0.550 4 (7.01)Occlusion form, n (%)Nonischemic 7 (70) 29 (61.7) 0.890 36 (63.1)Ischemic 3 (30) 18 (38.2) 0.890 21 (36.84)Central corneal thickness (μm)Mean±SD 531.8±12.82 555.31±28.62 0.001 551.19±27.94 Median (range) 532.5 (509-552) 552 (493-624) 0.017 548 (493-624)
Table 2 Characteristics of the 10 patients with central/hemicentral RVO associated with OAG

OAG: Open angle glaucoma; CRVO: Central retinal vein occlusion; RVO: Retinal vein occlusion; IOP: Intraocular pressure; ODH: Optic disc hemorrhage; BP: Blood pressure; c/d: Cup-to-disc ratio; OPP: Ocular perfusion pressure; PEXG: Pseudoexfoliative glaucoma; FCBT: Fixed combination bimatoprost/timolol; B: Bimatoprost; Tc: Trabeculectomy.
Case SexAge,y Diagnosis Treatment Initial Final Initial Final 1 F 61 Hemicentral OPP=100 mm Hg 26 18 -4.1 -4.5 c/d=0.5 522 High-pressure glaucoma FCBT RVO Cardio-vascular disease 2 M 73 CRVO OPP=95 mm Hg 24 16.5 -3.8 -4 c/d=0.5 ODH Occlusion form Progression factors IOP(mm Hg)Mean deviation(decibels)Posterior ocular pole Corneal thickness(μm)536 High-pressure glaucoma B Cardio-vascular disease 3 M 59 CRVO OPP=100 mm Hg 27 18.5 -4.4 -4.12 c/d=0.6 529 High-pressure glaucoma FCBT 4 F 59 CRVO OPP=96 mm Hg 21 14.5 -3.9 -4.1 c/d=0.5 ODH 541 PEXG FCBT OPP=90 mm Hg 536 High-pressure glaucoma B 5 M 53 CRVO Systolic BP 120 mm Hg 19 13 -4.6 -4.5 c/d=0.7 509 Normal-pressure glaucoma FCBT 6 F 65 CRVO OPP=95 mm Hg 20.5 14 -3.9 -3.84 c/d=0.6 545 Normal-pressure glaucoma FCBT Migraine 7 F 46 CRVO Systolic BP 110 mm Hg 18.5 12.5 -4.5 -4.82 c/d=0.7 552 Normal-pressure glaucoma FCBT Migraine 8 M 41 CRVO Pseudoexfoliation 29 20 -5 -5.5 c/d=0.8 520 PEXG Tc OPP=70 mm Hg 9 M 58 CRVO Pseudoefoliation 31 220 -5.5 -5.2 c/d=0.7 528 PEXG Tc OPP=80 mm Hg 10 F 51 CRVO Pseudoexfoliation 27 18 -4.5 -4.32 c/d=0.7 ODH
The ocular hypotensive medication used was a fixed combination of 0.03 bimatoprost/0.5% timolol (FCBT; Ganfort,Allergan, Inc., Irvine, CA, USA) in 6 patients (patients 1,3, 5, 6, 7, and 10) and 0.03% bimatoprost (B) (Lumigan,Allergan, Inc., Irvine, CA. USA) in 2 patients (patients 2 and 4). Trabeculectomy (Tc) was performed in 2 patients (patients 8 and 9). After these treatments, IOP decreased significantly from 24.3±4.36 mm Hg to 16.55±2.85 mm Hg (Table 3),a reduction of 31.89% compared with baseline values.Glaucoma progression was not present in any of the cases, and all perimetric and morphologic indices had non-significant changes (Table 3).
