INTRODUCTION
Ever since the Ocular Hypertension Treatment Study(OHTS) showed that decreased central corneal thickness(CCT) is an independent risk factor that is strongly associated with glaucoma conversion in eyes with ocular hypertension(OHT), CCT became a significant parameter that assume a critical role in OHT and glaucoma management, and gained a particularly wide clinical and investigative attention among. OHTS investigators suggested that thin CCT leads to underestimation of measured intraocular pressure (IOP)when using Goldmann applanation tonometer (GAT), and thick CCT leads to overestimation of IOP measurement[1].Using different measurement techniques, this CCT to IOP relationship has been investigated broadly by many several studies later on[2-5]. They all concluded that any significant variation in CCT may affect true IOP readings, and this was discussed in the adult corneas only. Furthermore, many studies concluded that, in adults, CCT is thicker in patients with OHT than in glaucoma or normal individuals[2-3,6]. However, these studies discussed the relationship of CCT to IOP in adults, and little is known about the CCT to IOP relationship in children. In addition, the distribution of CCT and IOP among children and adults is different. The racial factor has also proved to be an essential socio-demographic parameter affecting CCT and IOP. In different studies of Caucasian and coloured adults and children, CCT has been reported to be thinner in healthy eyes of coloured adults[1,7] and coloured children[8-9]. Currently, few data available about the normal value distribution of CCT and IOP in children of the Middle East[10-11] and none from the Arab people. To the best of the author’s knowledge, no data available in published literature about the normal value distribution of CCT and IOP, and their interrelation in children of Palestine.Therefore a representative school-based cross-sectional study was primarily conducted to determine the distributions of CCT and IOP in emmetropic eyes of healthy children of Palestine.A second specific objective was to investigate the following:1) the correlation between CCT and IOP in children; 2) the influence of age and gender on CCT and IOP in children; 3)the intereye asymmetry of CCT and IOP in children.
SUBJECTS AND METHODS
Ethical Approval The ethics committee of Palestinian health research council has approved the proposal of the study and provided a written ethical approval (No. PHRC/HC/161/16-Helsinki Approval). The study protocol was conducted in accordance with the tenets of Declaration of Helsinki.Written informed consent was obtained from either parents or the legal guardian of the child. Of importance, any child brought a signed informed consent from his or her parents or legal guardian but did not cooperate well or simply refused to complete the examination was not included in the current study and was transported back to his or her school. In other words, children’s assent to participation in the current study was properly obtained.
Study Design and Distribution of Participants This study was government schools-based cross sectional study performed in Gaza Strip in coastal western Palestine. This study was supported through a cooperative agreement with the ministry of education and higher education of Palestine and the Islamic University of Gaza, faculty of health sciences department of optometry. According to demographic distribution of schools in Gaza Strip, target schools (n=27) from all governorates were selected randomly to be sampled in this study, and were stratified by class, age, gender. Sample size determination and distribution to cover all governorates in Gaza Strip was statistically attained by the equation of Steven Thompson with the level of significance of 0.05, and then was collected accordingly[12]. A sample size of 400 students of both gender was statistically considered as a representative cluster sample of school-based population (n=79 552). However, sample size was increased by more than 30% (n=178) in order to increase powerful of statistical analysis and reduce type II error. Based on the inclusion criterion of an age 7, 9, 11, 13 and 15y, only 578 children were enrolled in the study.
Examination Procedures and Eligibility Criteria
Refraction examination First, all enrolled students underwent the measurement of distance uncorrected visual acuity using Snellen E-chart (Good-lite Co., Elgin, IL, USA) in special rooms at schools, and only those who attain 6/6 vision in each eye were included in the study. The students were then transported to the clinic to receive optometric and ophthalmic examinations. All ophthalmic imaging studies and nonocular parameters documentation were taken after complete refraction tests. Non-cycloplegic refraction was then measured with an auto-keratorefractometer (RC-5000 Advanced, Tomey,USA). The average of four reliable readings was considered as the valid reading. Finally, all students underwent subjective refraction. To minimize the impact of refractive status on CCT and IOP, the study included only emmetropic eyes of healthy students only. Emmetropia here was defined as those with refractive status between [-0.25 diopter sphere (Ds) to+1.00 Ds and -0.25 diopter cylinder (Dc) to -0.75 Dc] by auto-keratorefractometer, and attained 6/6 vision in each eye without any optical correction.
