INTRODUCTION
Outer retinal tubulations (ORTs) were first identified by Curcio et al[1] in a histopathologic investigation of autopsy eyes showing advanced age-related macular degeneration (AMD). The morphologic characteristics of ORTs were described as tubular structures which appeared as circular or ovoid hyperreflective borders surrounding hyporeflective spaces on spectral-domain optical coherence tomography (SDOCT) scans[2].
ORTs were documented as response to focal disruptions of the outer retina, always located in the outer nuclear layer (ONL) of the retina. It was postulated that ORTs develop in the evolution process of scrolling of the external limiting membrane (ELM),invagination of the disrupted remaining photoreceptors and downward displacement or collapse of the inner nuclear layer (INL), outer plexiform layer (OPL), and ONL, resulting in the lateral conjunction by the involving cells[2-6]. The hyperreflective borders of ORTs were proposed histologically to be mitochondria of degenerating cone photoreceptors which translocated from the inner segments to the cell bodies and ELM which delimited the ORT lumen[6-8]. Previous studies have revealed that ORTs can be considered as interconnecting tubes of Müller glia cells and surviving photoreceptor cells in histopathological rearrangement over disciform scars associated with AMD[1,7]. Besides these, critical histologic characteristics of ORTs also include absence or degeneration of the underlying retinal pigment epithelium (RPE). ORTs usually contain malformed photoreceptor outer segments which represent as hyperreflective material in lumina[9].
ORTs have been described in numerous different retinal diseases mainly associated with subretinal fibrosis or choroidal neovascularization (CNV), such as neovascular AMD, angioid streaks, myopic neovascularization, multifocal choroiditis and pseudoxanthoma elasticum[1-2,10-13]. It has also been reported in some cases associated with various retinal degenerative diseases without CNV, including non-neovascular AMD,retinitis pigmentosa, pattern dystrophy, Stargardt disease, acute zonal occult outer retinopathy, cone dystrophy, central serous choroidopathy and gyrate atrophy[14-18].
SD-OCT is a promising instrument which performs highresolution cross-sectional images noninvasively in diagnosing and following up retinal disorders. Detailed exploration of cross-sectional SD-OCT B-scan images has provided information on different configurations of ORTs. In addition,the morphology of ORTs may resemble a branching network with en-face SD-OCT. The formed morphologies are composed of subtypes including closed and open ORTs. Schaal et al[7] have described two ORT histological configurations:“open form”, which has horizontally elongated cross sections and hyporeflective cavity with curving ELM without complete encircling at the ends and nonphotoreceptor cells on the outer aspect and “closed form”, which are hyperreflective circular or oval with ELM on the outer border and photoreceptors completely encircling the lumen. The forming ORTs which precede the formed ORTs are proposed as scrolling of the ELM over a free edge without hyporeflective core[3-7].
However, there have been few studies investigating the long period demographics of ORTs in eyes with diabetic macular edema (DME) under treatment with intravitreous anti-vascular endothelial growth factor (VEGF) injections and integrating the significance of observations with disease severity and prognostic value in Chinese people to date. This is thus investigated in this study.
SUBJECTS AND METHODS
Ethical Approval All research and measurements complied with the tenets of the Declaration of Helsinki, and this study was approved by the Medical Research Ethics Committee and Institutional Review Board of Wuhan General Hospital of Guangzhou Military Region. Written informed consents were obtained from all participants.
Patients This retrospective and descriptive study involved the investigation of 435 eyes from 228 patients with DME,enrolled in Ophthalmology Department of Wuhan General Hospital of Guangzhou Military Region, between March 2016 and January 2018. The diagnoses of DME were made on the basis of the stereo fundus photograph, SD-OCT, and fluorescein angiography results. Cataracts were minimal to absent in all objects investigated.
Inclusion criteria were as follows: from 18 years old and above; best corrected visual acuity (BCVA) ≥23 in Early Treatment Diabetic Retinopathy Study (ETDRS). Visual impairment is caused by DME secondary to proliferative or non proliferative diabetic retinopathy (DR) in type 1 or type 2 diabetes mellitus. DME is characterized by posterior retinal thickening or rigid exudative deposition, which may be located in the area 500 microns away from the fovea, or less than 500 microns. Glycated hemoglobin level (HbAlc) of the selected subjects was less than 8.0%.
Exclusion criteria were macular disease associated with CNV, retinal dystrophies or retinal degeneration rather than DME; eye diseases, such as retinal detachment, uncontrolled glaucoma; retinopathy such as retinal vein occlusion(RVO) caused by other diseases than diabetes; patients with uncontrollable systemic disease; treatment for DME with intravitreal anti-VEGF injections in the study eye within 6mo before baseline; treatment with ocular steroid injections or implant within 6mo before baseline; evidence or history of any intraocular surgeries; panretinal photocoagulation or focal macular laser treatment within at last 3mo before baseline;and patients with poor fixation whose image qualities were not sufficient.