Table 3 Descriptive and discriminating statistics in the 10 patients with central/hemicentral RVO associated with OAG
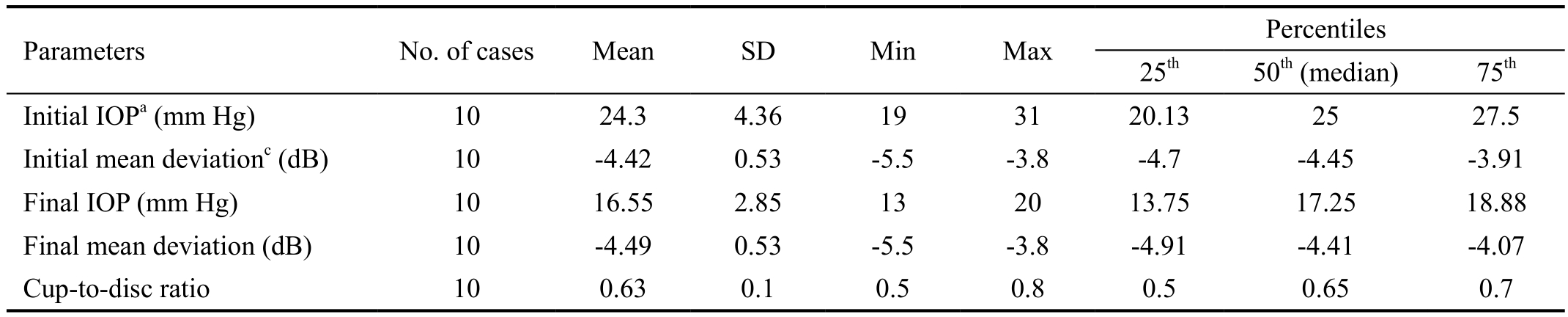
OAG: Open angle glaucoma; RVO: Retinal vein occlusion; IOP: Intraocular pressure; Db: Decibels. aInitial vs final IOP: P<0.0001 for the mean and P=0.023 for the median; cInitial vs final mean deviation: P=0.470 for the mean and P=0.900 for the median.
Parameters No. of cases Mean SD Min Max Percentiles 25th 50th (median) 75th Initial IOPa (mm Hg) 10 24.3 4.36 19 31 20.13 25 27.5 Initial mean deviationc (dB) 10 -4.42 0.53 -5.5 -3.8 -4.7 -4.45 -3.91 Final IOP (mm Hg) 10 16.55 2.85 13 20 13.75 17.25 18.88 Final mean deviation (dB) 10 -4.49 0.53 -5.5 -3.8 -4.91 -4.41 -4.07 Cup-to-disc ratio 10 0.63 0.1 0.5 0.8 0.5 0.65 0.7
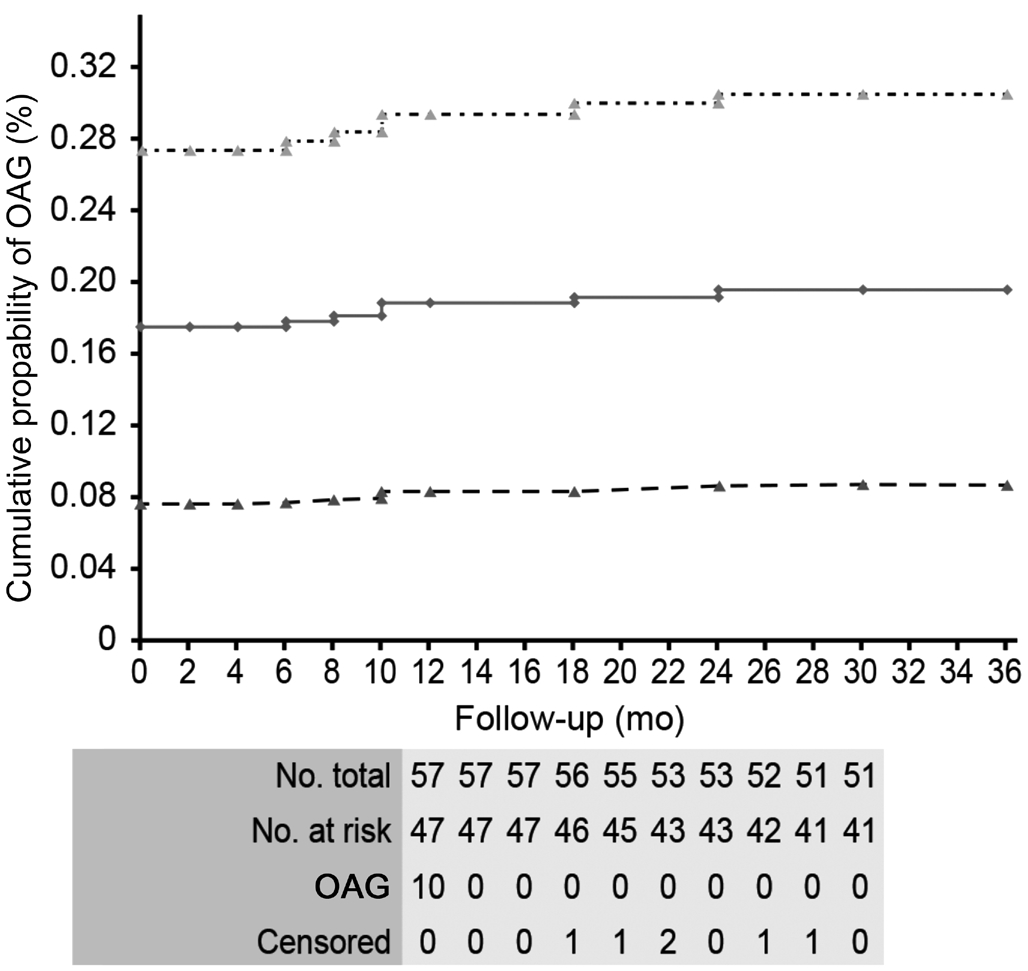
Figure 1 The Kaplan-Meier estimate of the cumulative prevalence of OAG during the entire course of the study in 57 patients with acute unilateral central/hemicentral RVO.
DISCUSSION
Although the number of OAG patients in our series remained the same throughout the follow-up period, the cumulative prevalence of OAG in patients with unilateral acute central/hemicentral RVOs increased from 17.5% to 19.6% (95%CI:8.7-30.5) due to the exclusion of 6 central/hemicentral RVO patients from the study (3 patients with nonischemic forms were lost to follow-up; 1 ischemic patient withdrew from the study; and 2 ischemic patients died). The cumulative prevalence of OAG in these central/hemicentral RVO patients was much greater than that of the general population older than 40y (2.1%, varying from 0.3% in persons aged 40 years old to 3.3% in persons aged 70 years old)[12]. Hayreh et al[5] reported that the global prevalence of OAG was 9.9% in central/hemicentral RVO patients. The greater cumulative prevalence of OAG in our series compared to that of Hayreh et al[5] can be explained by the difference in the number of patients investigated (57 vs 674) and the duration of the follow-up period (36 vs 21mo).
