INTRODUCTION
Type 3 neovascularization, also called retinal angiomatous proliferation, is a subtype of neovascular agerelated macular degeneration (AMD) that is characterized by intraretinal neovascularization[1-2]. Eyes with type 3 neovascularization exhibit characteristic features that distinguish them from those with the other subtypes of neovascular AMD, including a thin choroid[3-4], lack of choroidal vascular hyperpermeability[5], and high incidence of pseudodrusen[6].
Previously, type 3 neovascularization has been considered to be refractory to treatment[7-9]. However, treatment outcomes have improved markedly with the advent of anti-vascular endothelial growth factor (VEGF) therapy[10-14]. However,the response to treatment differs between patients with type 3 neovascularization and those with the other subtypes of neovascular AMD. In type 3 neovascularization, the incidence of geographic atrophy is higher[15], and the treatment outcome of subretinal hemorrhage secondary to type 3 neovascularization is very poor[16].
Although many studies have reported treatment outcomes of type 3 neovascularization, no previous study has focused on the events that may cause abrupt visual deterioration during the management of type 3 neovascularization, although this information may be useful to establish treatment plans and discuss disease prognosis with patients.
In the present study, we investigated the incidence and associated retinal findings of abrupt visual loss during anti-VEGF treatment for type 3 neovascularization. We additionally evaluated the influence of these events on long-term visual prognosis.
SUBJECTS AND METHODS
Ethical Approval This single-institution, retrospective study was approved by the Institutional Review Board of Kim’s Eye Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was not obtained in this retrospective study.
Patients Herein, patients diagnosed with treatment-naïve unilateral type 3 neovascularization between January 2010 and August 2015 and followed up for at least 12mo were included.The exclusion criteria were: 1) a history of treatment for choroidal neovascularization; 2) a symptom duration >6mo;3) severe media opacity precluding clear fundus photographs;4) fovea-involving scarring; 5) a concomitant retinal vascular disorder.
Examinations All patients underwent baseline examinations,including slit-lamp examination, best-corrected visual acuity(BCVA) measurement, fundus photography, optical coherence tomography (OCT), fluorescein angiography, and indocyanine green angiography (ICGA). Fluorescein angiography and ICGA images were obtained using Spectralis HRA-OCT®(Heidelberg Engineering, Heidelberg, Germany). OCT images were obtained using either Spectralis HRA-OCT® (Heidelberg Engineering, Heidelberg, Germany), RS 3000® (Nidek Co., Ltd., Tokyo, Japan), or Spectral OCT® (Ophthalmic Technologies Inc., Toronto, Canada).
To select type 3 neovascularization cases, 2 independent examiners (Kim JH and Chang YS) analyzed the OCT and ICGA images of patients diagnosed with neovascular AMD during the study period. Type 3 neovascularization cases were identified using a previously suggested method[17].Patients with characteristic hyper-reflective lesions in the outer retinal layer associated with intraretinal edema with or without subretinal fluid or subretinal pigment epithelial fluid were diagnosed with type 3 neovascularization. The presence of type 3 neovascularization was further confirmed using angiography results if focal hyperfluorescence with late leakage was observed at the site of neovascularization. Staging of the lesion was performed based on the previously suggested criteria[18]: stage 1=intraretinal hyperreflective focus, cystoid macular edema (CME) without external limiting membrane(ELM)/ellipsoid zone (EZ) disruption, stage 2=intraretinal hyperreflective focus, CME, ELM/EZ disruption, with or without retinal pigment epithelium (RPE) disruption, and stage 3=intraretinal hyperreflective focus, CME, ELM/EZ disruption, RPE disruption, and serous pigment epithelial detachment (PED), with or without subretinal fluid.
Treatment and Follow-up The patients were initially treated with either ranibizumab (0.5 mg/0.05 mL of Lucentis®;Genentech, Inc., South San Francisco, CA, USA) or aflibercept(2.0 mg/0.05 mL of Eylea®; Regeneron, Tarrytown, NY, USA).All patients received three monthly injections. After initial treatment, the patients were followed-up every 1-2mo. During the first 12mo, the follow-up interval was extended up to 3mo at the discretion of the physician. Subsequently, the followup interval was extended up to 4mo. Clinical examinations,OCT and BCVA measurement were performed at each follow-up visit. During the follow-up period, retreatment was performed as needed using either ranibizumab, aflibercept, or bevacizumab (1.25 mg/0.05 mL of Avastin®; Genentech). In some patients, a proactive regimen was employed during the treatment course at the discretion of the treating physician.
The cost, benefits and potential complications of the treatment were fully discussed with the patients. Further treatment was not performed when the patient refused despite sufficient explanation that their visual acuity could deteriorate and the chance to recover their vision may decrease without further treatment. Further treatment was also discontinued at the physician’s discretion for cases in which it was not beneficial.Patients who discontinued treatment were then followed up every 2-5mo.
Outcome Measures Abrupt visual loss was defined as a loss of 5 or more lines in BCVA compared to the previous visit.The incidence, timing and associated findings of abrupt visual loss were identified. Patients who experienced abrupt visual loss were included in the abrupt visual loss group, whereas the remaining patients were included in the non-abrupt visual loss group. Characteristics, including age, sex, hypertension,diabetes mellitus, stage of disease, incidence of drusen and pseudodrusen, maximum height and width of PED, subfoveal choroidal thickness, type of anti-VEGF drug used for loading injection, baseline BCVA, number of anti-VEGF injections and follow-up period were compared between the 2 groups.In addition, the BCVA at the baseline, at 12mo and at the final follow-up were compared between the 2 groups. When the patient failed to visit the hospital at exactly 12mo, data from the follow-up visit closest to that time point were used for analysis. When tear in RPE develops, the stage of the tear was classified according to the previously suggested method[19];grade 1=diameter smaller than 200 µm, grade 2=diameter between 200 µm and 1 disc diameter, grade 3=diameter greater than 1 disc diameter, and grade 4=grade 3 tears that involved the foveal center. The maximum height and width of PED were defined as the maximum value of each parameter, as measured based on the entire raster scan of OCT images (Figure 1).