Ophthalmic Examinations All patients underwent standard eye examinations, including BCVA, slit-lamp biomicroscopy,intraocular pressure measurement and fundus examination. An increase in BCVA greater than or equal to five letters is defined as improvement, while decrease in BCVA greater than or equal to five letters is defined as deterioration. BCVA fluctuation within five letters is defined as stable.
Spectral-domain Optical Coherence Tomography Commercially available high-axial resolution SD-OCT(3D-OCT-2000; TOPCON, Tokyo, Japan) was used in this study to obtain and estimate retinal architecture after pupillary dilation at each visit. A 6-mm raster scan of the axial retinal sections (comprised of 512×128 lines) was manipulated in every eye studied.
High-resolution baseline and follow-up images which centred at fovea and achieved by vertical and horizontal scans over consecutive visits were analyzed separately by two professional investigators to elucidate the OCT images, identify the presence of ORT and evaluate its prevalence, situation in the retinal layers and morphologic feature. Simultaneously, the evolution of ORT over time, central retinal thickness (CRT),type of fluid and subfoveal photoreceptor integrity on OCT imaging was also registered in overall eyes. Discrepancies were referred to a senior specialist for final determination.
The collapsed ORTs were defined as becoming applanate presentation with detectable hyperreflective borders from round or oval appearances on follow-up scans. The disappeared ORTs had no identifiable hyperreflective boudaries on monitoring scans compared with baseline. The recurrent ORTs were suggested as reappearing with identifiable hyperreflective borders on follow-up scans. The steady ORTs were detected as having no transformation in appearances from baseline to the end of follow-up span.
Anti-vascular Endothelial Growth Factor Treatment Enrolled patients received anti-VEGF therapy with ranibizumab or conbercept (0.5 mg/0.05 mL) injected intravitreally after diagnostic decision in a pro re nata (PRN) regimen.
Follow-up Each patient was reviewed regularly, at least once every three months. The minimum follow-up period was 6mo.
Statistical Analysis All statistical analysis were performed using SPSS version 20.0 (IBM Inc., Chicago, Illinois, USA).Descriptive analysis was done foremost. Categorical variables were presented in the form of frequencies and percentages and continuous variables in the form of mean±standard deviation(SD). A Chi-square test was applied to compare the frequency of binary categorical variables. Differences were reported with 95% confidence intervals. A P value<0.05 was considered as statistically significant.
RESULTS
Four hundred and thirty-five eyes with DME from 228 patients were recruited, unilaterally from 21 patients, bilaterally from 207 patients. Totally 228 patients included 127 males and 101 females, with a mean age±SD of 57.6±16.3 (range 19-79)years old. Treatment was with ranibizumab in 139 eyes of 77 patients and conbercept in 296 eyes of 151 patients. The follow-up duration was through 6-19mo. The average number of anti-VEGF injection was 5.04±1.79 (range 2-10) in the total cohort. During follow-up the mean anti-VEGF injection times between patients without and with ORTs were 5.62±1.38 and 4.85±1.12, respectively (P=0.26). ORTs were identified in 108 eyes of 435 eyes with an overall estimated incidence rate of 24.83% at baseline.

Figure 1 Different configurations of ORTs in different formative stages on OCT profiles from eyes with DME A: A SD-OCT scan of an eye with DME showed the development of ORT (arrowhead),hyperreflective dense granules in the ONL. INL, OPL and ONL were pulled down near the ORT, which was called OPL subsidence sign(arrows); B: A SD-OCT scan of an eye with DME displayed a long,ovoid and open ORT structure (arrowhead) evolving into a smaller ORT;C: An area of closed ORT (arrowhead) was identified by an oval structure located in ONL, OPL and INL with a mixed reflectivity lumen.
The ORT mean number in this investigation was1.40±0.27(ranged from 1 to 4) on any particular SD-OCT line scan.ORT mean vertical height was 101.26±24.93 μm (ranged from 46 μm to 192 μm), ORT mean width on horizontal OCT B-scan sections was 103.84±38.67 μm (ranged from 39 μm to 374 μm). Detailed exploration of cross-sectional SD-OCT B-scan images has provided information on different configurations of ORT: forming, open and closed (Figure 1).