Case-control CRVO studies[1,3-4] have shown a significant association of CRVO with a history of OAG and have highlighted that glaucoma is one of the major risk factors that is independent and predictive for CRVO occurrence. However,in a longitudinal analysis of the risk factors associated with CRVO, Stem et al[6] did not identify OAG among the main factors for CRVO occurrence, although their study showed an adjusted hazard ratio of 1.50 (95%CI: 1.30-1.72; P<0.0001)relative to individuals without this condition, indicating an approximately 50% risk of CRVO. Likewise, Zhou et al[7]did not find a significant association between the incidence of RVO and higher IOP or OAG.
Venous occlusions may appear during the early or late stages of glaucoma. In our study, glaucoma, including POAG and PEXG, had been already diagnosed and treated when venous occlusions occurred, and the functional and morphologic glaucomatous damage was mild (mean deviation: -4.42 dB±0.53). Increased IOP and glaucomatous cupping may be causally associated with CRVO produced at the level of optic disc excavation[17]. Elevated IOP results in a retrodisplacement of the lamina cribrosa with deformation of the channels that pass through it, exerting an adverse local hemodynamic influence. These events can compress the vein, predisposing patients to occlusions at the level of the lamina cribrosa[17].Glaucomatous cupping presumably causes a loss of the support tissue of the retinal vein, exposing the tissue to IOP changes.In addition, the vein is distorted as it bends around the highpitched rim of the cupping[17-18].