Statistical Analysis For statistical analysis, BCVA values were converted to the logarithm of minimum angle of resolution(logMAR). According to the suggestion by Holladay[20] the visual acuity of counting fingers was converted to logMAR value 2 and the visual acuity of hand motion was converted to logMAR values of 3.
The data are presented as the mean±standard deviation where applicable. The statistical analyses were performed using a commercially available software package (SPSS version 12.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences in the values between the 2 groups were analyzed using the independent-samples t-test, Chi-square test, or Fisher’s exact test. Differences in BCVA between the 2 groups at the 3 time points were analyzed using the independent-samples t-test with Bonferroni correction. P-values<0.05 were considered to be statistically significant.
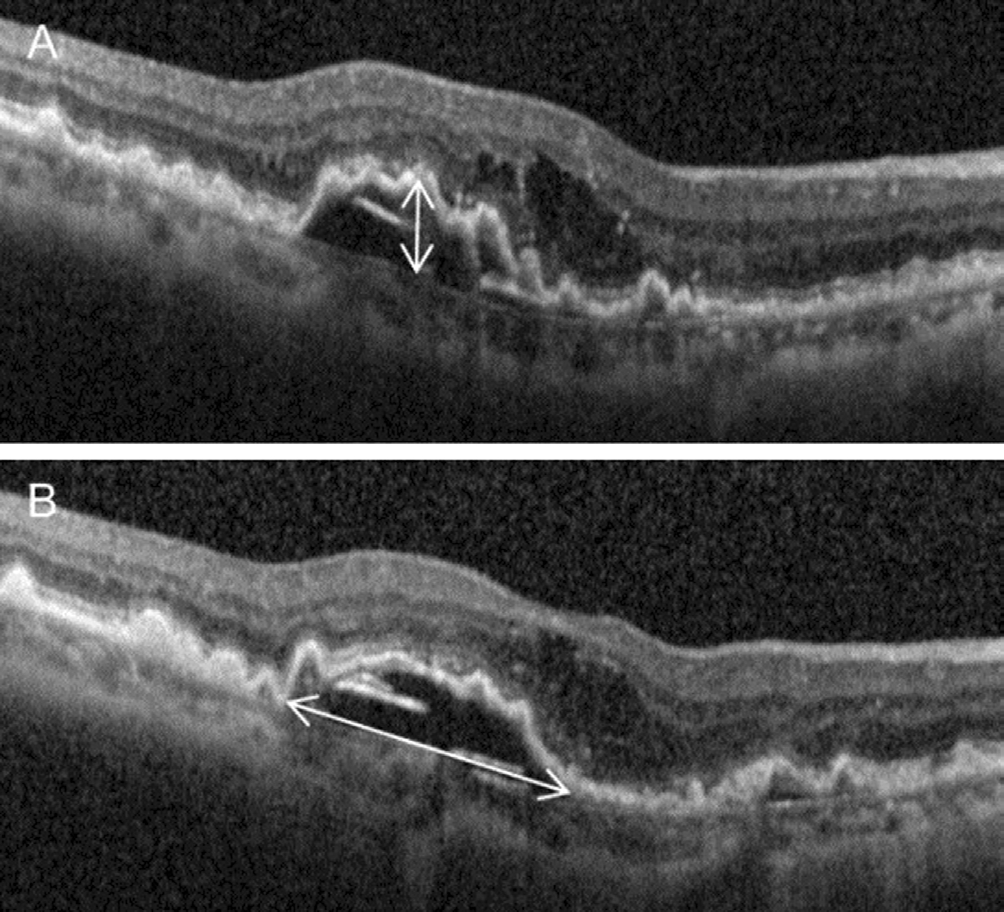
Figure 1 A representative figure showing measurement of maximum height and width of PED Panels A and B comprise different OCT raster scan sections from a single patient. A: Maximum height; B: Maximum width.
RESULTS
A total of 137 patients met the eligibility criteria (Table 1).BCVA was 0.77±0.34 (Snellen equivalent: 20/117) at the baseline, 0.67±0.43 (20/93) at 12mo, and 1.17±0.69 (20/295)at the final follow-up (Figure 2A). One hundred and twentysix eyes (91.9%) were initially treated with ranibizumab and 11 eyes (8.0%) were treated with aflibercept. During the follow-up period, a mean of 8.2±3.7 anti-VEGF injections were administered. Treatment was discontinued for 34 patients(24.8%) during the follow-up period after full discussion with the patients. Among them, the BCVA of 31 patients was 20/400 or worse when the treatment was discontinued.
Twenty-two eyes (16.1%) were included in the abrupt visual loss group and the remaining 115 eyes (83.9%) were included in the non-abrupt visual loss group. In the abrupt visual loss group, ranibizumab was used for loading injections in 20 eyes(90.9%) and aflibercept was used in 2 eyes (9.1%). Switching of the anti-VEGF agent was not performed before the development of visual loss. Table 2 summarizes the results of comparing characteristics between the abrupt visual loss group and the non-abrupt visual loss group. In the abrupt visual loss group, the mean follow-up period was 46.2±20.3mo and the mean timing of abrupt visual loss was 19.6±13.9 (range: 2-51) mo after the diagnosis (Figure 3).