The OCT scans indicated the content of ORT structures contained multiple granules of variable reflectivity in certain cases. From observations, ORTs were prone to locate in the proximity of the lesions of exudation and/or cystoid macular edema (Figure 2), which showed appearance of round or ovoid hyporeflective lumina with hyperreflective boundaries and demonstrated the distinguishing features from cystoid macular edema. Adjacent to the ORT focus there were multiple intraretinal cysts without hyperreflective borders in INL and ONL usually. Disruption of the integrity of subfoveal photoreceptor, intraretinal exudative lesions combined with subretinal liquid (SRF) and/or intraretinal liquid (IRF) on SDOCT was simultaneously indicated. Among the eyes with presence of ORTs, there were forming ORTs in 15 eyes, open ORTs in 30 eyes and closed ORTs in 63 eyes. The new ORTs in DME eyes presumably stemmed from the intraretinal fluid that disturbed the outer retinal structure and the gradual damage and reorganization of the photoreceptor layer, whether prior to or after anti-VEGF treatment. Furthermore, the formation process of ORT leaded to the focal downward displacement of OPL and INL toward the RPE adjacent to the lesion (Figure 1).During the follow up span of treatment with anti-VEGF for DME, 45 eyes had steady ORTs and 63 eyes had dynamic variants in ORTs. Table 1 summarizes different configurations of ORTs in eyes with ORTs. Concerning the proportion of forming ORTs and closed ORTs, there was a statistically significance between eyes with steady ORTs and eyes with variant ORTs (P=0.006, P=0.017 respectively). In the eyes with stable ORTs, there are higher proportion of closed ORTs and fewer proportion of forming ORTs. Regarding the proportion of open ORTs, patients with steady ORTs showed no significant difference compared with patients with variant ORTs (P=0.356).
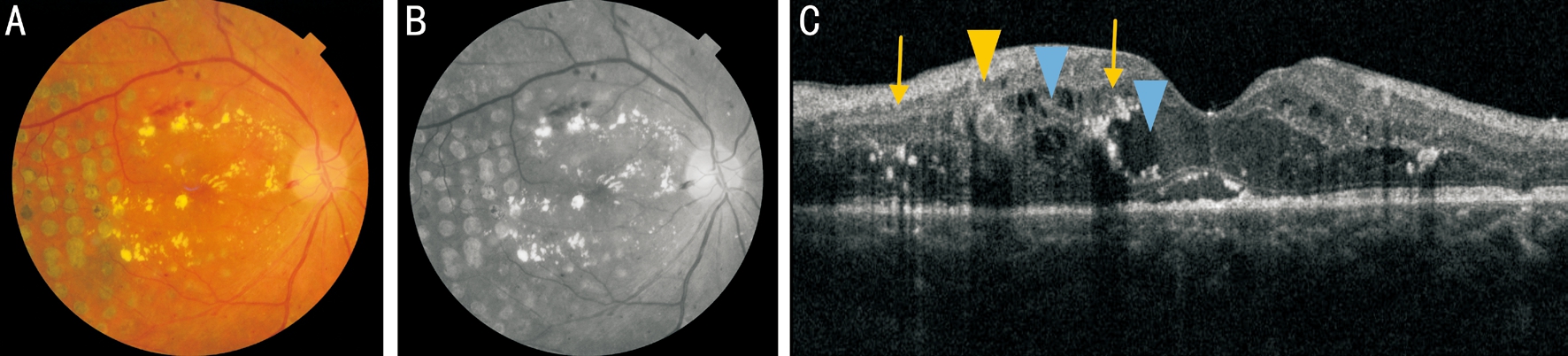
Figure 2 Ocular examinations of a 64-year-old man presenting with DME A: Color fundus photography; B: Infrared image; C: The corresponding SD-OCT B-scan section showed an ORT in OPL and INL (yellow arrowhead) and cystoid macular edema with distinguishing characteristics (blue arrowheads). The ORT was prone to locate in near proximity of the lesions of cystoid macular edema and exudation (arrows).

Figure 3 Eyes with steady or collapsed ORTs in patients with DME A: Two small ORTs (arrowheads) were present in the ONL adjacent to the fovea with evidence of subretinal and intraretinal fluid (BCVA=35 letters, CRT=739 μm); B: Three months after injections with conbercept,the macular edema decreased and the ORTs (arrowheads) remained steady with location of edema regions (BCVA=50 letters, CRT=285 μm);C: An ORT was present in INL and OPL nasal to the fovea (arrowhead) adjacent to cystic intraretinal fluid (BCVA=65 letters, CRT=196 μm);D: Two months after starting of conbercept treatment, the ORT collapsed (arrowhead), together with the remission of the intraretinal leakage(BCVA=70 letters, CRT=167 μm).
Table 1 Different configurations of ORTs in eyes with ORTs n (%)

ORT: Outer retinal tabulation.
