INTRODUCTION
F ungal keratitis (FK) is an infectious keratopathy with resulting in a high rate of blindness. This disease is extremely harmful and serious, even in many cases requiring eye removal. The cause of FK is clear and obvious. At present,the main treatment method is to tuse antibiotics to kill fungi,but the treatment effect is not ideal[1]. The development of new drugs for the treatment of FK should not only consider effective anti-fungal and pathogen removal, but also taking into account the regulation of the immune process of fungi and the body[2].
Among current known antifungal drugs, amphotericin B has an immunomodulatory effect, but at present it mainly targets clinical usage to achieve its antifungal effects. How to better exert its immunomodulatory effect requires further related immunological research. In recent years, more and more studies have confirmed that cyclosporine A and FK506 and other immunosuppressants have a therapeutic effect on FK[3-4]. Our experimental drug rapamycin has both anti-fungal and immunosuppressive effects and is a theoretical and ideal anti-fungal drug.
However, at present, rapamycin has only systemic preparations and there is not ophthalmic preparations. At the same time,the systemic administration of rapamycin has low intraocular drug concentrations and poor medication efficacy, which limits the research and application of rapamycin in ophthalmology.In this experiment, a good drug carrier-liposomes was used. Its constituent material was a phospholipid bilayer membrane. Similar to a biofilm, a lipid-soluble drug-rapamycin was encapsulated in its lipophilic portion or lipid bilayer. The obtained liposome eye drops of rapamycin is an ideal ophthalmic formulation.
Then, an animal model of rat FK was made and the therapeutic effect of rapamycin liposome eyedrops on FK was observed.Slit lamp microscope, immunohistochemistry and semiquantitative reverse transcription polymerase were performed.The purpose was to provide experimental basis and possible theoretical support for the clinical treatment of FK.
MATERIALS AND METHODS
Ethical Approval All Wistar rats were approved by Qingdao Medicine Inspecting Institute, Shandong province. They were healthy, ophthalmic diseases free, and treated in accordancewith the guidelines provided in Scientific and Technological Commission of China for the Use of Animals for Lab Research.
Preparation of Eye Drops A film dispersion method was taken to prepare rapamycin liposomes. The results of the entrapment efficiency were optimized by using orthogonal methods. The mass ratio of phospholipids to cholesterol and the mass ratio of drug to phospholipid the hydration time and drug concentration were used as the investigation factors.Four factors and three levels of orthogonal experiments were designed to optimize the liposome preparation process. The resulting liposomes were sealed in ampoules, placed in a refrigerator at 4℃, used for a period of one month, expired,and reconstituted. The prepared liposomes were subjected to encapsulation efficiency measurement and quality evaluation: transmission electron microscopy was performed for morphological observation, and the Zetasizer 3000 laser scattering force measuring instrument was used to measure the average particle diameter and particle size distribution. The prescriptions and processes optimized by orthogonal design were: the mass ratio of phospholipid to cholesterol was 1:0,the mass ratio of phospholipid to drug was 15:1, the hydration time was 1.5h, and the drug concentration was 2 mg/mL. The preparation recipes were as follows: rapamycin 20 mg, lecithin 300 mg, absolute ethanol, phosphate buffered saline (PBS; pH 7.0) 10 mL, the amount of rapamycin, lecithin, and dissolved in water-ethanol, at 40℃, a layer of film was vacuum-rotated and rotary evaporated to ethanol-free taste. The 10 mL of PBS(pH 7.0) was added and the rapamycin liposome suspension was hydrated.
Animal Model Production Totally 96 healthy Wistar rats were randomly divided into four groups: normal control group (A), FK blank control group (B), FK blank liposomes control group (C), and 30 FK rapamycin liposome treatment group (D). Groups B, C, and D were first prepared as FK animal models. Three days before the experiment, the eyes were given levofloxacin eye drops 4 times a day, and levofloxacin ointment was applied at night. General anaesthesia was performed by peritoneal injection of chloral hydrate 3 mL/kg,and local anesthesia was performed with 0.4% oxybuconine hydrochloride. Preoperative skin preparation, mucous membranes were washed with anerithine conjunctival sac,routinely disinfected, sterile hole towel was placed, and the ring was approximately 2 mm in diameter and positioned in the central region of the cornea under an eye surgery microscope.The cornea was completely scraped with a sterile surgical blade. The epithelial layer within the central clasp reaches the shallow stromal layer. The prepared Aspergillus fumigatus mycelium was applied to the surface of the cornea, and the contact lens overlaid with the parafilm sealing membrane was overlaid. The sub-conjunctival injection of 0.5 million units of gentamycin injection and the conjunctival sac were performed.Levofloxacin ointment were used, eyelids were sutured with 5-0 thread. Twenty-four hours after surgery, the eyelid suture was removed and the eyelid was opened.
