INTRODUCTION
Intraocular pressure (IOP), pressure within the eye, is exerted on the eye wall to prevent the collapse of the eyeball, higher than atmospheric pressure[1]. Elevated IOP has been certified to be associated with glaucoma[2], which is characterized by a disorder of blood supply and optic disc cupping[3]. IOP is the only modifiable risk factor for glaucoma,and lowering IOP prevents the development and progression of the disease[4]. A large number of studies have shown the relationship between the IOP and several health problems,such as age, abdominal obesity, high blood pressure, diabetes and hyperglycemia, most of them share a single common mechanism that contributes to metabolic syndrome (MetS)[5-7].MetS is a complex disorder defined by a cluster of interconnected risk factors, including abdominal obesity, raised blood pressure, hypertriglyceridemia, hyperglycemia, and low highdensity lipoprotein (HDL) cholesterol level[8]. The combination of these factors is often attributed to Gerald Reaven, who popularized the term “Syndrome X” in 1988[9]. MetS is an important health problem worldwide which is associated with cardiovascular disease, type 2 diabetes mellitus, cancer, as well as ophthalmic disease[10-12]. Approximately 22.9% of the adults met the criteria for MetS from 2000 to 2010 in the United States[13]. Two of numerous and similar definitions for MetS are applied frequently, the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATPIII)[14] and the International Diabetes Federation (IDF)[15].
Several studies have suggested an epidemiological link between IOP and MetS[16-25], however, there is no synthesis of observational data that explores the relationship between them. Therefore, we conducted a Meta-analysis to certify the association between the MetS components and IOP.
MATERIALS AND METHODS
Search Strategy The present Meta-analysis was reported on the basis of the proposed MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines[26] and the Preferred Reporting Items for Systematic Reviews and Metaanalyses (PRISMA)[27]. Two independent observers (Wang YX and Tao JX) searched the following databases till November 30th, 2017: PubMed and Embase. The databases were searched by using the keywords “intraocular pressure OR IOP OR glaucoma,” AND “metabolic syndrome”. In order to search all potential studies, we didn’t restrict the starting year at initial retrieval. The reference lists of all retrieved articles were cross checked manually.
Inclusion and Exclusion Criteria The inclusion criteria in this Meta-analysis were as follows: 1) studies reporting the correlation between MetS and IOP; 2) studies describing the diagnostic criteria of MetS; 3) studies describing the range of the normal IOP; 4) studies where Pearson’s correlation coefficients or standardized betas or odds ratios (ORs) were reported; 5) studies depending on human-beings; 7) studies published as full-text articles in English. If the studies met the following selection criteria, they would be excluded:1) meeting abstracts, case reports, reviews, Meta-analyses,comments and animal studies; 2) studies lacking necessary data for calculation, such as the Pearson’s correlation coefficients or standardized betas or ORs of the IOP and MetS,and the number of the patients included in the studies; 3)studies undertaken on patients with other disorders; 4) studies published in the language different from English.
Study Selection and Data Extraction All publications were categorized using Endnote X8 for Mac. Two reviewers (Wang YX, and Tao JX) screened all pertinent titles and abstracts for studies and then basing on full-text review was second performed. Articles were selected from included studies depending on first author, study design, study date, country of origin, sample size, participant age, gender, outcome ascertainment and diagnostic criteria of MetS. If a study did not clearly mention any above key points, we considered that it had been not performed.
Measurement of Intraocular Pressure IOP was measured by non-contact tonometry in all the included studies except 3 studies, 2[19-20] of which used Goldmann applanation tonometer and 1[22] of which used Kowa KT-800 tonometer for measurement. Moreover, all the measurements were conducted in the morning to minimize the effect of diurnal variation except 1[22] study conducted in the afternoon. All the above ophthalmological measurements were performed by trained and experienced ophthalmologists or nurses.
Definition of Metabolic Syndrome Definition of MetS in this study was based on the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III)[14] or the International Diabetes Federation (IDF) criteria[15].
NCEP ATP III criteria (the presence of any three or more of the following five syndromes): 1) abdominal obesity: waist circumference >102 cm (men) and >88 cm (women); 2)hypertriglyceridemia: serum triglyceride (TG) level ≥150 mg/dL or drug treatment for elevated TG; 3) low HDL-cholesterol:<40 mg/dL in men and <50mg/dL in women or drug treatment for low HDL-cholesterol; 4) high blood pressure: systolic blood pressure (SBP) ≥130 mm Hg and/or diastolic blood pressure (DBP) ≥85 mm Hg or drug treatment for elevated blood pressure (high blood pressure); 5) high fasting glucose(FBS): serum glucose level ≥100 mg/dL or on treatment for diabetes.