Slit-lamp examination and glaucoma screening Slitlamp examination was performed for all students by one experienced ophthalmologist to examine the anterior and posterior segments of the eye with strict adherence to the study protocol and guidelines. Glaucoma screening had the following four main examination stages. The first, slit lamp examination of the anterior segment, where any suspect anterior chamber dysgenesis of any form, significant ectropion uveae, iris hypoplasia, and congenital cataract of any form were all excluded from the study. The second, biomicroscopic indirect ophthalmoscopy using Volk 78 double aspheric lens to examine the fundus and the optic nerve head (ONH) in particular. A comprehensive ONH examination was carried out, where asymmetric cup-to-disc ratio>0.2, suspect cupping appearance, cupping that violates the ISNT rule (neuroretinal rim area: inferior>superior>nasal>temporal), dislocated cups,neuroretinal rim thinning or notching, peripapillary atrophy of any type, and congenital ONH or macular anomalies were all excluded from the study. Third, spectral domain optical coherence tomography imaging (RTVue; Optovue, Inc.,Fremont, CA, USA) for retinal nerve fiber layer thickness,ONH, and macular parameters were all reviewed, studied and documented. Forth, measured IOP. Only IOPs≤21 mm Hg in both eyes were included in the study.
Measurement of central corneal thickness and intraocular pressure To alleviate fear or anxiety and to cancel the effect of contact method ocular procedures on measurements of ocular parameters obtained by noncontact methods, all noncontact ocular parameters measurements were first acquired and then followed by measurement of CCT and IOP as the final phase of examination. The measurement of CCT for all participants was performed by one experienced optometrist using ultrasound pachymeter (Sonomed Pacscan 300A+, USA).One drop of 0.4% oxybuprocaine hydrochloride was instilled in the conjunctival sac of each eye for corneal anesthesia.The tip of the ultrasound pachymeter probe was disinfected with 75% alcohol swab and dried. After confirming fixation,the probe touched gently to the cornea without pressure or indentation, and perpendicular to the central corneal surface.CCT of the right eye was measured first and then the same procedure was performed in the left eye within 15s. The average of five reliable measurements was recorded for each eye. In all eyes, the difference between the maximum and minimum reading for the same eye was less than 10 µm. IOP measurement by GAT (Optisal, S.L., USA), was performed for all participants under topical anesthesia using one drop of 0.4% oxybuprocaine hydrochloride instilled in each eye and followed by instillation of appropriate amount of fluorescein.Using slit lamp, the cornea was first re-examined for any surface abnormalities. The tonometer was set at 10 mm Hg before each reading. After confirming fixation, slight upper eyelid elevation by the examiner’s finger, without pressing on the globe, was required for all participants in the same manner to avoid blink reflex and IOP re-evaluation. The prism of GAT was gently and slowly introduced to central corneal surface until appropriate fluorescein stained semicircles appear fixed and respect the horizontal midline then the examiner starts introducing variable forces until the inner margins of the semicircles just touch each other. The left eye was measured first and then the same procedure was applied to the right eye.The interval time between the measurements of left and right eye was ≤15s. Each eye was measured twice, but in case of any variation in IOP measurement in the same eye, a third measurement was taken, and such instances were very rare in this study. All participants had their IOPs measured by the author himself, and all measurements of CCT and IOP were taken between 11:00 a.m.-13:00 p.m. at different days and within a two-month period only.
Inclusion and exclusion criteria Inclusion criteria of the study included emmetropia, healthy children of an age 7, 9,11, 13 and 15y, uncorrected distant visual acuity of 6/6 in each eye, a refractive status between (-0.25 Ds to +1.00 Ds and -0.25 Dc to -0.75 Dc) by auto keratorefractometer, and the availability of binocular measurements. Exclusion criteria included a positive history of any type of prior systemic or ocular medical or surgical treatment, positive history of head or eye trauma, glaucoma suspect of any form, uncorrected visual acuity <6/6 in either eye, refractive errors outside the limits of (-0.25 Ds to +1.00 Ds and -0.25 Dc to -0.75 Dc), GAT measured IOP>21 mm Hg in either eye, previous contact lens use, were all excluded from the study.
Statistical Analysis SPSS software, version 22.00 (SPSS, Inc.,Chicago, IL, USA), was used for data analysis. Frequencies and percentile were used to represent demographics as age,gender and class. Histogram chart was used to represent quantitative and qualitative data of IOP and CCT variables.Values are reported as mean±standard deviation, and P<0.05 was considered statistically significant. One-way ANOVA variance analysis was used to compare CCT and IOP values between the age groups. The paired sample t-test was used to compare CCT and IOP values for right and left eyes, and theindependent sample t-test was used to compare CCT and IOP values between male and female groups. Pearson correlation coefficient was used for measuring the relationship between CCT and IOP. Simple linear regression analysis and coefficient of determination R2 were used to measure the effect of CCT on IOP for both eyes.
Table 1 Demographic characteristics of study population n (%)

?
RESULTS
Demographic Characteristics Overall, 578 school children were considered for enrollment, however, 53 (9.16%) children were excluded due to the criteria outlined above. Reasons for exclusion from analysis were: refractive errors (19 subjects),ocular trauma (7 subjects), informed consent disapproval or poor assent (17 subjects), and systemic pathology (10 subjects). In total, the study included 1050 eyes of 525 (90.83%)emmetropic healthy school children aged 7, 9, 11, 13 and 15y.The demographic characteristics of the study population are described in Table 1.