Slit Lamp Observation After removing the eyelid suture and the contact lens, fully wash the corneal lesion with sterile saline, wipe the surface of necrotic attachments, scrape the lesion and normal tissue with a sterile disposable microsurgical blade under sterile conditions. The corneal tissue of the communicator was partially used for observation of 10%potassium hydroxide wet tablets, and some of them were inoculated into fungal culture medium for fungal culture.Animal models establish successful criteria: 1) Form a typical corneal ulcer; 2) At least one of the potassium hydroxide wettablets or fungal cultures is a positive result. After successful modeling, the corneal lesions were observed under the slit lamp, scored and photographed. Then according to the grouping situation, the corresponding processing was given,and the slit lamp was observed, scored and photographed at 1,3, 5, 7 and 14d after successful modeling.
At the 24th hour after modeling, 6 rats in each group were sacrificed with chloral hydrate excess; afterwards, 6 rats in three groups B, C, and D were sacrificed in the same manner at 3, 5, 7 and 14d after modeling. The killed rats were aseptically removed the eyeball, and the cornea was divided into two parts and one half was fixed with 4% formaldehyde solution for immunohistochemical observations; the other half was placed in an EP tube containing 1 mL of cell lysate and treated with autoclaved DEPC-water inactivation enzyme. Stored at 80℃for RT-PCR.
Immunohistochemistry and PCR Experiments The cornea of experimental Wistar rat was placed in a 40 g/L formalin solution, fixed, routinely dehydrated, xylene was transparent,embedded after dipping, 5 μm continuous sections, and applied to 0.1% poly-L-lysine on slides, fish tablets, 60℃ baked overnight.Hematoxylin and eosin (HE) staining was then performed.
The rat monocyte chemotactic protein-1 (MCP-1) DNA sequence was searched from the GeneBank and Primer Premier 5.0 software was used to design the primers. The primers were synthesized by Shanghai Shenggong Bioengineering Service Co., Ltd. according to the sequence. The sequences are MCP-1:upstream primer, 5'- CAGGTCTCTGTCACGCTTCT -3',downstream primer: 5'- CTAGTATTCATGGAAGGGAATAG -3',amplified fragment size: 527bp, first strand cDNA synthesis,PCR amplification, DNA electrophoresis and analysis were performed.
Statistical Analysis The experimental data was indicated as mean and standard deviation (SD) values. G* power software was used to calculate the required sample size. SPSS 17.0 statistical software was used to statistically process the clinical scores and the protein and relative mRNA expression of MCP-1 in each group of FK. Two-factor mixed-design ANOVA was utilized to test statistically significant differences. The ANOVA was conducted to compare the group (groups A to D) and time (0, 1, 3, 5, 7 and 14d). The pairwise comparison between each group using LSD test. P<0.05 is set to get statistical significance.
RESULTS
To calculate the required sample size, G* power software was used with the following inputs; a power study of 85%; number of groups of 4; a significance level of 5%; an effect size of 0.25; and with a statistical test of one-way analysis of variance(ANOVA). The required sample size was 264 subjects (30 cases in each group).
Slit Lamp Observation On the 1st day after inoculation of the fungus, the conjunctiva of the rat showed obvious mixed hyperemia, marked edema on the cornea, white infiltration and turbidity at the inoculation site, and the boundary of the invaded part was clear. On the third day, ulcers began to appear on the surface of the cornea. Thin areas of the infiltrated areas were covered with dry moss. The surface was dry and rough.The cornea of a few infiltrated areas began to thin, and there was no evidence of posterior elastic layer bulging and corneal perforation. On the 5th day of infection, there were obvious corneal ulcer lesions, corneal ulcer lesions started to contract,the borders became clear, and the moss and necrotic tissue fell off. Part of the posterior corneal stretch membrane or perforation of the cornea occurred. On the 7th day, new blood vessels began to appear in the center of the cornea at the corneal limbus, and the infiltrative lesions of the cornea were further reduced. The corneal ulcer was gradually improved,and the range began to be limited, gradually being replaced by vascular scar tissue. On the 14th day, the lesions was further shrank and gradually healed to scars. Compared with groups B and C, the degree of lesions at each time point was reduced in group D, and there was no significant difference between groups B and C. The slit lamp observation of the groups B and D on the third day are shown in Figure 1.