IDF criteria (the presence of any two or more of the following five syndromes): 1) abdominal obesity: waist circumference>94 cm (men) and >80 cm (women); 2) hypertriglyceridemia:serum TG level ≥150 mg/dL or drug treatment for elevated TG; 3) low HDL-cholesterol: <40 mg/dL in men and <50 mg/dL in women or drug treatment for low HDL-cholesterol; 4)high blood pressure: SBP≥130 mm Hg and/or DBP≥85 mm Hg or drug treatment for elevated blood pressure (high blood pressure); 5) high FBS: serum glucose level ≥100 mg/dL or on treatment for diabetes.
Statistical Analysis Statistical data (ORs, standardized betas) on the relation of IOP and MetS were extracted from each article and converted to Pearson correlation coefficients (r)[28-29]. After unifying units into Pearson correlation coefficient, we transformed the Pearson correlation coefficient into the normal distribution variable Z by Fisher’s transformation, and calculated the 95% confidence interval(95%CI) for correlation coefficients Z, which assessed the relationship between IOP and MetS components.Heterogeneity was evaluated by Chi-squared test. If the I2>50%, the random-effect model was applied, which meant significant heterogeneity. Otherwise, the fixed-effect model was used. Publication bias was assessed by using Begg’s test and Egger’s test. All statistical tests were taken as significant with P-values<0.05. All analyses were conducted via Stata/SE 14.0 (Stata Corp, TX, USA).
RESULTS
Literature Search A total of 295 potential publications were obtained based on the initial search strategy (Figure 1). After removing duplicates, our primary search found 239 relevant articles. Twenty-four citations were fully reviewed because of 199 publications were excluded on the basis of the titles and abstracts by two investigators. Among the remaining articles,14 were excluded due to without accurate standard or effective data. Of the 14 studies, 6 studies described the relationship of glaucoma and MetS, 3 studies researched the correlation of IOP with other clinical conditions but not MetS, 2 studies were the review and comment respectively, 1 study didn’t describethe MetS and IOP, 1 study demonstrated the ORs but not relevant to IOP and MetS, and 1 study missed the definition of MetS. Finally, 10 (Japan, n=2; Korea, n=5; Taiwan, China,n=2; Turkey, n=1) articles[16-25] fulfilled our inclusion and exclusion criteria, consisting of 4 studies[17-19,24] about Pearson correlation coefficients, 5 studies[16,20-22,25] about standardized betas and 1 study[23] about OR.
Table 1 Basic information of the included studies in this Meta-analysis
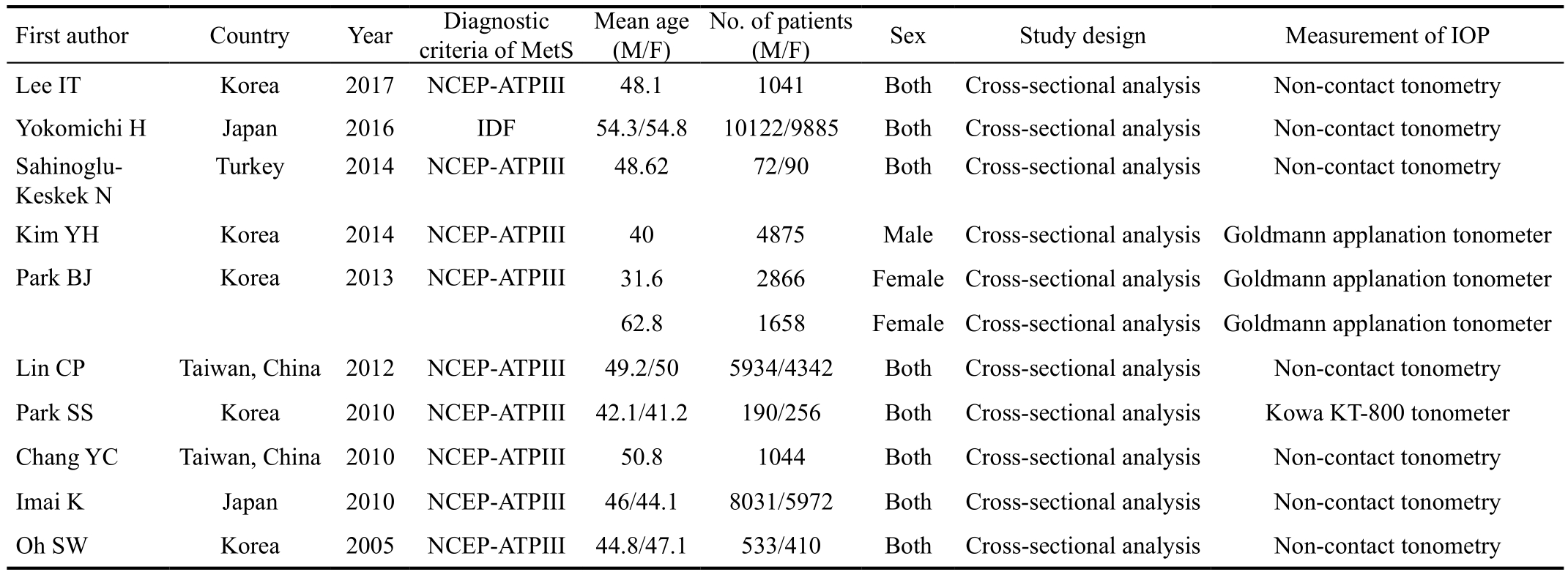
NCEP-ATP III: National Cholesterol Education Program Adult Treatment Panel III; IDF: International Diabetes Federation.