Different configurations of ORTs Eyes with steady ORTs Eyes with variant ORTs P Forming ORTs (15 eyes) 2 (4.44) 13 (20.64) 0.006 Open ORTs (30 eyes) 9 (20.00) 21 (33.33) 0.356 Closed ORTs (63 eyes) 34 (75.56) 29 (46.03) 0.017

Figure 4 Eyes with increased, decreased or disappeared ORTs in patients with DME A: Initially, an ORT (arrowhead) was mainly noted in INL together with subretinal fluid and intraretinal cysts (BCVA=50 letters, CRT=548 μm); B: One month after initial ranibizumab injection,the retinal macular edema decreased, but the ORT structure (arrowhead) increased in size, with the reflectance increase of the ORT tube wall and lumen (BCVA=55 letters, CRT=339 μm); C: Before anti-VEGF treatment, OCT showed DME and two ORTs (arrowheads) near proximity of fovea with evidence of intraretinal cysts and retinal incrassation (BCVA=44 letters, CRT=314 μm); D: After one month of conbercept treatment,the temporal ORT (arrowhead) disappeared, while the nasal lateral ORT (arrowhead) decreased in size compared with baseline, with cystic intraretinal fluid at the fovea (BCVA=50 letters, CRT=302 μm).

Figure 5 An eye with ORTs showing repeated changes in a 69-year-old patient with DME A: Two small ORTs (solid arrow) showed presence nasal to the fovea adjacent to cystic intraretinal fluid (BCVA=70 letters, CRT=364 μm); B: Two months after starting ranibizumab treatment, the ORT which was closer to the fovea disappeared, meanwhile the other ORT increased in size (solid arrow). The macular edema decreased to some extent (BCVA=80 letters, CRT=327 μm); C: After two months without anti-VEGF therapy subsequently, the remaining ORT disappeared with evidence of slight aggravation of macular edema (BCVA=74 letters, CRT=341 μm); D: The ORTs (solid arrow) reappeared nine months after initial injection with presence of intraretinal and subretinal fluid due to DME (BCVA=74 letters, CRT=332 μm).
Among the 45 eyes with steady ORTs, although the macular edema subsided and the exudate was absorbed gradually after anti-VEGF treatment for DME, the number and morphology of ORT structures maintained stable (Figure 3A, 3B). The presence of ORT merely without thickening of the retina resulting from DME did not require further anti-VEGF retreatment. In 4 cases, the configurations of ORT structures appeared to increase after anti-VEGF therapy (Figure 4A, 4B),yet the morphology of ORT structures in 7 eyes with DME appeared to decrease after treatment (Figure 4C, 4D). Longer ovoid ORTs were observed to evolve into small round ORTs during observation infrequently. Among the 63 eyes with ORT changes during the treatment period, 9 eyes had collapsed ORTs during anti-VEGF treatment (Figure 3C, 3D) and did not represent ORT reappearance in cases of fluctuations of the disease. Moreover, ORTs in 16 eyes disappeared gradually after several intravitreal anti-VEGF injections (Figure 4C, 4D).In particular, although 2 eyes ceased treatment and edema aggravated, ORTs disappeared after several months of review.Four eyes manifested recurrent ORTs but no recurrence of edema. The ORTs of other eyes showed repeated changes during monitoring period, some cases disappeared eventually,nevertheless the other cases reappeared finally (Figure 5). In a summary, from observations, regardless of whether anti-VEGF treatment continued, or whether macular edema subsided, ORT structures might recur. It was worth mentioning that when DME aggravated, in some cases the reflectivity of ORT tube walls and lumina decreased and the ORT structures became even deformed. While DME was alleviated, the reflectivity of ORT tube walls and lumina tended to increase (Figure 4A, 4B). Interestingly, it was noteworthy that the ORTs were detected under the fovea or extrafoveal lesions and not usually restricted to ONL, which was defined by Zweifel et al[2]. ORTs possibly located in ONL, OPL and/or INL in eyes with DME(Figures 3-5).
The mean BCVA at baseline of the 435 eyes was 59±11 (24-80)in ETDRS, and the mean central foveal thickness was 429.31±67.64 μm (316-748) at baseline. All enrolled eyes were divided by the absence or presence of ORTs into two groups. Comparison of BCVA (variation) between eyes with and without ORTs is summarized in Table 2. There was no statistical significance between two groups at baseline in meanBCVA (P=0.087). The study showed that eyes without ORTs had significantly better mean final BCVA and more mean BCVA change than those eyes with ORTs in DME patients after anti-VEGF therapy (P=0.023, P=0.009 respectively).
Table 2 Comparison of visual acuity (variation) between eyes with and without presence of ORTs mean±SD

BCVA: Best corrected visual acuity; ORT: Outer retinal tubulation; SD: Standard deviation.
BCVA Eyes with ORTs (108 eyes) Eyes without ORTs (327 eyes) P BCVA at baseline 57±16 61±12 0.087 BCVA at the end of follow-up 60±11 76±18 0.023 BCVA variation 3±12 15±13 0.009
Table 3 Comparison of BCVA fluctuation between eyes with and without presence of ORTs n (%)

ORT: Outer retinal tabulation; BCVA: Best corrected visual acuity.