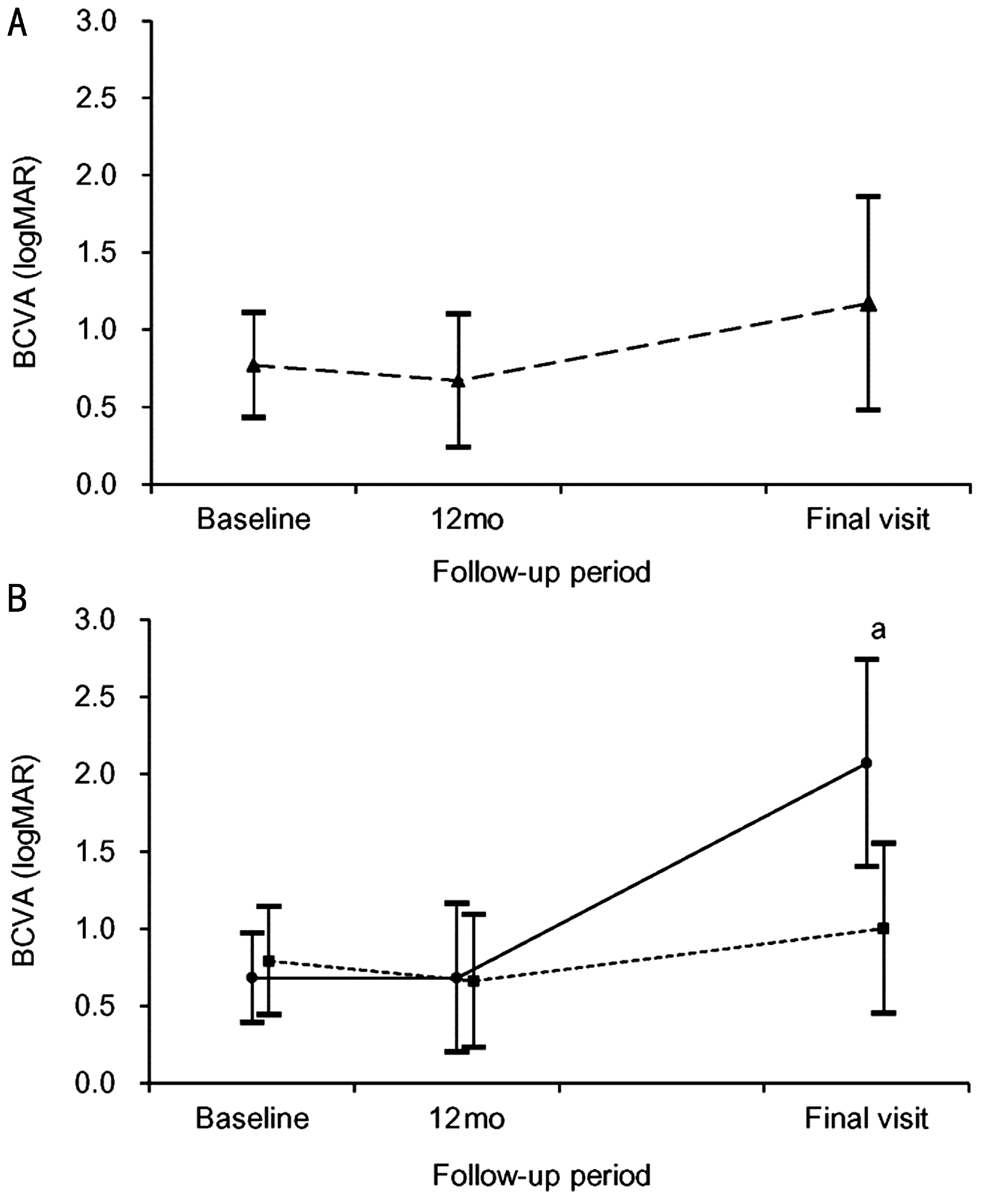
Figure 2 Changes in logMAR BCVA, according to the followup period A: Changes in all 137 patients; B: Changes in the abrupt visual loss group (n=22, solid line, closed circle) and non-abrupt visual loss group (n=115, dashed line, closed square). aSignificant difference between the 2 groups (Mann-Whitney U test with Bonferroni’s correction, P<0.001).
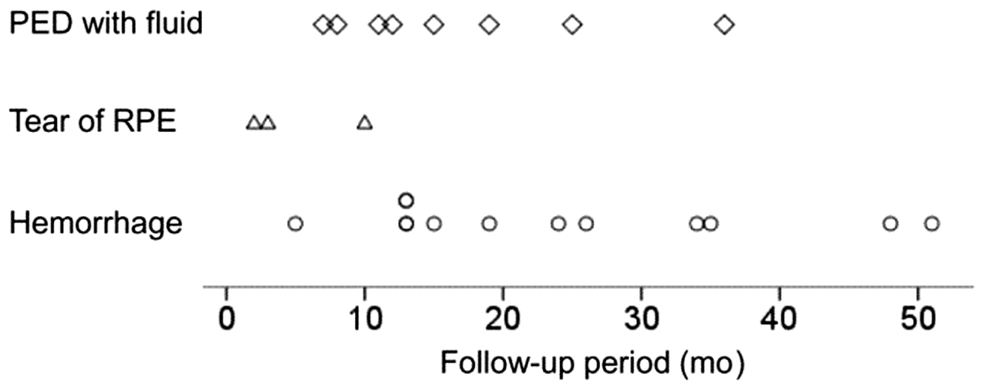
Figure 3 Timing of the development of abrupt visual loss,stratified according to etiology.