First author Country Year Diagnostic criteria of MetS Mean age(M/F)No. of patients(M/F) Sex Study design Measurement of IOP Lee IT Korea 2017 NCEP-ATPIII 48.1 1041 Both Cross-sectional analysis Non-contact tonometry Yokomichi H Japan 2016 IDF 54.3/54.8 10122/9885 Both Cross-sectional analysis Non-contact tonometry Sahinoglu-Keskek N Turkey 2014 NCEP-ATPIII 48.62 72/90 Both Cross-sectional analysis Non-contact tonometry Kim YH Korea 2014 NCEP-ATPIII 40 4875 Male Cross-sectional analysisGoldmann applanation tonometer Park BJ Korea 2013 NCEP-ATPIII 31.6 2866 FemaleCross-sectional analysis Goldmann applanation tonometer 62.8 1658 Female Cross-sectional analysis Goldmann applanation tonometer Lin CP Taiwan, China 2012 NCEP-ATPIII 49.2/50 5934/4342 Both Cross-sectional analysis Non-contact tonometry Park SS Korea 2010 NCEP-ATPIII 42.1/41.2 190/256 Both Cross-sectional analysis Kowa KT-800 tonometer Chang YC Taiwan, China 2010 NCEP-ATPIII 50.8 1044 Both Cross-sectional analysis Non-contact tonometry Imai K Japan 2010 NCEP-ATPIII 46/44.1 8031/5972 Both Cross-sectional analysis Non-contact tonometry Oh SW Korea 2005 NCEP-ATPIII 44.8/47.1 533/410 Both Cross-sectional analysis Non-contact tonometry
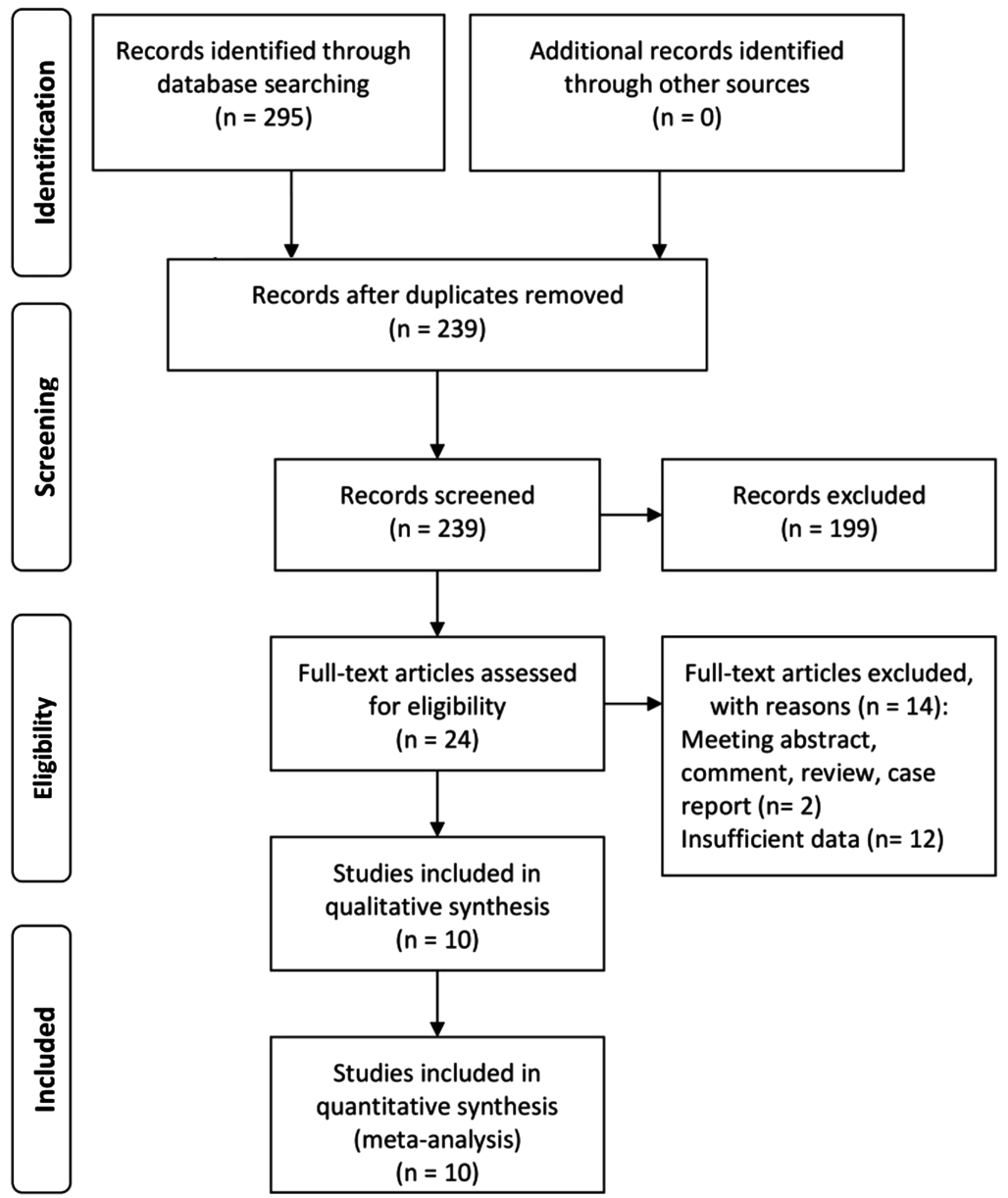
Figure 1 Flow diagram for articles included in this Meta-analysis.
All of the studies included in this Meta-analysis reported the relationship between MetS components and IOP (Table 1). The extracted data from these individual studies were summarized which included 57 321 participants.
Metabolic Syndrome and Intraocular Pressure The relationship between MetS and IOP were reported in 3 studies[16,23-24] which included 16 088 participants. The pooled Z between MetS and IOP for these studies with data was 0.47(95%CI: 0.15-0.79, P=0.005; Figure 2G) and exhibited notable heterogeneity (I2=99.7%, P<0.001).