The perimetric and morphologic progression of OAG was not seen in any of the 10 patients, although the risk factors for progression were present in almost all of them, namely,higher IOP (patients 1-4, 8-10); age ≥60y (patients 1, 2, and 6);pseudoexfoliation (patients 8-10); optic disc hemorrhages at the beginning of the study (patients 2, 4, and 10); lower ocular systolic perfusion pressure (OPP; ≤125 mm Hg; patients 1-4,6, 8-10); cardiovascular disease history (patients 1 and 2);lower systolic blood pressure (BP; patients 5 and 7); thinner CCT (<550 µm; patients 1-6, 8-10); and history of migraine linked to sex (patients 6 and 7).
The lack of glaucoma progression in our series can be explained in two ways: the predefined target percentage of IOP decrease from the initial values under the influence of treatment (30%) for each of the 3 forms of OAG and the relatively short follow-up period (3y). We chose the percentage of 30% for the reduction in IOP because the target value of 25%, as set by the Early Manifest Glaucoma Trial (EMGT)[19],proved to be insufficient for the prevention of progression.In a previous study, progression occurred during an 11-year follow-up in 40% of patients with high-pressure glaucoma, in 55% of patients with normal-pressure glaucoma, and in 100%of PEXG patients[16,19]. In addition, we believe that glaucoma progression would have appeared if the follow-up period had been longer in our study. The EMGT noted an average progression time of 66mo in OAG patients treated with betaxolol plus argon laser trabeculoplasty, which was 18mo longer than that in the untreated control group.
We treated two patients with 0.03% bimatoprost (patients 2 and 4; Table 2) who had been treated just before the study with the prostaglandin analogue latanoprost (Xalatan, Pfizer Inc.,New York, USA). Because clinically adequate IOP control(e.g. an IOP reduction of 30%) could not be achieved with this medication, we replaced latanoprost with bimatoprost,which decreased their IOPs by 31.5% and 30.05% at the end of the follow-up period, respectively, compared with the initial values. The fact that bimatoprost and its hydrolysis product(e.g. the active free bimatoprost acid) are active metabolites in the eye can explain the increased efficiency of bimatoprost,particularly among patients who do not respond adequately to latanoprost[20]. Further, latanoprost is a prodrug; our patients’lack of response might have involved poor deesterification of the prodrug to the pharmacologically active free fatty acid[20].Six central/hemicentral RVO patients with glaucoma (2 patients with high-pressure glaucoma, 3 patients with normalpressure glaucoma, and 1 patient with PEXG; patients 1, 3, 5,6, 7, and 10; Table 2) were treated with FCBT. The decrease in IOP relative to the initial values was >30% in all cases. The superiority of the Ganfort solution compared with the other two available fixed prostaglandin combinations [Duotrav(Alcon Laboratories, Inc., Fort Worth, TX, USA) and Xalcom(Pfizer, Inc., New York, NY, USA)] has been demonstrated by Centofanti et al[21].
The analysis of the risk factors for the progression of the 3 forms of glaucoma encountered in our series revealed several interesting pieces of data. In the case of the 4 patients with high-pressure glaucoma (patients 1-4; Table 2), the main risk factors were increased IOP, lower OPP, cardiovascular disease history, and thinner CCT. An IOP decrease of 30% compared with baseline, as well as a related increase in the OPP, were the essential factors for preventing progression in our series.Over an 11-year follow-up period, EMGT[19] reported 40%progression among treated cases of high-pressure glaucoma after an IOP reduction of only 25% compared with the initial values.