Table 1 Baselines characteristics of the included patients (n=137)mean±SD, n (%)
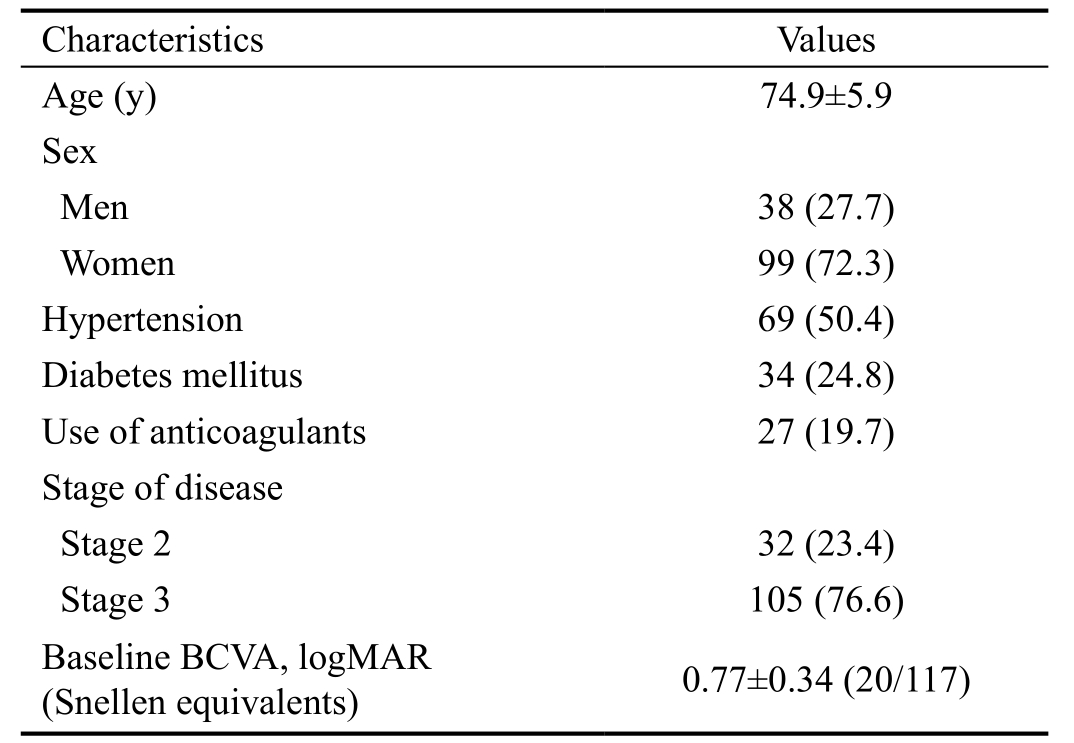
BCVA: Best-corrected visual acuity.
?
Table 2 Comparison of characteristics between the abrupt visual loss group and the non-abrupt visual loss group mean±SD, n (%)
BCVA: Best-corrected visual acuity; VEGF: Vascular endothelial growth factor; PED: Pigment epithelial detachment.Statistical analysis was performed using athe independent-samples t-test, bthe Chi-square test, cthe independent-samples t-test with Bonferroni correction, dthe Fisher’s exact test; efor 105 stage 3 eyes.
Characteristics Abrupt visual loss group(n=22)Non-abrupt visual loss group(n=115) P Age (y) 76.0±5.2 74.8±6.0 0.310a Sex 0.958b Men 6 (27.3) 32 (27.8)Women 16 (72.7) 83 (72.2)Hypertension 11 (50.0) 58 (50.4) 1.000b Diabetes mellitus 6 (26.3) 28 (24.3) 0.771b Use of anticoagulants 6 (27.3) 21 (18.3) 0.381d Stage of disease 0.636b Stage 2 6 (27.3) 26 (22.6)Stage 3 16 (72.7) 89 (77.4)Drusen 21 (95.5) 100 (86.9) 0.468d Pseudodrusen 18 (81.8) 91 (79.1) 1.000d Maximum height of PEDe 282.3±132.3 231.3±116.3 0.118a Maximum width of PEDe 2034.9±991.0 1662.8±830.9 0.113a Subfoveal choroidal thickness 148.9±71.8 144.9±51.2 0.754a Anti-VEGF drug for loading injections 1.000d Ranibizumab 20 (90.9) 106 (92.2)Aflibercept 2 (9.1) 9 (7.8)BCVA, logMAR (Snellen equivalent) 0.68±0.29 (20/95) 0.79±0.35 (20/123) 0.429c No. of anti-VEGF injections 8.9±3.7 8.0±3.7 0.281a Follow-up period (mo) 46.2±20.3 41.7±18.6 0.338a
The retinal findings associated with the visual loss were subretinal hemorrhage in 11 eyes (50.0%), development or increase in the height of PED with fluid in 8 eyes (36.4%), and tears of the RPE in 3 eyes (13.6%). PED accompanied all 11 subretinal hemorrhage cases, and all 3 tears of the RPE were classified as grade 4. Figure 4 shows representative cases of abrupt visual loss. The mean size of subretinal hemorrhage in these 11 eyes was 12.9±4.7 disc areas. All eyes that exhibited hemorrhage were treated with anti-VEGF therapy. Vitrectomy was performed in 4 eyes in which vitreous hemorrhage developed following anti-VEGF therapy. In 1 eye, silicone oil injection was performed at the end of vitrectomy. In the remaining 3 eyes, vitrectomy alone was performed to clear the media. Tissue-plasminogen activator injection or pneumatic displacement was not performed.
During the study period, a mean of 8.9±3.7 anti-VEGF injections were administered. The mean BCVA was 0.68±0.29(20/95) at baseline, 0.68±0.48 (20/95) at 12mo, and 2.07±0.67(20/2349) at final follow-up (Figure 2B). At baseline, BCVA was ≤20/200 in 7 eyes (31.8%), >20/200, <20/50 in 10 eyes(45.5%), and ≥20/50 in 5 eyes (22.7%) (Figure 5A).
At final follow-up, BCVA was measured as ≤20/200 in all 22 eyes (100%). More specifically, BCVA was worse than 20/1000 in 16 eyes (72.7%). Treatment was discontinued for 16 patients (72.7%). At baseline, 6 eyes were diagnosed with stage 2 disease and the remaining 16 eyes were diagnosed with stage 3 disease. During the follow-up period, development of serous PED, which indicates progression to stage 3 disease,was noted at least once on OCT before the development of abrupt visual loss in all 6 eyes that were initially diagnosed with stage 2 disease. In all 22 eyes, proactive treatment was not performed before the development of abrupt visual loss.