BCVA fluctuation Eyes with ORTs (108 eyes) Eyes without ORTs (327 eyes) P Improvement 49 (45.37) 166 (50.76) 0.149 Stabilization 34 (31.48) 63 (19.27) 0.016 Deterioration 25 (23.15) 98 (29.97) 0.284
Table 4 Comparison of CRT between eyes with and without presence of ORTs at the end of follow-up μm

ORT: Outer retinal tabulation; CRT: Central retinal thickness.
CRT Eyes with ORTs (108 eyes) Eyes without ORTs (327 eyes) P CRT in vision-improved eyes 243.89±43.17 257.21±52.43 0.589 CRT in eyes with stable vision 254.41±56.68 261.85±63.92 0.425 CRT in vision-impaired eyes 270.82±64.59 303.76±57.84 0.072
Table 5 Morphological characteristics of eyes with and without presence of ORTs n (%)

ORT: Outer retinal tabulation; OCT: Optical coherence tomography.
Fluid and subfoveal photoreceptor integrity Eyes with ORTs (108 eyes)Eyes without ORTs (327 eyes)Total eyes (435 eyes) P OCT fluid 0.096 Intraretinal only 79 (73.15) 207 (63.30) 286 (65.75)Both intraretinal and subretinal 29 (26.85) 120 (36.70) 149 (34.25)Subfoveal photoreceptor integrity 0.013 Complete 32 (29.63) 162 (49.54) 194 (44.60)Impaired 76 (70.37) 165 (50.46) 241 (55.40)
Comparison of BCVA fluctuation between two groups is summed up in Table 3. In terms of the proportion of eyes with improved vision and eyes with decreased vision, there was no significant difference between two groups (P=0.149, P=0.284 respectively). The proportion of stable vision in eyes with ORTs was significantly higher than that in eyes without ORTs,showing statistical significance (P=0.016).
Regarding CRT in eyes with different visual changes, there was no statistically significance between two groups (P=0.589,P=0.425, P=0.072 respectively), which is summarized in Table 4.Table 5 summarizes morphological characteristics of eyes with and without ORTs. There was no significant difference between the two groups regarding fluid on SD-OCT (P=0.096).Nevertheless, there was statistical significance between the two groups concerning the disruption of subfoveal photoreceptor integrity (P=0.013). The damage of photoreceptor integrity in eyes with ORTs was more serious than that in eyes without ORTs.
DISCUSSION
However, most of recent scholars agree that ORT formation is a result of the degeneration of photoreceptor cells[2,11,16].Despite the pathophysiological mechanism of ORT formation being not completely clear, ORTs have been detected in pathologies primarily involving the RPE and choroid or photoreceptors proposing that ORTs are at the final stage in patients with chronic disorders of the photoreceptors irrespective of treatment whether they are neovascular or degenerative in etiology[15]. The sequence of events in ORT formation may be associated with loss of the interdigitation of the outer segments with RPE, the sublethal damage to the photoreceptors, disruption of tight junctions, subsequently reorganizing and invaginating the ellipsoid portion of the photoreceptor inner segment junction, the ELM scrolling to form their hyperreflective border as outlined, shaping a tubular structure ultimately and reassembling into smaller round tubulations over time[2,15]. Furthermore, Panorgias et al[18] proposed that the ELM, the ellipsoid portion of the photoreceptor inner segment, and RPE were loss surrounding the ORT in geographic atrophy. ORTs might arise from SRF and/or IRF that presumably injured the outer retinal layers.Impairment of tight junctions with neighboring cells, loss of interdigitation with RPE, and loss of focal cells were due to the destruction of the photoreceptor cell layer by SRF and/or IRF,which was in conformity with Freund’s research[2,19].