Mean age was 11.13±2.8y. The study population consisted of 257 (48.95%) males and 268 (51.05%) females. Mean CCT values were 542.2±37.4 (range: 449-656) μm and 544.3±39.2(range: 446-657) μm for right and left eyes, respectively. Mean IOP values were 12.5±2.2 and 12.3±2.2 mm Hg for right and left eyes, respectively, and with a range of 7-19 mm Hg in both eyes. Based on the result of normality test, values of CCT and IOP were normally distributed, as can be seen in Figures 1 and 2,respectively.
Distribution of Central Corneal Thickness and Correlation with Gender and Age Distribution of CCT by gender and age in right and left eyes are presented in Tables 2 and 3,respectively. In males, mean CCT values for right and left eyes were 542.5±35.3 and 543.5±34.9 μm, respectively. In females,mean CCT values for right and left eyes were 541.9±39.4 and 545.1±43.0 μm, respectively. There were no statistically significant differences between CCT and gender (P>0.05,Table 2), or CCT and age (P>0.05, Table 3).
Distribution of Intraocular Pressure and Correlation with Gender and Age Mean IOP values by gender and age are presented in Tables 2 and 3, respectively. In males, mean IOP values for right and left eyes were 12.3±2.3 and 12.2±2.2 mm Hg,respectively. In females, mean IOP values for right and left eyes were 12.6±2.2 and 12.4±2.2 mm Hg, respectively. There were no statistically significant differences between IOP and gender (P>0.05, Table 2), or IOP and age (P>0.05, Table 3).

Figure 1 Histogram showing the distribution of CCT of the right eye in Palestinian children according to age (n=525). CCT is normally distributed.

Figure 2 Histogram showing the distribution of IOP of the right eye in Palestinian children according to age (n=525). IOP is normally distributed.
Table 2 Distribution of CCT and IOP in children according to gender mean±SD

CCT: Central corneal thickness; IOP: Intraocular pressure. aIndependent sample t-test for testing the mean difference of CCT due to gender,P>0.05; cIndependent sample t-test for testing the mean difference of IOP due to gender, P>0.05.
CCT (µm) IOP (mm Hg)Female Male Female Male Right eyea Left eyea Right eyea Left eyea Right eyec Left eyec Right eyec Left eyec 7 98 (18.67) 542.7±35.8 545.9±34.8 552.1±40.7 551.7±38.6 13.0±1.7 12.8±1.9 12.7±2.3 12.7±2.3 9 99 (18.86) 536.4±33.6 538.1±35.1 538.8±29.0 540.3±28.8 12.4±2.1 12.0±2.0 12.9±2.1 12.6±2.1 11 105 (20.00) 539.9±34.9 542.0±35.5 541.7±34.0 542.0±34.8 12.4±2.2 12.3±2.1 11.8±2.4 11.8±2.2 13 118 (22.48) 544.1±32.4 545.0±32.2 541.7±35.4 543.6±35.4 12.7±2.5 12.4±2.5 12.2±2.1 12.1±2.2 15 105 (20.00) 540.1±37.6 541.1±37.3 537.7±36.0 539.4±36.2 12.6±2.7 12.7±2.1 12.1±2.6 11.9±2.4 Total 525 (100.00) 541.9±39.4 545.1±43.0 542.5±35.3 543.5±34.9 12.6±2.2 12.4±2.2 12.3±2.3 12.2±2.2 Age n (%)
Table 3 Distribution of CCT and IOP in children according to age mean±SD

CCT: Central corneal thickness; IOP: Intraocular pressure. aOne way ANOVA test for mean difference of CCT due to age, P>0.05; bOne way ANOVA test for mean difference of IOP due to age, P>0.05.
Variables 7y (n=98) 9y (n=99) 11y (n=105) 13y (n=118) 15y (n=105) Total (n=525)CCTa (µm)Right eye 547.6±38.5 540.7±44.1 540.8±34.3 543.0±33.7 539.0±36.7 542.2±37.4 Left eye 548.9±36.7 546.3±52.5 542.0±34.9 544.4±33.7 540.3±36.7 544.3±39.2 IOPb (mm Hg)Right eye 12.8±2.0 12.6±2.1 12.1±2.3 12.5±2.3 12.4±2.4 12.5±2.2 Left eye 12.7±2.1 12.3±2.1 12.0±2.2 12.2±2.4 12.3±2.3 12.3±2.2
Correlation Between Central Corneal Thickness and Intraocular Pressure Using Pearson correlation coefficient,a positive correlation was observed between CCT and IOP of the right eye (P=0.000, R=0.358) and CCT and IOP of the left eye (P=0.000, R=0.324), as can be seen in Table 4. Linear regression analysis indicated that for every 100 μm increase in CCT, measured IOP increases by 0.024 mm Hg (P=0.000,R2=0.138) and 0.022 mm Hg (P=0.000, R2=0.121) for right and left eyes, respectively (Figure 3).