In the non-abrupt visual loss group, the mean follow-up period was 41.9±18.7mo and patients received a mean of 8.0±3.7 anti-VEGF injections. During the study period, one patient(0.9%) underwent a vitrectomy because of the development of vitreous hemorrhage and 8 patients (6.9%) underwent cataract surgery. Treatment was discontinued for 18 patients(15.7%). The mean BCVA was 0.79±0.35 (20/123) at baseline,0.66±0.43 (20/91) at 12mo, and 1.00±0.55 (20/200) at the final follow-up (Figure 2B). At baseline, BCVA was ≤20/200 in 53 eyes (46.1%), >20/200, <20/50 in 40 eyes (34.8%) and ≥20/50 in 22 eyes (19.1%) (Figure 5B). At final follow-up, BCVA was ≤20/200 in 71 eyes (61.7%), >20/200, <20/50 in 21 eyes(18.3%) and ≥20/50 in 24 eyes (20.9%). BCVA (P=0.429) at baseline and BCVA at 12mo (P=1.000) did not differ between the abrupt visual loss group and the non-abrupt visual loss group, whereas BCVA at final follow-up was significantly worse in the abrupt visual loss group (P<0.001).
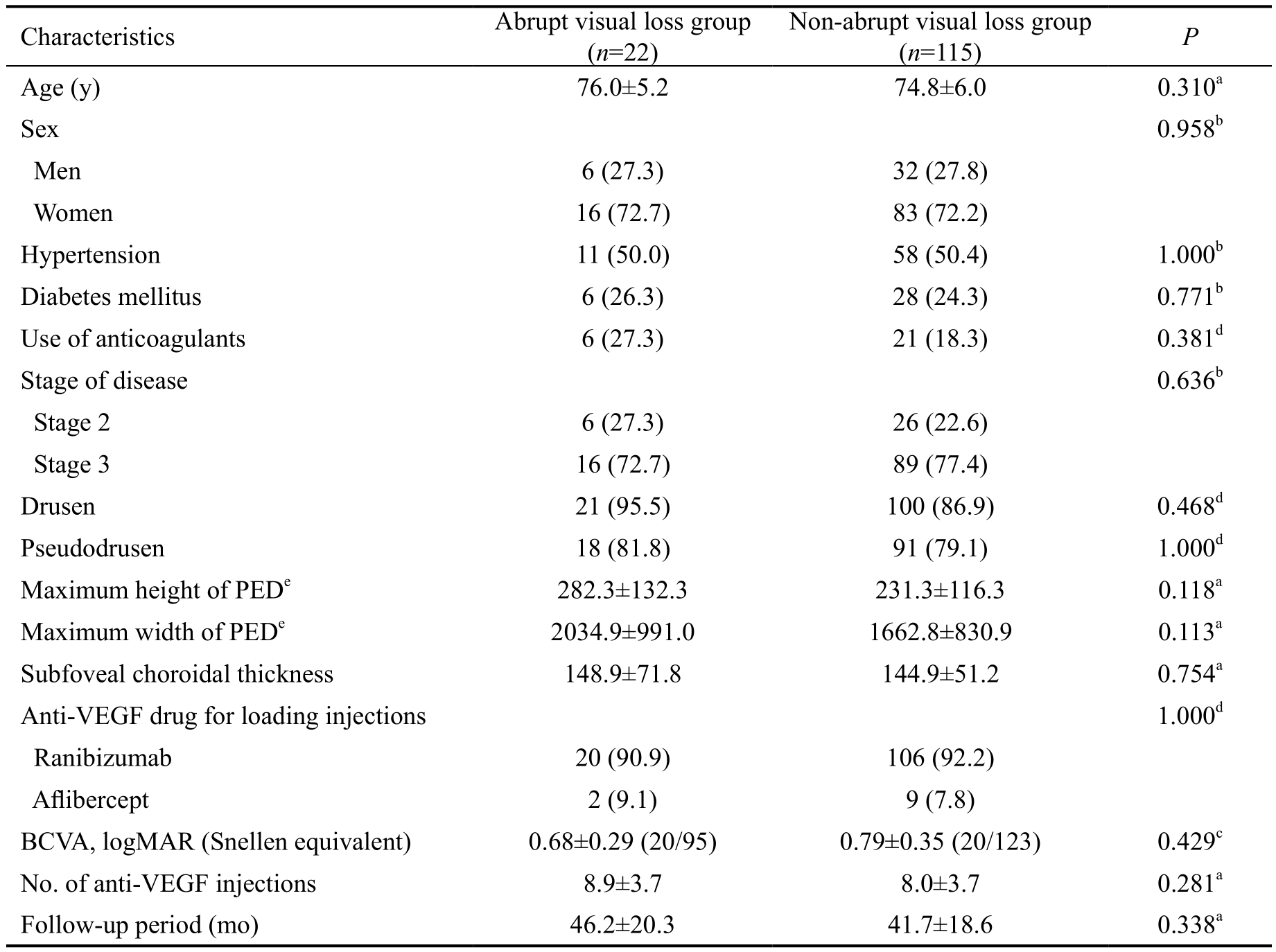
Figure 4 Clinical course of 3 patients with abrupt visual loss Each case shows the development of subretinal hemorrhage (A-D; A-C: at the baseline, D: when abrupt visual loss developed), PED with fluid (E-H; E-G: at the baseline, H: when abrupt visual loss developed), and tears in the RPE (I-M; I-K: at the baseline, L, M: when abrupt visual loss developed). A, D, E, I, L: Fundus photography image; B, F, J: Indocyaninegreen angiography image; C, G, H, K, M: OCT image.