Disease severity such as serious disruption of photoreceptor layers and/or RPE, seems enough to trigger the formation of ORTs[15,20-21]. The reports published previously suggested that degenerating photoreceptors appeared to organize in a tubular organization and neighbouring RPE and glial cells partake in the formation of tubular structures[2]. The presence of ORTs may be of prognostic value concerning disease severity. ORTs are presumed to be indicative of underlying critical and chronic DME that arise secondary to reorganization of retinal layers after anti-VEGF therapy. In the case of retinal edema subsiding and without exudative recurrences, the clinical significance of ORT is that its appearance does not predict the continuous activity of the disease, thus there is no need for excessive treatment[11,22]. The ORT can be distinguished from cystoid intraretinal macular edema due to its high reflective boundary and insensitivity to anti-VEGF treatment, and the latter are therapeutic implications of exudative process in disorders such as DME, RVO and AMD. The cystic edema of the retina is often located in INL, showing a number of small capsule cavity and honeycomb. There is no high reflex boundary in the edematous cystic cavity and it will disappear after anti-VEGF treatment. However, ORTs always change slowly over time,even in cases of accepting intravitreal anti-VEGF injections[2].Several configurations in ORTs were reported in this study,as described by Schaal et al[7]. The sequential evolution of ORTs began with the development of forming ORTs. Forming ORTs were recognized in a zone of injured ELM and ellipsoid zone and subsequently evoluted into large open ORTs, which divided into multiple smaller open ORTs. Smaller open ORTs were prone to evolve into closed ORTs in morphology. Hua et al[4] proposed the presence of a novel SD-OCT sign called“cynapsis”, defined as the collapse of the INL, OPL, and ONL which separates each ORT. Similarly, the OPL subsidence sign[23] defined as the downward displacement of INL and OPL in the proximity of ORTs was illustrated by Preti et al[3], which was predominantly associated with the ORT formation. It is presumed that Müller cells which formed tight junctions with inner segments of the photoreceptors to comprise the ELM were intricately involved in the process of ORT formation.During the process the inward scrolling of the ELM and ellipsoid zone presumably dragged the Müller cells toward the outer retina. Furthermore, it is confirmed that ORTs will decrease spontaneously in the process of evolution over time and end-stage ORTs are histologically comprised of an ELM circle constituted by Müller cells without photoreceptors[7,24].When we focus our research on DME, the incidence of ORTs may not be as low as previously reported. The objective of this investigation was to review the developing process of ORTs in eyes with DME after anti-VEGF treatment and to correlate ORT diversification with presence or absence of fluid, the integrity of subfoveal photoreceptor, disease reactiveness.We also went further and explored patient demographic characteristics and the association between the morphological features and prognostic value of ORTs. This investigation corroborates results from previous researcher[2,11,21,25] that ORTs are characteristically correlated with lower BCVA and worse prognosis resulting from severer disruption of subfoveal photoreceptor integrity. This is supported by the integrity of the subfoveal photoreceptor where we found that 70.37% of eyes with ORTs had been damaged. The significant differences expatiated in BCVA, disruption of integrity of subfoveal photoreceptor between patients with and without ORTs may be due to the pathogenesis of the ORT formation, secondary to photoreceptor damage or RPE tears. The correlation of the photoreceptor integrity to visual acuity has been previously described[26-27]. Moreover, the higher proportion of stable vision in eyes with ORTs compared with eyes without ORTs confirmed that ORTs is one of the manifestations of the terminal stage of the disease. However, there was no significant difference in CRT between the two groups at the end of follow-up. This should be associated with the fact that CRT changes are due to the effect of anti-VEGF drugs rather than the presence of ORTs in the process of edema subsidence and visual acuity recovery induced by DME with anti-VEGF drugs. Accordingly, it is not surprising that patients who developed ORTs displayed lower final BCVA and lower treatment benefits in terms of BCVA variation compared with patients without ORTs. It is undeniable that in various types of retina choroid diseases, the ORT is a typical degeneration manifestation of severe injury of RPE and choroid. ORTs may be indicative of underlying severity and chronicity of the DME in the eyes underwent anti-VEGF treatment.
In our study variations of ORTs in 63 DME eyes ranged from disappearance, reappearance, collapse, diminution to enlargement. During follow-up 58.33% of ORTs were in dynamic changes. Such discoveries obviously document that ORTs may not be stable as reported previously[2,25], which was in accordance with the results of the study of Hua et al[4] and Espina et al[28]. Observations suggest that part of ORTs presumably disappeared in condition of retinal edema aggravation and reappeared after edema fades away.
Concerning the proportion of forming ORTs and closed ORTs,there was a statistically significance between eyes with steady ORTs and eyes with variant ORTs. This is related to forming ORTs are in the early stage of ORT formation, while closed ORTs are at the end stage of ORT formation.
From observations, when the DME edema was aggravated,the reflectivity of ORT tube walls and lumina was reduced and ORTs even deformed in some cases. While the DME edema was relieved, the reflectivity of ORT tube walls and lumina tended to increase. Whether or not DME causes the changes in the reflectance of the ORT lumina and walls, it is a phenomenon worthy of further observation. In consideration of their response to anti-VEGF treatment, it is not completely excluded that ORTs contain no vascular-related components,although it was suggested that ORTs were composed of degenerating photoreceptor cells as elaborated by Schaal et al[7]. To some extent, this conclusion is consistent with what was reported by Espina et al[28]. Another explanation may hypothesize that there was communicating structures between the adjacent retinal vessels or tissue and ORTs, and anti-VEGF therapy could eliminate the exudation of liquid into ORT network and then transiently collapse the ORTs[2]. The variations of ORTs could be associated with spontaneous and coincident findings or anti-VEGF therapy.