Results of Immunohistochemical Staining
Monocyte chemotactic protein-1 protein Normal control group: the expression of brown-yellow granules of MCP-1 was very weak in the normal corneal epithelium. In each FK group: At 1d after FK, inflammatory cell infiltration began to be observed in the stromal layer of the cornea. The expression of MCP-1 was enhanced in the corneal epithelium and stromal layer. After 3d of FK, a large number of inflammatory cells infiltrated in the stromal layer of the cornea, visible in the corneal epithelium and stromal layers. MCP-1 expression was significantly enhanced. Five days after FK, the infiltration of inflammatory cells in the corneal stromal layer was less than that on the 3rd day, and the expression of MCP-1 was weakened on the 3rd day. On the 7th day after FK, the number of fibroblasts in the lesions was increased. There was still a small amount of inflammatory cells infiltrating. The expression of MCP-1 was weaker than that on the 5th day. On the 14th day,the lesions were basically healed, and part of the cornea was replaced by fiber scar tissue. Basically, no inflammatory cell infiltration, only a very small amount of MCP-1 expression.The expression of MCP-1 in group D was lower than that in group B and C at each time point, and there was no difference between groups B and C. The expressions of the groups B and D at various time points are shown in Figure 2.
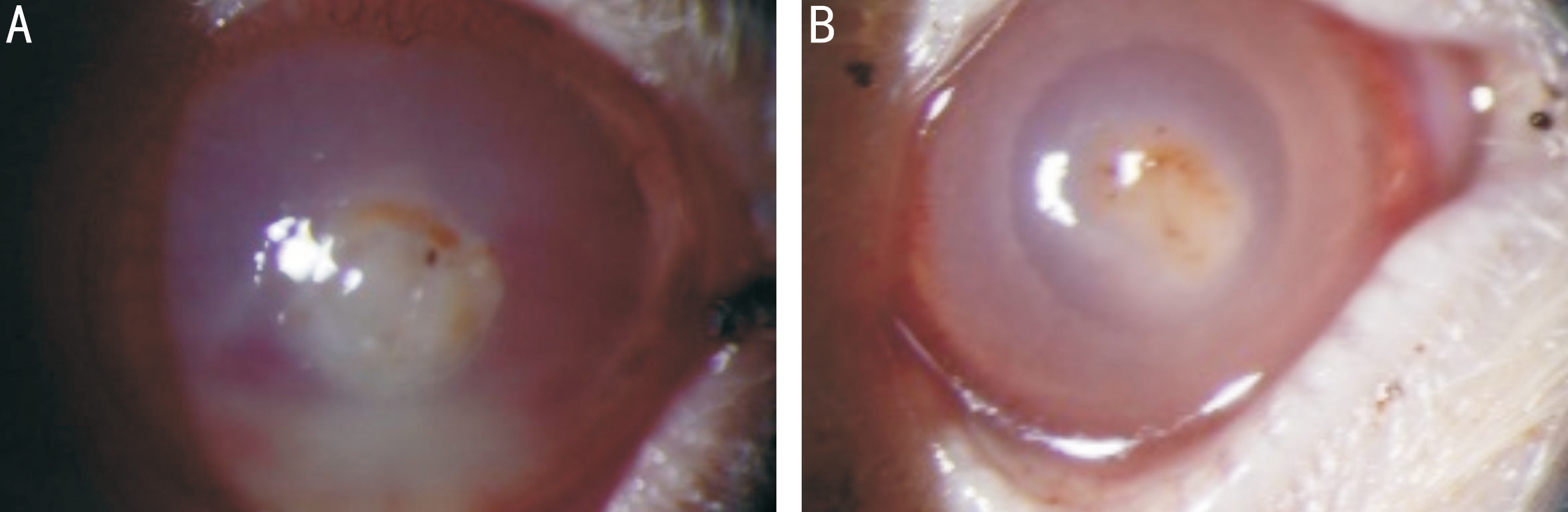
Figure 1 Fissured lamp observation of FK blank control group (A) and rapamycin treatment group (B) 3d after FK infection.