In the 3 cases of normal-pressure glaucoma (patients 5-7; Table 2), the risk factors for progression were lower systolic BP and migraines. With the current ocular hypotensive medications used worldwide (prostaglandins and carboanhydrase inhibitors), we were able to reduce IOP by 30% compared with the initial values, an extremely useful therapeutic construct adopted for the prevention of progression. Surgical treatment was unnecessary in all cases to achieve this target. IOP is considered a part of the pathogenetic process and represents a progression factor in at least some cases of normal-pressure glaucoma[22]. The protective effect of IOP reduction is amplified by the subsequent increase in the OPP. The Collaborative Normal-Tension Glaucoma Study[22-23]reported 12% perimetric progression during a 5- to 7-year follow-up in normal-tension glaucoma patients treated with medication (excluding beta blockers and alpha-adrenergic agonists), laser trabeculoplasty, and trabeculectomy, during which a 30% reduction of IOP compared with the initial values was achieved in almost all cases. The percentage of progression reported by this study (12%) can be explained by the fact that, in patients treated with filtering surgery,a decrease in IOP of only 20% compared with the initial values was admitted, and no secondary surgical procedure was necessary. Alternatively, these values may be accounted for by the fact that these patients presented via a pressureindependent mechanism of the glaucomatous lesion. After an 11-year follow-up, the EMGT[19] reported 55% progression in treated patients with normal-pressure glaucoma, in whom a decrease in IOP of only 25% compared with the initial values was achieved.
In the 3 patients with PEXG (patients 8-10; Table 2), the increased IOP, pseudoexfoliation, and lower OPP were the essential factors of progression. The fact that we managed to decrease IOP by 30% contributed decisively to the prevention of this progression. EMGT[19] noted glaucoma progression in all patients (100%), with PEXG (8 cases) occurring after a progression time of 25mo, after the IOP had already been reduced by 25% compared with baseline. The more severe clinical course of PEXG is well known, as its IOP values are higher and more difficult to therapeutically influence than the average POAG case. Visual field damage is typically greater in these patients than in those with POAG, reflecting the likely greater effects of IOP on the optic nerve[24]. The two basic components of pseudoexfoliation syndrome, e.g. exfoliation and pigmentation, play important roles in the occurrence of PEXG. The exfoliation material and pigment granules accumulate in the trabecular meshwork and Schlemm’s canal,causing decreased aqueous humor outflow and significantly elevated IOPs. Unlike those with POAG, patients with pseudoexfoliation syndrome do not respond to topical steroid administration; instead, their reaction is rather similar to that of the normal population. In addition, the pathomechanism of PEXG interferes in some cases with the pathomechanism of POAG. Vascular factors represent potential possible pathomechanisms contributing to the increased progression of PEXG[24-25].
The main limitation of our study was the lack of a control group from the general population to determine the cumulative prevalence of the OAG and the disease progression. Monitoring the pressure behaviors, as well as structural and functional parameters, could have revealed whether OAG evolves differently in patients with vs without central/hemicentral RVOs. Similarly, the observation period of 3y was too short to assess the progression of OAG in the treated patients.
In conclusion, the high value of the cumulative prevalence of OAG in central/hemicentral RVO patients denotes that OAG is a risk factor for the appearance of venous occlusions. The treatment of glaucoma, which achieved an IOP reduction of 30% compared with the initial values, prevented the progression of OAG over the 3-year follow-up period.
ACKNOWLEDGEMENTS
Conflicts of Interest: Călugăru D, None; Călugăru M,None.
1 The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol 1966;114(4):545-554.
2 Coscas G, Dhermy P. Occlusions Veineuses Retiniennes. Paris: Masson;1978:30-50; 77-116; 119-141; 143-150; 277-345; 357-403; 405-439.
3 Shahsuvaryan ML, Melkonyan AK. Central retinal vein occlusion risk profile: a case-control study. Eur J Ophthalmol 2003;13(5):445-452.
4 Koizumi H, Ferrara DC, Bruè C, Spaide RF. Central retinal vein occlusion case-control study. Am J Ophthalmol 2007;144(6):858-863.