It is worth noting that ORTs usually exist in ONL at the macular RPE lesion or above the RPE and the choroidal fibrous scar, adjacent to the retinal cystoid edema area in macular pathologies accompanied by degeneration or CNV, such as AMD in which ORTs appear most frequently.Nevertheless in this study, the majority of ORTs exist in INL,OPL and/or ONL, which might be supplementary for previous investigations. The reasons for this difference should be explored from the different pathophysiological mechanisms of the above diseases. As we all know, DME originates from DR, and the latter belongs to retinal microangiopathy which does not damage the choroid primarily. It is believed that the accumulation of liquid in the inner retina of the macular region is the main cause of the destruction of blood-retinal barrier(BRB) under high glucose environment[29]. The damage of BRB structure and function balance leads to the change of capillary permeability, the obvious expansion of the outer space of the retina, the exudation of water and protein of the capillary, and accumulation in OPL and INL of the retina,resulting in macular edema.
In terms of the tissue structure of the macular retina, photoreceptor cells account for almost half of the thickness of the retina in nature. The axons and nuclei of the photoreceptor cells constitute OPL and ONL respectively, and the INL of the inner layer is the nucleus of the bipolar cells. The dense line of 1/3 in OPL is the synapse and desmosomes connection between photoreceptor fibers and bipolar cells. There are only internal limiting membrane (ILM), ONL and other outer retina and no OPL at the macular fovea of retina. However, OPL is the main interstitial layer of retina. When retinal hemorrhage or edema is manifested due to the retinal leakage in DME, liquid and exudate mainly focus on OPL. When the OPL at parafovea is swelled, there is a cystic edema in the macular area.Degeneration of photoreceptor cells can involve ascending bipolar cells and horizontal cells, which is based on the structural characteristics of retina. Accurate ORT localization is difficult because of the complexity and atypia of severe structural damage to retina and choroidal tissue. Therefore,some ORT cases may be referred to as the outer layer of the retina in previous studies.
However, there was no such a report on the morbidity and morphological character of ORTs in DME cases of Chinese after intravitreal anti-VEGF therapy so far. We observed the reflectivity of ORT tube walls and lumina varied during anti-VEGF therapy. Additionally, we suggested besides the ellipsoid zone of the photoreceptor inner segment and RPE, the ONL, OPL and INL all participated in the developing process of ORTs simultaneously. The comprehension of the prevalence and progression of ORTs are of substantial importance for future clinical practice.
In conclusion, ORTs have a high incidence and changes over time in DME with anti-VEGF treatment and may be located at various retinal layers. Persistent ORT can be as a negative biomarker of outcome of DME.
The present analysis has the following limitations that must be taken into account. ORT is localized in the macular region,especially near the fovea, which is related to the scan range of OCT. Objectively, we ignore the ORTs outside the macular.The study is limited to the retrospective nature of our study.There is no data on en face OCT scan and angiography.Prospective long-term follow-up studies, especially the addition of en face OCT analysis and fluorescein angiography,will give us a better understanding of ORTs.
ACKNOWLEDGEMENTS
Foundation: Supported by the Hubei Provincial Health and Family Planning Commission Joint Fund Project (No.WJ2018H0071).
Conflicts of Interest: Huang XL, None; Song YP, None;Ding Q, None; Chen X, None; Hong L, None.
1 Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in agerelated macular degeneration. Invest Ophthalmol Vis Sci 1996;37(7):1236-1249.
2 Zweifel SA, Engelbert M, Laud K, Margolis R, Spaide RF, Freund KB.Outer retinal tubulation: a novel optical coherence tomography finding.Arch Ophthalmol 2009;127(12):1596-1602.
3 Preti RC, Govetto A, Filho RGA, Cabral Zacharias L, Gianotti Pimentel S, Takahashi WY, Monteiro MLR, Hubschman JP, Sarraf D.Optical coherence tomography analysis of outer retinal tubulations:sequential evolution and pathophysiological insights. Retina 2018;38(8):1518-1525.
4 Hua R, Liu LM, Hu YD, Chen L. The occurrence and progression of outer retinal tubulation in Chinese patients after intravitreal injections of ranibizumab. Sci Rep 2015;5:7661.
5 Litts KM, Ach T, Hammack KM, Sloan KR, Zhang YH, Freund KB,Curcio CA. Quantitative analysis of outer retinal tubulation in age-related macular degeneration from spectral-domain optical coherence tomography and histology. Invest Ophthalmol Vis Sci 2016;57(6):2647-2656.
6 Litts KM, Messinger JD, Freund KB, Zhang YH, Curcio CA.Inner segment remodeling and mitochondrial translocation in cone photoreceptors in age-related macular degeneration with outer retinal tubulation. Invest Ophthalmol Vis Sci 2015;56(4):2243-2253.
7 Schaal KB, Freund KB, Litts KM, Zhang YH, Messinger JD,Curcio CA. Outer retinal tubulation in advanced age-related macular degeneration: optical coherence tomographic findings correspond to histology. Retina 2015;35(7):1339-1350.