Metabolic Syndrome Components and Intraocular Pressure The five components of MetS constituted the following analyses. Firstly, the Meta-analysis on the studies reporting on waist circumference and IOP showed pooled Z of 0.08 (95%CI: 0.04-0.12, P<0.001, I2=95.0%, P<0.001;Figure 2A) which indicated positive correlation. At the same time, IOP was positively correlated with hypertriglyceridemia and high FBS according to the pooled Z of 0.16 (95%CI:0.11-0.21, P<0.001, I2=97.1%, P<0.001; Figure 2C) and 0.12(95%CI: 0.08-0.16, P<0.001, I2=95.4%, P<0.001; Figure 2F),respectively. Furthermore, high blood pressure as a component of MetS was separated into two parts, SBP and DBP, in order to demonstrate the results more accurately. The pooled Z was 0.16 (95%CI: 0.10-0.22, P<0.001, I2=96.3%, P<0.001; Figure 2D) for SBP and 0.30 (95%CI: 0.20-0.40, P<0.001, I2=99.0%,P<0.001; Figure 2E) for DBP and which exhibited the positive correlations between IOP and hypertension. However, the analysis of low HDL-cholesterol showed a pooled Z of -0.03(95%CI: -0.06-0.01, P=0.145, I2=91.5%, P<0.001; Figure 2B)that meant no statistical significance.
Publication Bias Begg’s test[30] and Egger’s test[31] were used to evaluate the possible publication bias. For MetS and IOP, the P values for Begg’s and Egger’s test were 0.734 and 0.538 (Figure 3F). For MetS components and IOP, the P values of waist circumference, TG, SBP, DBP, and fasting glucose for Begg’s test were 0.945, 0.300, 0.721, 0.283 and 1.000, respectively. The P values for Egger’s test were 0.518,0.211, 0.972, 0.125 and 0.844 (Figure 3), respectively. All the data above showed no statistical significance, which meant no evidence of significant publication bias of this Metaanalysis.