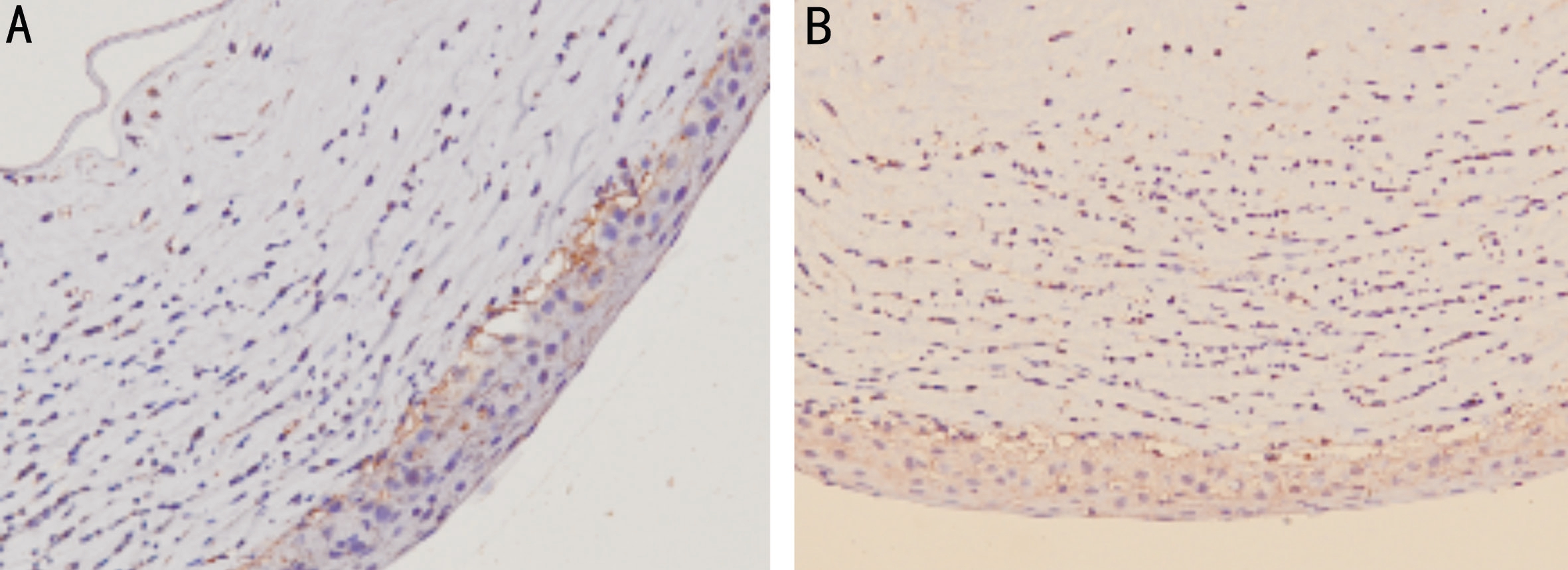
Figure 2 Expression of MCP-1 protein in the blank control group (A) and rapamycin treatment group (B) 3d after FK infection.
Table 1 Comparison of optical density values of MCP-1 positive cells in each group mean±SD
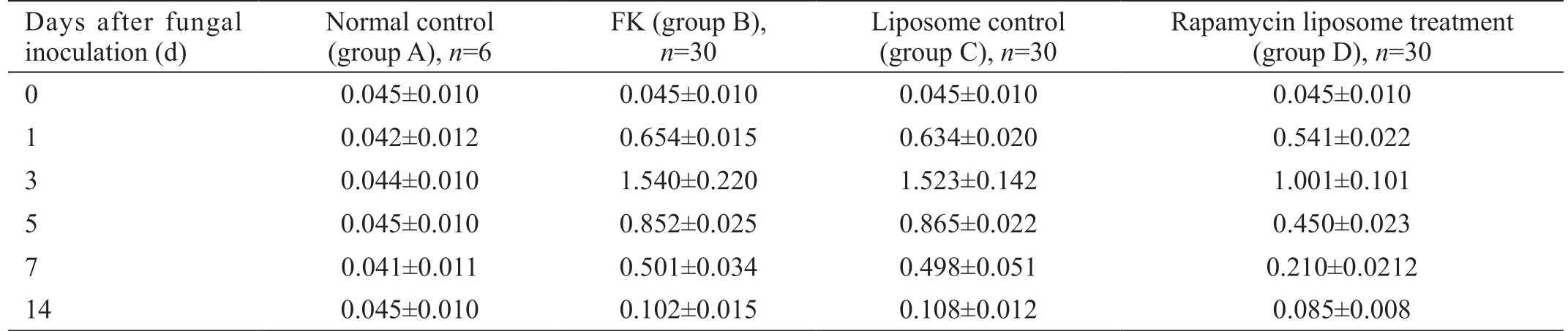
Days after fungal inoculation (d)Normal control(group A), n=6 FK (group B),n=30 Liposome control(group C), n=30 Rapamycin liposome treatment(group D), n=30 0 0.045±0.010 0.045±0.010 0.045±0.010 0.045±0.010 1 0.042±0.012 0.654±0.015 0.634±0.020 0.541±0.022 3 0.044±0.010 1.540±0.220 1.523±0.142 1.001±0.101 5 0.045±0.010 0.852±0.025 0.865±0.022 0.450±0.023 7 0.041±0.011 0.501±0.034 0.498±0.051 0.210±0.0212 14 0.045±0.010 0.102±0.015 0.108±0.012 0.085±0.008
Table 2 Comparison of MCP-1 mRNA relative expression levels in rat corneas of different groups mean±SD

Days after fungal inoculation (d)Normal control(group A), n=6 FK (group B),n=30 Liposome control(group C), n=30 Rapamycin liposome treatment(Group D), n=30 0 0.083±0.010 0.083±0.010 0.083±0.010 0.083±0.010 1 0.083±0.012 25.620±0.011 24.220±0.013 18.320±0.021 3 0.083±0.010 43.440±0.200 42.360±0.012 30.210±0.120 5 0.083±0.011 31.100±0.030 30.200±0.010 20.101±0.021 7 0.083±0.010 20.20±0.004 20.98±0.011 11.302±0.010 14 0.083±0.010 5.020±0.025 5.260±0.012 3.021±0.010
The optical density of MCP-1 positive cells in each group is shown in Table 1.
After rat corneal fungal infection, the overall F value was 213.07 at each time point in group B, P<0.01 and P<0.01 for the pairwise comparison. The change trend was: the expression of MCP-1 protein began to increase on the 1st day after corneal fungal infection, peaked on 3rd day, decreased on 5th day, and fell to the lowest on 14th day, but still higher than normal corneas. At each time point, the expression of MCP-1 protein in group D was lower than that in the first two groups,and there was no change in the expression of MCP-1 protein in group C comparing with group B. In other words, the expression of MCP-1 protein in the rapamycin-treated group was significantly inhibited, and the interference of the blank liposomes was ruled out, that is, the effect was rapamycin's own drugs.
Reverse Transcription Polymerase Chain Reaction Results