Figure 3 Scattergrams of CCT versus IOP in Palestinian children (n=525), where the CCT is the independent variable A: The right eye;B: The left eye.
Table 4 Correlations between CCT and IOP in children
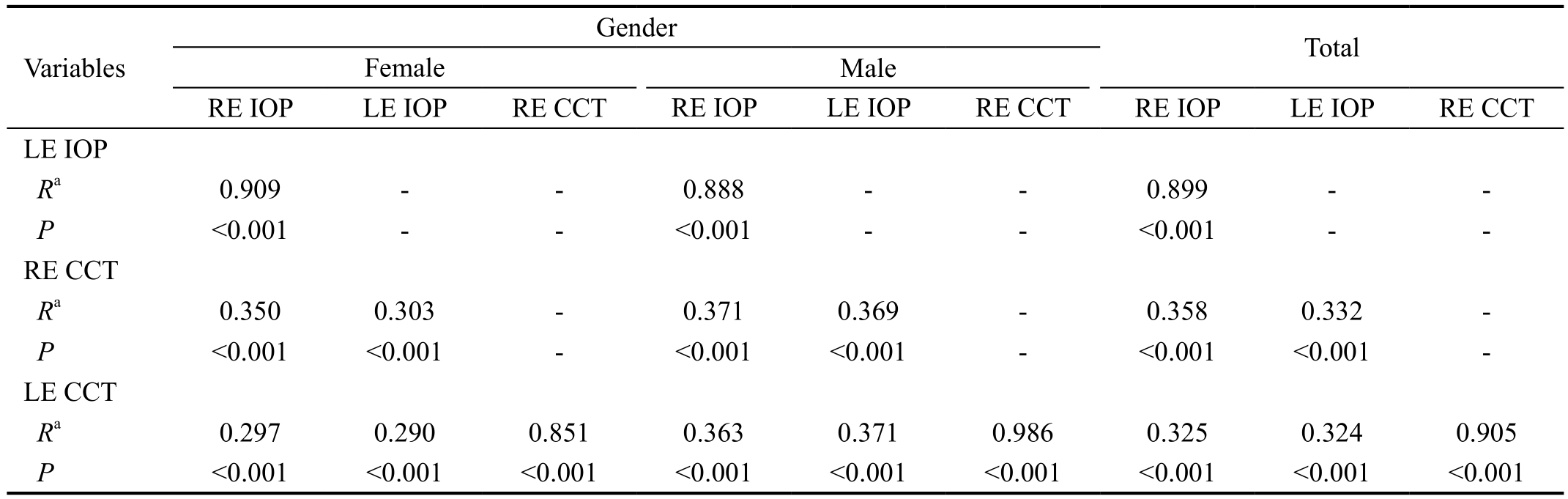
RE: Right eye; LE: Left eye; IOP: Intraocular pressure; CCT: Central corneal thickness; R: Pearson correlation. aCorrelation is significant at the 0.01 level (2-tailed).
Gender Variables Total Female Male RE IOP LE IOP RE CCT RE IOP LE IOP RE CCT RE IOP LE IOP RE CCT LE IOP Ra 0.909 - - 0.888 - - 0.899 - -P<0.001 - - <0.001 - - <0.001 - -RE CCT Ra 0.350 0.303 - 0.371 0.369 - 0.358 0.332 -P<0.001 <0.001 - <0.001 <0.001 - <0.001 <0.001 -LE CCT Ra 0.297 0.290 0.851 0.363 0.371 0.986 0.325 0.324 0.905 P<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001
Intereye Asymmetry of Central Corneal Thickness and Intraocular Pressure By using paired sample t-test, there was statistically significant difference in mean CCT values between right and left eyes (2.1±16.8 µm, P=0.004) in favor for the left eye. The range of intereye difference in CCT values was estimated to be 0-36 µm. A significant statistical difference in mean IOP values was also observed between right and left eyes (0.2±1.0 mm Hg, P=0.001) in favor for the right eye. The range of intereye difference in IOP values was estimated to be 0-5 mm Hg. Differences in mean CCT and IOP values between right and left eyes due to gender were also similarly significant,P<0.05. More details were shown in Table 5.
DISCUSSION
Ultrasound pachymeter and GAT are the gold standard measurement of CCT and IOP, respectively[13-14]. In this cross sectional study, ultrasound pachymeter and GAT were used to obtain measurements of CCT and IOP, respectively, in Palestinian children for the first time. For this reason, there was a considerable limitation in comparing the values of this study with results of other domestic studies.