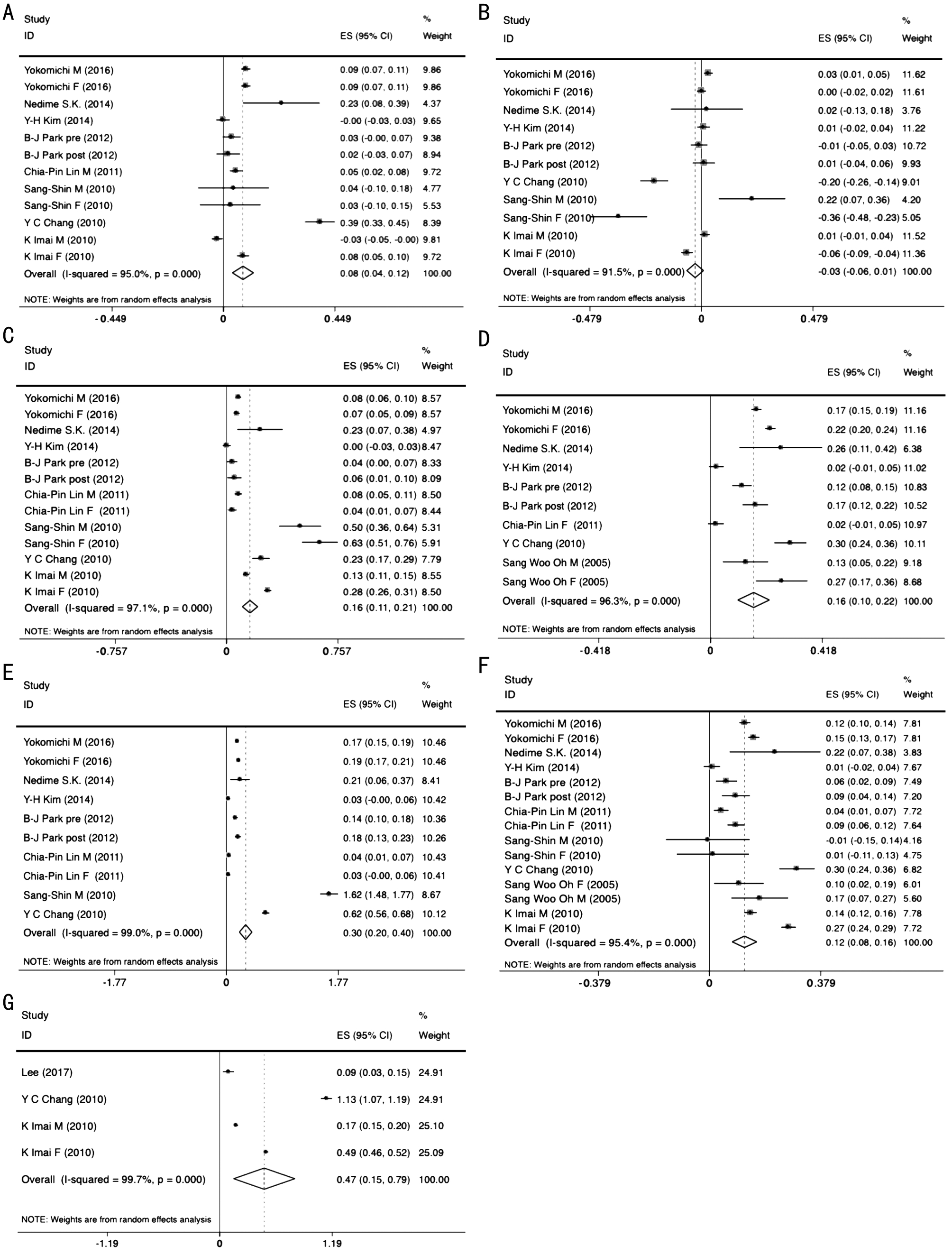
Figure 2 Forest plot of relationship between metabolic syndrome and intraocular pressure A: Waist circumference; B: HDL; C:Triglyceride; D: Systolic blood pressure; E: Diastolic blood pressure; F: Fasting glucose; G: Metabolic syndrome.
DISCUSSION
This is a Meta-analysis based on primary studies published between 2005 and 2017 that were identified through a comprehensive review. The relationship between IOP and MetS components were examined by this Meta-analysis roundly, in which 10 articles from 4 countries and regions were incorporated. For all we know, this is the most comprehensive Meta-analysis on IOP and MetS to data.