The extracted RNA was detected by UV spectrophotometer and its OD260/OD280 value was between 1.8 and 2.0, which proved that the extracted RNA had higher purity and less degradation.
Expression of Monocyte Chemotactic Protein-1 mRNA
The length of MCP-1 PCR product was 527 bp in normal corneas and in post-FK corneal tissues, and the length of PCR products in GAPDH was 173 bp. The expression of MCP-1 mRNA in corneal tissue of normal rats was increased, and the expression of MCP-1 mRNA began to increase on 1d after FK,peaked on 3d, decreased on 5d, then decreased gradually, and slightly higher than normal on 14d. Electrophoresis diagrams at various times are shown in Figure 3. The MCP-1 mRNA relative expression level values for each group are shown in Table 2.
MCP-1 mRNA was expressed on 1d after corneal fungal infection. It began to rise and peaked on 3d. It began to decline on 5d and fell to the lowest level on 14d, but it was still higher than the normal cornea. At each time point, the expression of MCP-1 mRNA in group D was lower than that in group C and D. The expression of MCP-1 mRNA was not changed in groups C compared with groups B. It can be seen that the expression of MCP-1 mRNA in the rapamycin-treated group was significantly inhibited, and the factors that interfered with blank liposomes were excluded, which was caused by the action of rapamycin's own drugs.
DISCUSSION
FK was first reported by Leber in 1879[5]. In the past, the incidence rate was not high. It was only gradually recognized and developed since the 1950s as a more harmful corneal infectious disease. If the diagnosis or treatment is not timely, it will cause severe corneal infection, leading to perforation of the cornea, blindness, and more serious need for enucleation[5]. China's current huge agricultural population creates high population base of peasants, which leads tomorecorneal vegetal trauma. In addition, the clinical abuse of glucocorticoids, the application of broad-spectrum antibiotics and the non-standard wearing of contact lenses cause FK.The incidence of FK is rising. More and more clinicians are paying attention to this disease. Due to the large variety of pathogenic fungi and complex etiology, the basic research on the pathogenesis of the disease is lagging behind. Clinically,there is a lack of safe and effective therapeutic drug. Therefore,related basic and clinical studies are imminent and necessary.