Distribution of Central Corneal Thickness and Correlation with Age, and Gender In this study, mean CCT values in school children were 542.2±37.4 (range: 449-656) and 544.3±39.2 (range: 446-657) µm for right and left eyes,respectively. In studies of the Middle East, mean CCT inTurkish children was reported to be 564.92±32, 557.91±34.2 and 564.92±32 µm[10,15-16]. The instruments used to measure CCT, and the age groups in these studies were very similar to the current study. Similarly, Mean CCT in Iranian children was reported to be 556.34±34.2 and 575.1±44.5 (range: 473-667)µm[11,17]. However, sample size, age groups, and measurement technique were all very different and were not similar to this study. Only one Iranian study[18] has reported a thinner mean CCT (513.47±34.5 µm) than those reported previously.However this study had a small sample size (n=131) and mean CCT was obtained with specular microscope which has been reported to produce a relatively thinner mean CCT compared to ultrasound pachymeter[19]. In Asian population, mean CCT in Mongolian[20], Japanese[21], Malay[22], and in Chinese[23]children was reported to be 521, 544.3±36.9, 530.87±30.7 and 554.19±35 µm, respectively. This contradiction in values of mean CCT in Asian studies is likely related to the large variations in sample size, age groups, and measurement techniques. Mean CCT has been reported to be between 544 and 563 µm in western Caucasian children[24-25] and between 559 and 573 µm in USA Caucasian children. While in African-American children mean CCT values were reported to be between 535 and 551 µm[9,26-27]. The above mentioned results suggest that mean CCT in Palestinian children of Arab origin is slightly thicker than that of African-American and Asian children, and similar or slightly thinner than that of Caucasian children population. This controversy in the results of CCT measurement in children, including the present study, can be attributed to a wide range of different extrinsic and intrinsic factors, such as: racial and ethnic composition of the child population, demographics, measurement technique, children assent, study design, sample size, expertise of the examiner,hereditability and genetics, validity of measurement, health status, instrument calibration, etc., so that each of these factors plays a key role in providing accurate and precise results. In a Meta-analysis study, the mean CCT in children was reported to be 553.69 µm (95%CI: 551.60-555.78) among all races[28].While the range of CCT values, measured solely by ultrasound pachymeter, in children was estimated to be between 429 to 666 µm[12-14,18-19,24,28-30]. The relationship between CCT and age in children was still controversial. In agreement with other reports, no correlation between CCT and age was observed in children[17-18,22-23]. The results of the current study is also strongly supported by Ehlers et al[29] who suggested that CCT reaches adult levels at around 3 years of age. Published studies that showed a correlation with age, either have a younger age group (6-12y) or a wider range of age (7mo to 18 years old), which is different from age group recruited in the present study (7 to 15 years old) [13-14,18-19,23-24,27,29-30].The measurement technique and ethnicity may also play a key role in this correlation. In agreement with other studies,no correlation between CCT and gender was observed in children[17-18,20,23-24,26-27]. Only few studies observed a correlation between CCT and gender, in which mean CCT values in males were slightly thicker than in females by 3.23 to 6.4 µm[10-11,22]. The author suggests that this correlation is clinically insignificant and may be negligible.
Table 5 Evaluation of intereye asymmetry for CCT and IOP in children
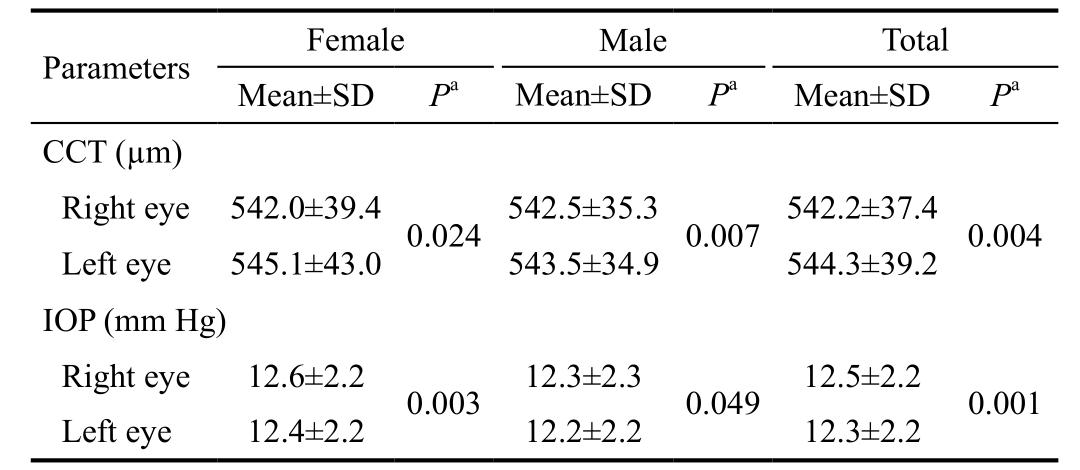
CCT: Central corneal thickness; IOP: Intraocular pressure. aP-value by paired samples t-test, P<0.05.