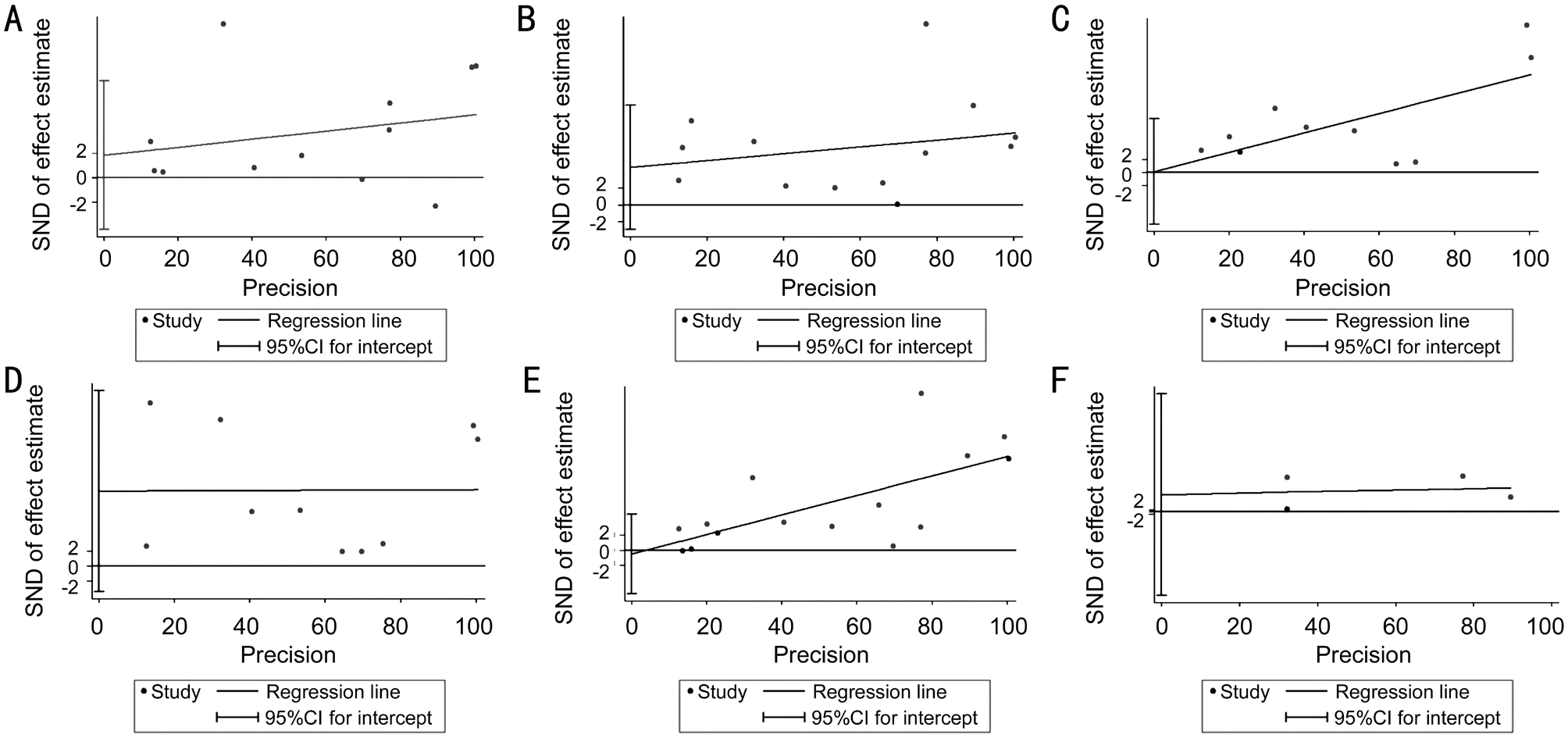
Figure 3 Egger’s test for metabolic syndrome and its components A: Waist circumference; B: Triglyceride; C: Systolic blood pressure; D:Diastolic blood pressure; E: Fasting glucose; F: Metabolic syndrome.
Our pooled Z of 0.47 indicated a higher prevalence of MetS in people with intraocular hypertension. In order to get to the bottom of the connection between them, we carried on the further study about MetS components and IOP. As it turned out, increased waist circumference, hypertriglyceridemia, high fasting glucose and high blood pressure were the parameters which drove the association between MetS and IOP.
Many reports have indicated that, IOP increases in people with obesity whose waist circumference over 102 cm (men)and 88 cm (women) because of a decrease in the squeous humor outflow due to an increase in orbital fat and episcleral pressure[32]. Blood viscosity may increase along with the raise of red cell count, hemoglobin and hematocrit, and consequently increased outflow resistance of episcleral veins in the obese population[33]. Moreover, hypertriglyceridemia also caused an increase in episcleral pressure and a decrease in aqueous humor outflow owing to accumulation of orbital adipose tissue[34-35].Although the influence of systemic hypertension on glaucoma is complex, several mechanisms are suggested. The majority of studies considered that high blood pressure increased the pressure of ciliary artery in ocular, which increased the IOP because it induced more aqueous humor to be produced[36].While, some thought chronically elevated blood pressure may result in arteriosclerosis, changes in the size of the precapillary arterioles, and capillary dropout leading to increased resistance to blood flow and, thus, reduced perfusion[37-38].