5 Hayreh SS, Zimmerman MB, Beri M, Podhajsky P. Intraocular pressure abnormalities associated with central and hemicentral retinal vein occlusion. Ophthalmology 2004;111(1):133-141.
6 Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology 2013;120(2):362-370.
7 Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, Yang H, Jonas JB. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing eye study. Ophthalmology 2013;120(4):803-808.
8 Călugăru D, Călugăru M. Intravitreal bevacizumab in acute central/hemicentral retinal vein occlusions: three-year results of a prospective clinical study. J Ocul Pharmacol Ther 2015;31(2):78-86.
9 Călugaru D, Călugăru M. Ranibizumab in preproliferative (ischemic)central retinal vein occlusion. The Rubeosis anti-VEGF (RAVE) trial.Retina 2015;35(10):59-61.
10 Călugaru D, Călugăru M. Retinal vein occlusion and the use of a dexamethasoene intravitreal implant (Ozurdex) in its treatment. Graefes Arch Clin Exp Ophthalmol 2016;254(12):2477-2478.
11 Călugăru D, Călugăru M, Ţălu Ş. Ocular hypertension in patients with central/hemicentral retinal vein occlusions: cumulative prevalence and management. Int J Ophthalmol 2018;11(7):1173-1178.
12 European Glaucoma Society. Terminology and Guidelines for Glaucoma.3rd ed. Savona Italy: Dogma; 2008:15-16; 20; 67-73; 111-113.
13 Boey PY, Mansberger SL. Ocular hypertension: an approach to assessment and management. Can J Ophthalmol 2014;49(6):489-496.
14 Chan PP, Leung CK, Chiu V, Gangwani R, Sharma A, So S, Congdon N. Protocol-driven adjustment of ocular hypotensive medication in patients at low risk of conversion to glaucoma. Br J Ophthalmol 2015;99(9):1245-1250.
15 Martínez A, Sanchez-Salorio M. Predictors for visual field progression and the effects of treatment with dorzolamide 2% or brinzolamide 1%each added to timolol 0.5% in primary open-angle glaucoma. Acta Ophthalmol 2010;88(5):541-552.
16 Heijl A, Leske MC, Bengtsson B, Bengtsson B, Hussein M, Early Manifest Glaucoma Trial Group. Measuring visual field progression in the early manifest glaucoma trial. Acta Ophthalmol Scand 2003;81(3):286-293.
17 Beaumont PE, Kang HK. Cup-to-disc ratio, intraocular pressure, and primary open-angle glaucoma in retinal venous occlusion. Ophthalmology 2002;109(2):282-286.
18 Klein BE, Meuer SM, Knudtson MD, Klein R. The relationship of optic disk cupping to retinal vein occlusion: the Beaver Dam Eye Study.Am J Ophthalmol 2006;141(5):859-862.
19 Leske MC, Heijl A, Hyman L, Bengtsson B, Dong LM, Yang ZM,EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007;114(11):1965-1972.
20 Gandolfi SA, Cimino L. Effect of bimatoprost on patients with primary open-angle glaucoma or ocular hypertension who are nonresponders to latanoprost. Ophthalmology 2003;110(3):609-614.
21 Centofanti M, Oddone F, Gandolfi S, Hommer A, Boehm A, Tanga L,Sangermani C, Sportelli V, Haustein M, Manni G, Rossetti L. Comparison of travoprost and bimatoprost plus timolol fixed combinations in openangle glaucoma patients previously treated with latanoprost plus timolol fixed combination. Am J Ophthalmol 2010;150(4):575-580.
22 Collaborative Normal Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normaltension glaucoma. Am J Ophthlmol 1998;126(4):498-505.
23 Caprioli J. The treatment of normal-tension glaucoma. Am J Ophthalmol 1998;126(4):578-581.
24 Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology 2001;108(6):1043-1049.
25 Harju M, Vesti E. Blood flow of the optic nerve head and peripapillary retina in exfoliation syndrome with unilateral glaucoma or ocular hypertension. Graefe’s Arch Clin Exp Ophthalmol 2001;239(4):271-277.