?
Intereye Asymmetry of Central Corneal Thickness in Children It has been reported that there are no significant differences in mean CCT values between right and left eyes of children[18,24]. However, mean CCT values in the present study were thicker in left eyes by 2.1±16.8 µm compared to right eyes. This is in agreement with other studies that confirmed a significant intereye asymmetry in children[10,20-22]. In the present study, CCT measurements were taken first in the right eye followed by the left eye; this intereye difference in CCT values may possibly be caused by the order of measurements, and this is in agreement with similar situation reported by Sakalar et al[10]; another justification is the measurement accuracy of the ultrasound pachymeter; otherwise the author could not find a reasonable clarification for the difference in CCT between the two eyes and suggests that this mild difference is clinically insignificant. In the present study, the calculated normal range for intereye difference in CCT is 0-36 µm.
Distribution of Intraocular Pressure and Correlation with Age, and Gender According to the results of the present study, the mean IOP in emmetropic healthy children of Palestine was 12.5±2.2 and 12.3±2.2 mm Hg for right and left eyes, respectively. The normal range, which is expected to contain 95% of the children population, was 7 to 19 mm Hg for both eyes. Mean IOP of children aged from birth to 19 years old measured in different techniques was 12.01±3.9 to 17.8±2.7 mm Hg in Turkey[10], Iran[18], China[30-31], Japan[21],Poland[25], India[32], Spain[24] and USA[26]. This difference in values supports the hypothesis of the existence of ocular structural variation among different ethnic and racial groups[9].In the Middle East, mean IOP in children reported from Turkey(14.21±2.95 mm Hg) and Iran (13.72±2.04 mm Hg) were relatively higher than mean IOP values reported in the present study (12.46±2.242 mm Hg)[10,18]. This is in part due to different measurement methods and studied age groups. However, as noted previously, both of Turkish and Iranian children had a higher mean CCT values compared to the present study, and it’s well established that eyes with thinner CCT have lower IOP values[1,33]. The IOP values using pneumotonometer in an Indian study (n=405) aged 0-12 years old was 12.03±3.5 and 12.01±3.9 mm Hg; and mean CCT values were 541 and 552 µm for the right and left eyes, respectively, which is very similar to criteria, methods, and results of the present study[32].This result indicates that IOP is influenced by variations in CCT values. As a matter of fact, direct comparisons between the studies discussed above, including the present study, can be very difficult and confusing since there is a large variation in methodologies, age group, sample size, race and ethnicity, and more importantly the great variance in the mean CCT values of these studies as mentioned previously. Using GAT, mean IOP values in Palestinian children of Arab origin is considerably lower than that of Iranian, Chinese, and Caucasian children of Turkey, Spain, Poland and USA, and slightly lower than that of Japanese children, and similar to that of Indian children population. The correlation between IOP and age or gender in children is controversial. Some investigators[10,30-32]suggested that IOP is positively correlated with age, while others[18,21,24-25] did not find any correlation between IOP and age, which is in agreement with the present study. In agreement with other studies, as well, the author did not find any statistically significant difference in the IOP due to gender in either eye[10,18,24-25]. Only few studies showed that IOP was significantly higher in female than in male children[30-31].
Intereye Asymmetry of Intraocular Pressure in Children It has been reported that there are no significant differences in mean IOP values between right and left eyes of children[10,24,32].However, some investigators confirmed a significant intereye asymmetry in IOP[30]. Mean IOP values in the present study were higher in right eyes by 0.2±1.0 mm Hg compared to left eyes which is clinically negligible and insignificant. This difference may be attributed to the order of measurements since IOP measurements were taken first in the right eye followed by the left eye. In adults, it has been suggested that intereye difference of 3 mm Hg is associated with a 6 percent probability and a difference of >6 mm Hg with a 57 percent probability of having glaucoma[34]. In the present study,the calculated normal range for intereye difference in IOP of children is 0-5 mm Hg. Therefore any greater variation between the two eyes in IOP is to be suspicious and should be carefully examined and monitored.
Correlation Between Central Corneal Thickness and Intraocular Pressure Using different measurement technique,numerous studies showed a positive correlation between CCT and measured IOP in children[9,15-16,18,20-23,25,27]. According to these studies, there has been a well-established quantitative relation between CCT and IOP ranging from 0.32 to 3.5 mm Hg increase in measured IOP for every 100 µm increase in CCT.The present study showed that measured IOP increases 0.024 and 0.022 mm Hg for the right and left eye, respectively, for every 100 µm increase in CCT. This variance in values of quantitative relation among these studies is likely attributed to methodologies, instrumentations, and time of measuring CCT and IOP values. In addition, effect of IOP fluctuation was not taken into consideration though proved to be significant[35].It’s, however, important to note that this quantitative relation between CCT and IOP should not be interpreted as a correction factor. On the other hand, Haider et al[26], as a unique exception, did not find any correlation between CCT and measured IOP. This result is most likely due to a small sample size (n=137) of different racial groups, and a wide range of age groups (7mo-18y).