It has been reported that hyperglycemia may also be closely associated with IOP. However, the etiologic links between them remain unclear, several hypotheses have been advanced.One possible explanation was that elevated blood glucose resulted in the fluid shifts into the intraocular space from the osmotic gradient[39]. Hyperglycemia caused microvascular damage and may affect vascular autoregulation of the retain and optic nerve, which could reduce blood flow and impair oxygen diffusion[40]. On the other hand, neuronal and glial functions as well as metabolism in the retina may also be impaired which caused progressive retinal ganglion cell death and optic disk excavation followed by the IOP elevation[41].
Glaucoma is a multifactorial disease that the etiology is still not completely understood, but multiple previous studies had noted a strong relationship between IOP and glaucoma. IOP was the only known modifiable factor among age, genetic,family history or other ones and induce lesion by causing structural and functional damage[42]. The median follow-up study of six years confirmed the findings: the post-baseline progression factors included IOP, with every 1 mm Hg increase in IOP leading to a 12%-13% higher risk of progression[43].In a Meta-analysis of randomized clinical trials, treating high IOP was associated with a significant reduction in glaucoma development (0.56 hazards ratio) for patients at high-risk of converting to primary open-angle glaucoma. Patients in the treatment arm of these trials did not progress to glaucoma diagnoses in 63%-91% of the cases[44]. At the same time, in the wake of changing lifestyles among more and more people,the prevalence of the MetS increased dramatically in the few years. According to our Meta-analysis the epidemic of the MetS or even its components have a huge impact on the IOP,that is to say, there may be a relationship between MetS and glaucoma. Lifestyle intervention may play an important role in the treatment of glaucoma by regulating the lower IOP.
Several limitations needed to be considered in our study.First, the majority of studies were from Asia, most of them were yellow race, selection bias was inevitable. Second,our findings were primarily based on results derived from cross-sectional analyses or observational studies, which may subject to unmeasured confounding and other potential biases.Third, significant heterogeneity was observed in the overall analysis. The heterogeneity could be explained by differences in populations, regions, gender, age, and confounding factors such as smoking of exposure ascertainment. Fourth, the absence of uniform diagnostic criteria of MetS may increase the incomparability among studies.
In conclusions, our analysis indicated that MetS and its components besides HDL were associated with IOP. Moreover,the treatment of the MetS may have a potential role in preventing elevated IOP, which may have benefit in several eye diseases.
ACKNOWLEDGEMENTS
Conflicts of Interest: Wang YX, None; Tao JX, None; Yao Y, None.
1 Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res 2016;55:108-148.
2 Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010.Br J Ophthalmol 2012;96(5):614-618.
3 Cohen LP, Pasquale LR. Clinical characteristics and current treatment of glaucoma. Cold Spring Harb Perspect Med 2014;4(6):a017236.
4 Ho H, Shi Y, Chua J, Tham YC, Lim SH, Aung T, Wong TY, Cheng CY. Association of systemic medication use with intraocular pressure in a multiethnic Asian population: the singapore epidemiology of eye diseases study. JAMA Ophthalmol 2017;135(3):196-202.
5 Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol 2007;52(2):180-195.
6 Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol 1997;115(12):1572-1576.
7 Lee YW, Min WK, Chun S, Lee W, Kim Y, Chun SH, Park H, Shin HB,Lee YK. The association between intraocular pressure and predictors of coronary heart disease risk in Koreans. J Korean Med Sci 2008;23(1):31-34.
8 Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab 2007;3(10):696-704.
9 Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37(12):1595-1607.
10 O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 2015;16(1):1-12.
11 Poh S, Mohamed Abdul RB, Lamoureux EL, Wong TY, Sabanayagam C. Metabolic syndrome and eye diseases. Diabetes Res Clin Pract 2016;113:86-100.
12 Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis.Diabetes Care 2012;35(11):2402-2411.
13 Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S.Prevalence and trends of metabolic syndrome in the adult US population,1999-2010. J Am Coll Cardiol 2013;62(8):697-703.
14 Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486-2497.
15 Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23(5):469-480.