8 Litts KM, Messinger JD, Dellatorre K, Yannuzzi LA, Freund KB,Curcio CA. Clinicopathological correlation of outer retinal tubulation in age-related macular degeneration. JAMA Ophthalmol 2015;133(5):609-612.
9 Tulvatana W, Adamian M, Berson EL, Dryja TP. Photoreceptor rosettes in autosomal dominant retinitis pigmentosa with reduced penetrance. Arch Ophthalmol 1999;117(3):399-402.
10 Giachetti Filho RG, Zacharias LC, Monteiro TV, Preti RC, Pimentel SG. Prevalence of outer retinal tubulation in eyes with choroidal neovascularization. Int J Retina Vitreous 2016;2:6.
11 Wolff B, Matet A, Vasseur V, Sahel JA, Mauget-Faÿsse M. En face OCT imaging for the diagnosis of outer retinal tubulations in age-related macular degeneration. J Ophthalmol 2012;2012:542417.
12 Ellabban AA, Hangai M, Yamashiro K, Nakagawa S, Tsujikawa A, Yoshimura N. Tomographic fundus features in pseudoxanthoma elasticum: comparison with neovascular age-related macular degeneration in Japanese patients. Eye (Lond) 2012;26(8):1086-1094.
13 Papastefanou VP, Nogueira V, Hay G, Andrews RM, Harris M, Cohen VM, Sagoo MS. Choroidal naevi complicated by choroidal neovascular membrane and outer retinal tubulation. Br J Ophthalmol 2013;97(8):1014-1019.
14 Sergouniotis PI, Davidson AE, Lenassi E, Devery SR, Moore AT,Webster AR. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology 2012;119(3):596-605.
15 Goldberg NR, Greenberg JP, Laud K, Tsang S, Freund KB. Outer retinal tubulation in degenerative retinal disorders. Retina 2013;33(9):1871-1876.
16 Iriyama A, Aihara Y, Yanagi Y. Outer retinal tubulation in inherited retinal degenerative disease. Retina 2013;33(7):1462-1465.
17 Kojima H, Otani A, Ogino K, Nakagawa S, Makiyama Y, Kurimoto M,Guo CR, Yoshimura N. Outer retinal circular structures in patients with Bietti crystalline retinopathy. Br J Ophthalmol 2012;96(3):390-393.
18 Panorgias A, Zawadzki RJ, Capps AG, Hunter AA, Morse LS, Werner JS. Multimodal assessment of microscopic morphology and retinal function in patients with geographic atrophy. Invest Ophthalmol Vis Sci 2013;54(6):4372-4384.
19 Jung JJ, Freund KB. Long-term follow-up of outer retinal tubulation documented by eye-tracked and en face spectral-domain optical coherence tomography. Arch Ophthalmol 2012;130(12):1618-1619.
20 Dirani A, Gianniou C, Marchionno L, Decugis D, Mantel I. Incidence of outer retinal tubulation in ranibizumab-treated age-related macular degeneration. Retina 2015;35(6):1166-1172.
21 Lee JY, Folgar FA, Maguire MG, Ying GS, Toth CA, Martin DF, Jaffe GJ, CATT Research Group. Outer retinal tubulation in the comparison of age-related macular degeneration treatments trials (CATT).Ophthalmology 2014;121(12):2423-2431.
22 Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab(avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36(4):331-335.
23 Wu ZC, Luu CD, Ayton LN, Goh JK, Lucci LM, Hubbard WC,Hageman JL, Hageman GS, Guymer RH. Optical coherence tomographydefined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology 2014;121(12):2415-2422.
24 Dolz-Marco R, Litts KM, Tan ACS, Freund KB, Curcio CA. The evolution of outer retinal tubulation, a neurodegeneration and gliosis prominent in macular diseases. Ophthalmology 2017;124(9):1353-1367.
25 Faria-Correia F, Barros-Pereira R, Queirós-Mendanha L, Fonseca S, Mendonça L, Falcão MS, Brandão E, Falcão-Reis F, Carneiro AM.Characterization of neovascular age-related macular degeneration patients with outer retinal tubulations. Ophthalmologica 2013;229(3):147-151.
26 Gamulescu MA, Panagakis G, Theek C, Helbig H. Predictive factors in OCT analysis for visual outcome in exudative AMD. J Ophthalmol 2012;2012:851648.
27 Chhablani J, Kim JS, Freeman WR, Kozak I, Wang HY, Cheng LY.Predictors of visual outcome in eyes with choroidal neovascularization secondary to age related macular degeneration treated with intravitreal bevacizumab monotherapy. Int J Ophthalmol 2013;6(1):62-66.
28 Espina M, Arcinue CA, Ma FY, Camacho N, Barteselli G, Mendoza N,Ferrara N, Freeman WR. Outer retinal tubulations response to anti-VEGF treatment. Br J Ophthalmol 2016;100(6):819-823.
29 Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res 2004;36(5):241-249.