Potential limitations for the present study should be mentioned.First, Gaza Strip in costal western of Palestine is not representative for whole Palestine. For the whole country,however, this study was the first one to report on CCT and IOP in children. Second, this cross sectional study provided only one time measurement for each child. Nevertheless, the absence of correlation between age and gender in IOP does not necessarily indicate that correlation is not existent. Third,the present study has not been able to compare the results with other domestic or international results of Arab children due to lack of similar studies. Forth, this study did not include a younger age group because Goldmann applanation tonometry and ultrasound pachymetry are very difficult to be performed in younger children. Fifth, only one examiner conducted IOP measurement in the current study which may lead to a limitation on investigating the correlation between measured IOP and gender, age, and CCT.
In this study, a population profile of CCT and IOP was established in Palestinian children for the first time. Mean CCT is comparable to some studies but differ from others.Mean IOP is considerably lower than that of majority children of other ethnic groups. CCT and IOP in emmetropic eyes of healthy children (7-15 years old) were positively correlated.Intereye difference of more than 36 µm in CCT, and 5 mm Hg in IOP should prompt evaluation for potential ocular disorders.In this study, CCT and IOP were not correlated to age or gender. Findings of this study can be used as a reference for diagnostic and clinical purposes.
AKNOWLEDGEMENTS
Foundation: Supported by Qatar Charity Under Ibhath Project for Research Grants, which is funded by the Cooperation Council for the Arab States of the Gulf throughout Islamic Development Bank.
Conflicts of Interest: Alkhodari HT, None.
1 Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the ocular hypertension treatment study (OHTS). Ophthalmology 2001;108(10):1779-1788.
2 Argus WA. Ocular hypertension and central corneal thickness.Ophthalmology 1995;102(12):1810-1812.
3 Hemdon LW, Choudhri SA, Cox T, Damji KF, Shields MB, Allingham RR. Central corneal thickness in normal, glaucomatous, and ocular hypertensive eyes. Arch Ophthalmol 1997;115(9):1137-1141.
4 Foster PJ, Alsbirk PH, Baasanhu J, Munkhbayar D, Uranchimeg D,Johnson GJ. Anterior chamber depth in Mongolians: variation with age,sex, and method of measurement. Am J Ophthalmol 1997;124(1):53-60.
5 Dohadwala AA, Munger R, Damji KF. Positive correlation between Tono-Pen intraocular pressure and central corneal thickness. Ophthalmology 1998;105(10):1849-1854.
6 Ventura AC, Bohnke M, Mojon DS. Central corneal thickness measurements in patients with normal tension glaucoma, primary open angle glaucoma, pseudoexfoliation glaucoma, or ocular hypertension. Br J Ophthalmol 2001;85(7):792-795.
7 La Rosa FA, Gross RL, Orengo-Nania S. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Arch Ophthalmol 2001;119(1):23-27.
8 Muir KW, Jin J, Freedman SF. Central corneal thickness and its relationship to intraocular pressure in children. Ophthalmology 2004;111(12):2220-2223.
9 Muir KW, Duncan L, Enyedi LB, Freedman SF. Central corneal thickness in children: racial differences (black vs. white) and correlation with measured intraocular pressure. J Glaucoma 2006;15(6):520-523.
10 Sakalar YB, Keklikci U, Unlu K, Alakus MF, Yildirim M, Dag U.Distribution of central corneal thickness and intraocular pressure in a large population of Turkish school children. Ophthalmic Epidemiol 2012;19(2):83-88.
11 Hashemi, H, Saatchia M, Khabazkhoobbc M, Emamian MH, Yektae A, Fotouhif A. Distribution of corneal thickness and its determinants in 6-12-year-old children in an Iranian general population. J Curr Ophthalmol 2017.
12 Thompson SK. Sampling, 3rd Edition 2012.
13 Sadoughi MM, Einollahi B, Einollahi N, Rezaei J, Roshandel D, Feizi S. Measurement of central corneal thickness using ultrasound pachymetry and Orbscan II in normal eyes. J Ophthalmic Vis Res 2015;10(1):4-9.
14 Lamparter J, Hoffmann EM. Measuring intraocular pressure by different methods. Ophthalmologe 2009;106(8) 676-682.
15 Gul A, Caglar C, Cinal A, Yasar T, Kilic A. Ocular biometry and central corneal thickness in children: a hospital-based study. Arq Bras Oftalmol 2014;77(3):152-154.
16 Yildirim N, Sahin A, Basmak H, Bal C. Effect of central corneal thickness and radius of the corneal curvature on intraocular pressure measured with the Tono-Pen and noncontact tonometer in healthy schoolchildren. J Pediatr Ophthalmol Strabismus 2007;44(4):216-222.