All 11 eyes with submacular hemorrhage were treated with anti-VEGF after the development of hemorrhage; 4 of these eyes underwent vitrectomy due to vitreous hemorrhage. The mean number of anti-VEGF injections after submacular hemorrhage was 3.7±2.3. Treatment was eventually discontinued in 7 eyes. Among these 11 eyes, mean logMAR BCVA at the final visit was 2.24±0.80; 8 eyes exhibited BCVA of counting fingers or hand motion.
The mean interval between the last injection before abrupt visual loss and the time when visual loss was noted was 7.4±6.1mo. The mean interval between the hospital visit immediately preceding visual loss and when visual loss was noted was 2.4±0.9mo. Except for 2 eyes in which abrupt visual loss developed during or immediately after the initial loading injections, fluid or hemorrhage was not noted during the hospital visit immediately before the development of visual loss. Thus, anti-VEGF injection was not performed in the remaining 20 eyes during the hospital visit immediately before visual loss.
DISCUSSION
In the present study, an abrupt loss of 5 or more lines in BCVA was noted in 16.1% of the patients with type 3 neovascularization over a 42.4±18.9mo follow-up period. The most common cause of abrupt visual loss was subretinal hemorrhage,observed in half of the cases in this study. The onset time of subretinal hemorrhage was widely distributed, varying from the early to late follow-up period, suggesting that this condition could develop at any time point during the treatment for type 3 neovascularization.
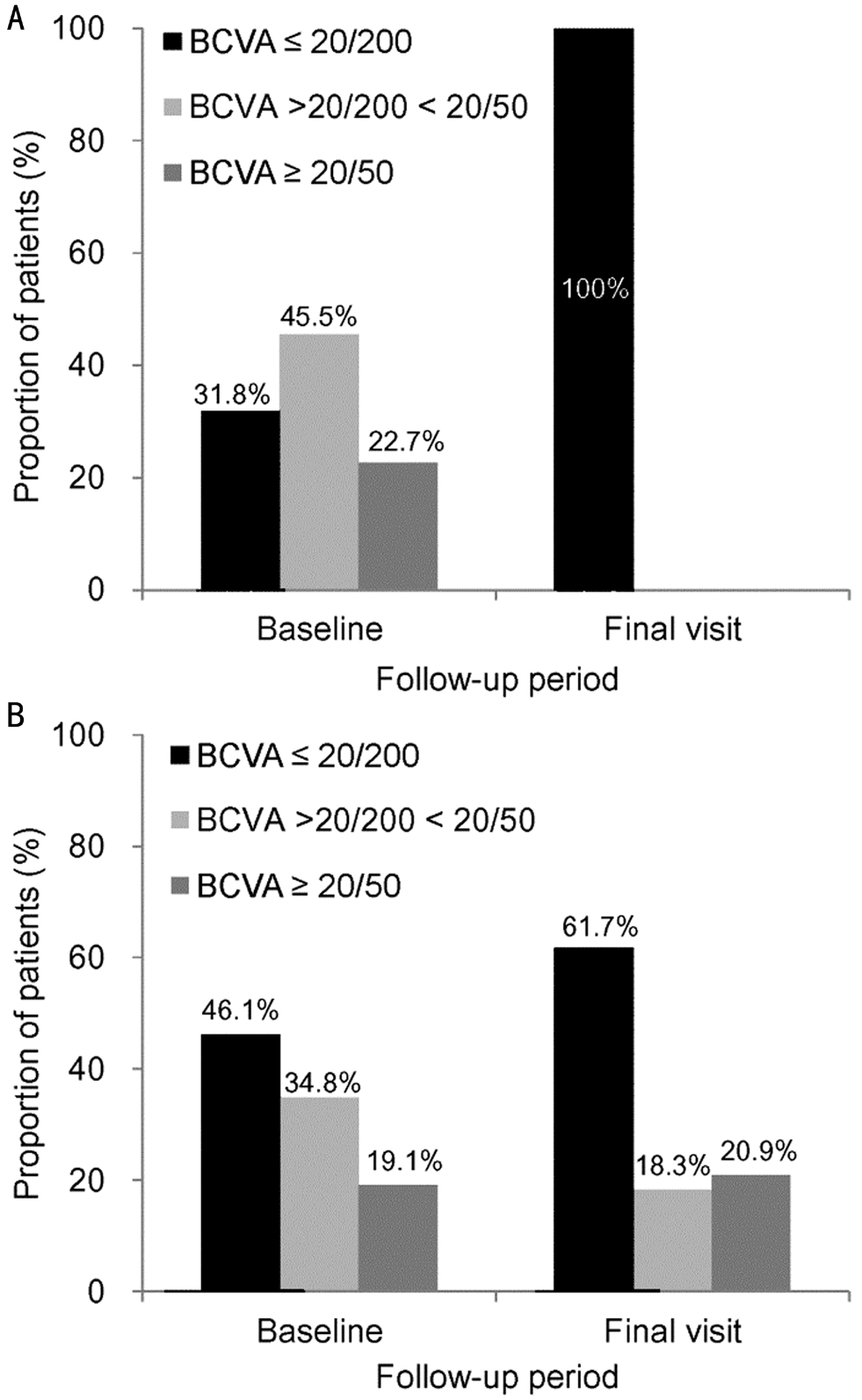
Figure 5 Proportion of patients stratified according to BCVA A:Abrupt visual loss group (n=22, final visit: 46.2±20.3mo); B: Nonabrupt visual loss group (n=115, final visit: 41.9±18.7mo).