16 Lee IT, Wang JS, Fu CP, Chang CJ, Lee WJ, Lin SY, Sheu WH.The synergistic effect of inflammation and metabolic syndrome on intraocular pressure: A cross-sectional study. Medicine (Baltimore)2017;96(36):e7851.
17 Yokomichi H, Kashiwagi K, Kitamura K, Yoda Y, Tsuji M, Mochizuki M, Sato M, Shinohara R, Mizorogi S, Suzuki K, Yamagata Z. Evaluation of the associations between changes in intraocular pressure and metabolic syndrome parameters: a retrospective cohort study in Japan. BMJ Open 2016;6(3):e010360.
18 Sahinoglu-Keskek N, Keskek SO, Cevher S, Kirim S, Kayiklik A,Ortoglu G, Saler T. Metabolic syndrome as a risk factor for elevated intraocular pressure. Pak J Med Sci 2014;30(3):477-482.
19 Kim YH, Jung SW, Nam GE, Do Han K, Bok AR, Baek SJ, Cho KH, Choi YS, Kim SM, Ju SY, Kim DH. High intraocular pressure is associated with cardiometabolic risk factors in South Korean men: Korean National Health and Nutrition Examination Survey, 2008-2010. Eye (Lond)2014;28(6):672-679.
20 Park BJ, Park JO, Kang HT, Lee YJ. Elevated intraocular pressure is associated with metabolic syndrome in postmenopausal women: the Korean National Health and Nutrition Examination Survey. Menopause 2013;20(7):742-746.
21 Lin CP, Lin YS, Wu SC, Ko YS. Age- and gender-specific association between intraocular pressure and metabolic variables in a Taiwanese population. Eur J Intern Med 2012;23(1):76-82.
22 Park SS, Lee EH, Jargal G, Paek D, Cho SI. The distribution of intraocular pressure and its association with metabolic syndrome in a community. J Prev Med Public Health 2010;43(2):125-130.
23 Imai K, Hamaguchi M, Mori K, Takeda N, Fukui M, Kato T, Kawahito Y, Kinoshita S, Kojima T. Metabolic syndrome as a risk factor for highocular tension. Int J Obes (Lond) 2010;34(7):1209-1217.
24 Chang YC, Lin JW, Wang LC, Chen HM, Hwang JJ, Chuang LM.Association of intraocular pressure with the metabolic syndrome and novel cardiometabolic risk factors. Eye (Lond) 2010;24(6):1037-1043.
25 Oh SW, Lee S, Park C, Kim DJ. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab Res Rev 2005;21(5):434-440.
26 Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Metaanalysis Of Observational Studies in Epidemiology (MOOSE) group.JAMA 2000;283(15):2008-2012.
27 Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G,Tierney JF; PRISMA-IPD Development Group. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 2015;313(16):1657-1665.
28 Peterson RA, Brown SP. On the use of beta coefficients in metaanalysis. J Appl Psychol 2005;90(1):175-181.
29 Bonett DG. Transforming odds ratios into correlations for metaanalytic research. Am Psychol 2007;62(3):254-255.
30 Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-1101.
31 Egger M, Davey Smith G, Schneider M, Minder C. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997;315(7109):629-634.
32 Shiose Y, Kawase Y. A new approach to stratified normal intraocular pressure in a general population. Am J Ophthalmol 1986;101(6):714-721.33 Mori K, Ando F, Nomura H, Sato Y, Shimokata H. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol 2000;29(4):661-666.
34 Klein BE, Klein R, Linton KL. Intraocular pressure in an american community. the beaver dam eye study. Invest Ophthalmol Vis Sci 1992;33(7):2224-2228.
35 Pertl L, Mossböck G, Wedrich A, Weger M, Königsbrügge O,Silbernagel G, Posch F. Triglycerides and open angle glaucoma-a metaanalysis with meta-regression. Sci Rep 2017;7(1):7829.
36 Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol 1975;59(12):717-720.
37 Shiose Y. The aging effect on intraocular pressure in an apparently normal population. Arch Ophthalmol 1984;102(6):883-887.
38 Chung HJ, Hwang HB, Lee NY. The association between primary open-angle glaucoma and blood pressure: two aspects of hypertension and hypotension. Biomed Res Int 2015;2015:827516.
39 Hennis A, Wu SY, Nemesure B, Leske MC; Barbados Eye Studies Group. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology 2003;110(5):908-914.
40 Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology 2015;122(1):72-78.
41 Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica 2005;219(1):1-10.42 Miglior S, Bertuzzi F. Relationship between intraocular pressure and glaucoma onset and progression. Curr Opin Pharmacol 2013;13(1):32-35.
43 Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M;Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120(10):1268-1279.
44 Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ 2005;331(7509):134.