17 Hashemi H, Yazdani K, Mehravaran S, KhabazKhoob M, Mohammad K, Parsafar H, Fotouhi A. Corneal thickness in a population-based, crosssectional study: the Tehran eye study. Cornea 2009;28(4):395-400.
18 Nejabat M, Heidary F, Talebnejad MR, Salouti R, Nowroozzadeh MH, Masoumpour M, Mahdaviazad H, Tajbakhsh Z, Keshtkar M, Jamali H, Khalili MR, Movahedan H, Roustaei N, Gharebaghi R. Correlation between intraocular pressure and central corneal thickness in Persian children. Ophthalmol Ther 2016;5(2):235-243.
19 Al-Ageel S, Al-Muammar AM. Comparison of central corneal thickness measurements by Pentacam, noncontact specular microscope,and ultrasound pachymetry in normal and post-LASIK eyes. Saudi J Ophthalmol 2009;23(3-4):181-187.
20 Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Central corneal thickness and intraocular pressure in a Mongolian population. Ophthalmology 1998;105(6):969-973.
21 Hikoya A, Sato M, Tsuzuki K, Koide YM, Asaoka R, Hotta Y. Central corneal thickness in Japanese children. Jpn J Ophthalmol 2009;53(1):7-11.
22 Heidary F, Gharebaghi R, Wan Hitam WH, Naing NN, Wan-Arfah N,Shatriah I. Central corneal thickness and intraocular pressure in Malay children. PLoS One 2011;6(10):e25208.
23 Wei W, Fan Z, Wang L, Li Z, Jiao W, Li Y. Correlation analysis between central corneal thickness and intraocular pressure in juveniles in Northern China: the Jinan city eye study. PLoS One 2014;9(8):e104842.
24 Bueno-Gimeno I, Gene-Sampedro A, Pinero-Llorens DP, Lanzagorta-Aresti A, Espana-Gregori E. Corneal biomechanics, retinal nerve fiber layer, and optic disc in children. Optom Vis Sci 2014;91(12):1474-1482.
25 Krzyzanowska-Berkowska P, Asejczyk-Widlicka M, Pierscionek B. Intraocular pressure in a cohort of healthy eastern European schoolchildren: variations in method and corneal thickness. BMC Ophthalmol 2012;12:61.
26 Haider KM, Mickler C, Oliver D, Moya FJ, Cruz OA, Davitt BV. Age and racial variation in central corneal thickness of preschool and schoolaged children. J Pediatr Ophthalmol Strabismus 2008;45(4):227-233.
27 Pediatric Eye Disease Investigator Group, Bradfield YS, Melia BM,Repka MX, Kaminski BM, Davitt BV, Johnson DA, Kraker RT, Manny RE, Matta NS, Weise KK, Schloff S. Central corneal thickness in children.Arch Ophthalmol 2011;129(9):1132-1138.
28 Farvardin M, Heidary F, Sayehmiri K, Gharebaghi R, Jabbarvand Behrooz M. A comprehensive meta-analysis on intraocular pressure and central corneal thickness in healthy children. Iran J Public Health 2017;46(6):724-732.
29 Ehlers N, Hansen FK, Aasved H. Biometric correlations of corneal thickness. Acta Ophthalmol 1975;53(4):652-659.
30 Jiang WJ, Wu JF, Hu YY, Wu H, Sun W, Lu TL, Wang XR,Bi HS, Jonas JB. Intraocular pressure and associated factors in children: the Shandong children eye study. Invest Ophthalmol Vis Sci 2014;29;55(7):4128-4134.
31 Yang DY, Guo K, Wang Y, Guo YY, Yang XR, Jing XX, Guo HK, Tao Y, Zhu D, Jonas JB. Intraocular pressure and associations in children. The gobi desert children eye study. PLoS One 2014;9(10):e109355.
32 Sihota R, Tuli D, Dada T, Gupta V, Sachdeva MM. Distribution and determinants of intraocular pressure in a normal pediatric population. J Pediatr Ophthalmol Strabismus 2006;43(1):14-18; quiz 36-37.
33 Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ,Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Kass MA. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120(6):714-720.
34 Williams AL, Gatla S, Leiby BE, Fahmy I, Biswas A, de Barros DM,Ramakrishnan R, Bhardwaj S, Wright C, Dubey S, Lynch JF, Bayer A, Khandelwal R, Ichhpujani P, Gheith M, Siam G, Feldman RM,Henderer JD, Spaeth GL. The value of intraocular pressure asymmetry in diagnosing glaucoma. J Glaucoma 2013;22(3):215-218.
35 Cheng J, Xiao M, Xu H, Fang S, Chen X, Kong X, Sun X. Seasonal changes of 24-hour intraocular pressure rhythm in healthy Shanghai population. Medicine (Baltimore) 2016;95(31):e4453.