To date, only limited knowledge is available regarding the development of subretinal hemorrhage secondary to type 3 neovascularization[16]. A recent study by Lee et al[16] showed that the visual prognosis of subretinal hemorrhage secondary to retinal angiomatous proliferation was very poor, with 17 of 18 eyes showing visual acuity worse than 20/1000 at the final follow-up examination. The exact reason for this markedly poor prognosis remains unknown. Recently, we reported marked degeneration in the outer retinal layers after resolution of subretinal hemorrhage[21], which may partially explain the limited recovery of visual function after hemorrhage[22-23]. Eyes with type 3 neovascularization are well known to exhibit a thin choroid[3] and high incidence of drusen and pseudodrusen[4-6].These findings suggest that perfusion and retinal function may already be compromised in these eyes. Thus, subretinal hemorrhage may induce more severe impairment of retinal function in type 3 neovascularization than in other subtypes of neovascular AMD, explaining the very poor visual outcome.
In the present study, all eyes with hemorrhage were primarily treated with anti-VEGF therapy; vitrectomy was performed in a subset of patients in order to clear the media. In addition,other treatment modalities (e.g. tissue-plasminogen activator injection or pneumatic displacement) were not used. Because abrupt visual loss was diagnosed based on visual acuity that was measured at the time of hemorrhage development, the treatment modality used in the present study did not influence the results regarding the incidence, timing, and causes of abrupt visual loss. However, it is possible that the lack of tissue-plasminogen activator injection or lack of pneumatic displacement may have affected the treatment outcome.
In the present study, tears in the RPE (grade 4) were found to be associated with abrupt visual loss in 13.6% of the patients.Sarraf et al[19] have previously shown that the prognosis of grade 4 RPE tears is very poor. This finding is similar to the poor prognosis noted in cases of RPE tears in our study. We attempted to identify factors predictive of abrupt visual loss.However, comparisons of characteristics between the abrupt visual loss and non-abrupt visual loss groups did not show significant differences, suggesting that this event may be difficult to predict.
A question stemming from these observations is whether it is possible to prevent or at least lower the risk of these catastrophic events. To answer this question, we focused on the potential association of these events with PED. In a study conducted by Su et al[18], the presence of serous PED was used to indicate an advanced stage of the disease. In the present study, PED was noted at least once on OCT images before the development of abrupt visual loss. The association of PED with tears in the RPE has already been firmly established;in fact, the etiology of tears in the RPE has been postulated to be a tensile force induced by contraction of choroidal neovascularization in PED[24]. PED was observed in all 11 cases of subretinal hemorrhage in this study. In a study conducted by Lee et al[16], a layered lamellar tissue complex was frequently noted in cases of fibrovascular PED before the development of subretinal hemorrhage, suggesting a close association between sub-RPE fibrovascular tissue and the development of hemorrhage. All the events associated with abrupt visual loss, except for subretinal hemorrhage and tears in the RPE, were accompanied by the development of an increase in the height of PED. This suggests that PED is associated, at least partially, with the events leading to abrupt visual loss.
PED is a frequently noted finding in type 3 neovascularization[18].We believe that if the development of PED can be impeded by treatment, the risk of abrupt visual loss may be reduced. PED in type 3 neovascularization generally responds well to anti-VEGF therapy[25]. Thus, continuous proactive treatment can be a useful therapeutic approach to impede PED development or resolve it immediately. The results of previous studies on treatment of type 3 neovascularization or retinal angiomatous proliferation using a treat-and-extend regimen support our postulation. In a study conducted by Matsumoto et al[26],significant improvement in visual acuity was noted during a 12-month follow-up period. In addition, no exudative change was noted throughout the follow-up period in 82%of the included eyes. In a study conducted by Castro-Navarro et al[27], improvement in visual acuity was noted in 6 of 8 patients during a 12-month follow-up period without development of tears in the RPE. In the present study, proactive treatment was not performed in any of the 22 eyes with abrupt visual loss. Additional studies are needed to elucidate whether long-term proactive treatment is truly effective in preventing abrupt visual loss.
It is noteworthy that abrupt visual loss developed during the fluid-free period in most of the patients. Abrupt visual loss may have been an “abrupt” event that developed within a short period in eyes that previously exhibited dry macula; in contrast, delayed treatment may have caused accumulation of retinal damage and eventually contributed to profound visual loss. In the present study, retreatment was primarily performed on an as-needed basis. However, strict monthly follow-up visits were not performed. In addition, the mean interval was 2.5mo between hospital visits immediately before abrupt visual loss and when visual loss was noted. Thus, lesion re-activation may not have been promptly detected in some patients, resulting in delayed treatment. Therefore, we suspect that the incidence of abrupt visual loss might be somewhat reduced if patients undergo more frequent follow-up. Additional controlled studies with frequent follow-up are needed to test this hypothesis.
This study had some limitations, such as its retrospective design and re-treatment being performed only on an asneeded basis in most cases. Although the reasons for treatment discontinuation were not accurately identified in this retrospective study, we postulate that poor visual outcome was the primary reason. The proportion of patients who discontinued treatment was higher in the abrupt visual loss group than in the non-abrupt visual loss group. However, the BCVA of 91.2% of the eyes was 20/400 or worse when the treatment was discontinued, suggesting that the influence of treatment discontinuation on the study results may not have been significant.
In summary, we investigated the incidence of abrupt visual loss, and factors associated with it, during anti-VEGF treatment for type 3 neovascularization. We found that loss of 5 or more lines of BCVA, indicating abrupt visual loss,was noted in 16.1% of the patients over a follow-up period of 42.4±18.9mo. The development of subretinal hemorrhage,tears in the RPE, and PED with fluid were found to be associated with abrupt visual loss. Once abrupt visual loss had developed, the visual prognosis was very poor, suggesting the importance of establishing a preventive method. We assume that proactive treatment may be useful in reducing the risk of these catastrophic events. However, considering the high risk of geographic atrophy in type 3 neovascularization[26], the advantages and disadvantages of this approach should be fully considered by the treating physician on a case-by-case basis.
ACKNOWLEDGEMENTS
Conflicts of Interest: Kim JH, None; Chang YS, None; Kim JW, None; Kim CG, None; Lee DW, None.
1 Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D,Matsumoto Y, Sorenson JA, Yannuzzi L. Type 3 neovascularization:the expanded spectrum of retinal angiomatous proliferation. Retina 2008;28(2):201-211.
2 Yannuzzi LA, Negrao S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J, Freund KB, Sorenson J, Orlock D, Borodoker N. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21(5):416-434.
3 Kim JH, Kim JR, Kang SW, Kim SJ, Ha HS. Thinner choroid and greater drusen extent in retinal angiomatous proliferation than in typical exudative age-related macular degeneration. Am J Ophthalmol 2013;155(4):743-749.
4 Kim JH, Lee TG, Chang YS, Kim CG, Cho SW. Short-term choroidal thickness changes in patients treated with either ranibizumab or aflibercept: a comparative study. Br J Ophthalmol 2016;100(12):1634-1639.
5 Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci 2015;56(3):1909-1915.
6 Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol 2018;63(6):782-815.
7 Boscia F, Furino C, Sborgia L, Reibaldi M, Sborgia C. Photodynamic therapy for retinal angiomatous proliferations and pigment epithelium detachment. Am J Ophthalmol 2004;138(6):1077-1079.
8 Bottoni F, Massacesi A, Cigada M, Viola F, Musicco I, Staurenghi G.Treatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation.Arch Ophthalmol 2005;123(12):1644-1650.
9 Nakata M, Yuzawa M, Kawamura A, Shimada H. Combining surgical ablation of retinal inflow and outflow vessels with photodynamic therapy for retinal angiomatous proliferation. Am J Ophthalmol 2006;141(5):968-970.
10 Parodi MB, Iacono P, Menchini F, Sheth S, Polini G, Pittino R,Bandello F. Intravitreal bevacizumab versus ranibizumab for the treatment of retinal angiomatous proliferation. Acta Ophthalmol 2013;91(3):267-273.
11 Parodi MB, Donati S, Semeraro F, Danzi P, Introini U, Viola F, Bottoni F, Pucci V, Musig A, Pece A, Azzolini C. Intravitreal anti-vascular endothelial growth factor drugs for retinal angiomatous proliferation in real-life practice. J Ocul Pharmacol Ther 2017;33(2):123-127.
12 Konstantinidis L, Mameletzi E, Mantel I, Pournaras JA, Zografos L,Ambresin A. Intravitreal ranibizumab (Lucentis) in the treatment of retinal angiomatous proliferation (RAP). Graefes Arch Clin Exp Ophthalmol 2009;247(9):1165-1171.
13 Oishi A, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Nakanishi H,Ueda-Arakawa N, Miyake M, Akagi-Kurashige Y, Hata M, Yoshikawa M, Kuroda Y, Takahashi A, Yoshimura N. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol 2015;159(5):853-860.e1.
14 Jacob J, Brie H, Leys A, Levecq L, Mergaerts F, Denhaerynck K,Vancayzeele S, Van Craeyveld E, Abraham I, MacDonald K. Sixyear outcomes in neovascular age-related macular degeneration with ranibizumab. Int J Ophthalmol 2017;10(1):81-90.
15 Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA,Jaffe GJ, Fine SL, Blodi B, Klein ML, Martin AA, Hagstrom SA, Martin DF. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121(1):150-161.
16 Lee JH, Lee MY, Lee WK. Incidence and risk factors of massive subretinal hemorrhage in retinal angiomatous proliferation. PLoS One 2017;12(10):e0186272.
17 Nagiel A, Sarraf D, Sadda SR, Spaide RF, Jung JJ, Bhavsar KV,Ameri H, Querques G, Freund KB. Type 3 neovascularization: evolution,association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina 2015;35(4):638-647.
18 Su D, Lin S, Phasukkijwatana N, Chen X, Tan A, Freund KB, Sarraf D. An updated staging system of type 3 neovascularization using spectral domain optical coherence tomography. Retina 2016;36 Suppl 1:S40-S49.
19 Sarraf D, Reddy S, Chiang A, Yu F, Jain A. A new grading system for retinal pigment epithelial tears. Retina 2010;30(7):1039-1045.
20 Holladay JT. Visual acuity measurements. J Cataract Refract Surg 2004;30(2):287-290.
21 Kim JH, Chang YS, Kim JW, Lee TG, Kim CG, Lee DW. Radiating hemorrhage in exudative age-related macular degeneration. Jpn J Ophthalmol 2016;60(6):466-475.
22 Kim JH, Chang YS, Lee DW, Kim CG, Kim JW. Quantification of retinal changes after resolution of submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2018;62(1):54-62.
23 Kim JH, Chang YS, Kim CG, Lee DW, Han JI. Hyperpigmented spots after treatment for submacular hemorrhage secondary to polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2018;256(3):469-477.
24 Nagiel A, Freund KB, Spaide RF, Munch IC, Larsen M, Sarraf D.Mechanism of retinal pigment epithelium tear formation following intravitreal anti-vascular endothelial growth factor therapy revealed by spectral-domain optical coherence tomography. Am J Ophthalmol 2013;156(5):981-988. e982.
25 Cho HJ, Kim KM, Kim HS, Lee DW, Kim CG, Kim JW. Response of pigment epithelial detachment to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol 2016;166:112-119.
26 Matsumoto H, Sato T, Morimoto M, Mukai R, Takahashi M, Hiroe T, Ehara K, Takayama M, Mimura K, Kishi S. Treat-and-extend regimen with aflibercept for retinal angiomatous proliferation. Retina 2016;36(12):2282-2289.
27 Castro-Navarro V, Cervera-Taulet E, Montero-Hernandez J, Navarro-Palop C. One-year outcomes of the treat-and-extend approach with aflibercept in age-related macular degeneration: effects on typical choroidal neovascularization and retinal angiomatous proliferation.Ophthalmologica 2016;236(4):215-222.