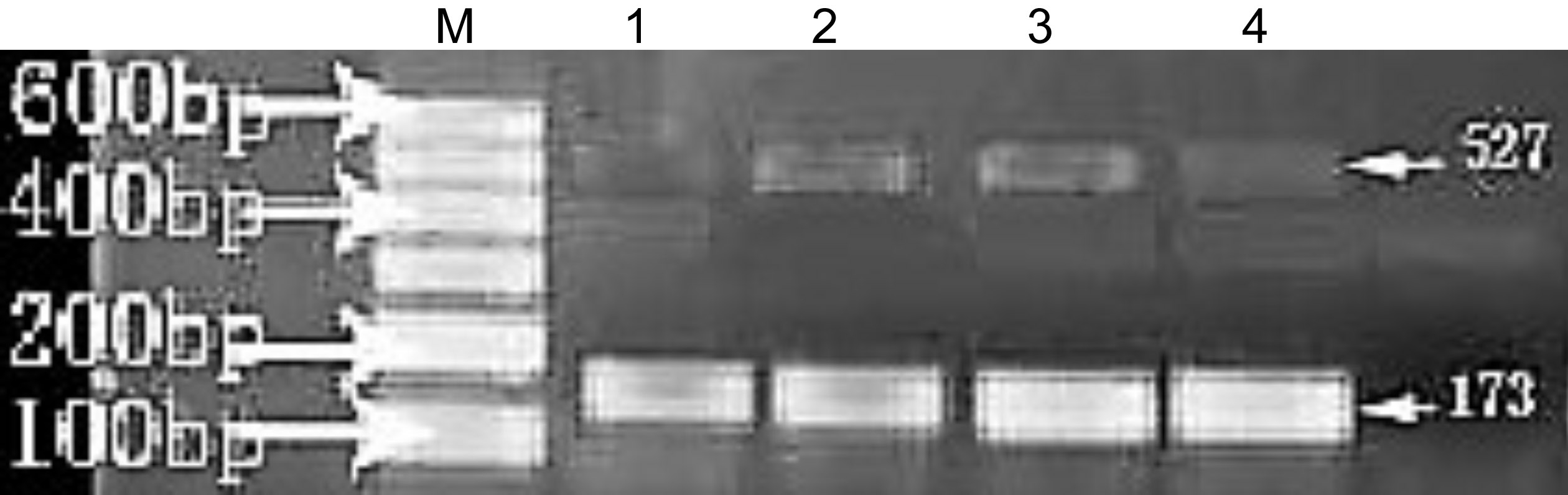
Figure 3 Rat corneal MCP-1 mRNA RT-PCR product agarose gel electrophoresis: lane M is marker, 173 bp of lanes 1, 2, 3, and 4 were GAPDH mRNA products, 527 bp of lanes 1, 2, 3, and 4 were MCP-1 mRNA product for blank control, blank control group,liposome control group and rapamycin treatment group on 3rd day after FK.
Pathogenic Mechanism of Fungi The pathogenesis of FK can be summarized as the following three points[6-10]: 1) Direct damage to the host cornea; degradation of the corneal tissue through the production of various enzymes and promotion of mycelial proliferation in the cornea; 2) Inflammation reaction was caused by the destruction of neutrophil enzyme;3) Immune factors: in the course of FK, various toxins and denatured corneal tissues secreted by fungi, fungi stimulate the body to produce an immune response, causing antigens. The deposition of antibody complexes, the accumulation of various immune cells, aggravate local tissue damage, and together with pathogenic fungi lead to fungal corneal pathological lesions.
It is learnt from the gross morphology observation of the diseased cornea and the results of histological examination thatdifferent fungi causes different methods and extent of damage, but the common features are listed as follows[11-13]:the fungus firstly make direct damages to the corneal tissue.After invading the cornea by the fungal spores, the mycelium germinates in the cornea, which makes mechanical damage to the corneal tissue; at the same time, some secretory,metabolites of the fungi and various enzymes were released,such as alkaline serine protease, secreting asparagus. The enzyme is an important chemokine, which can chemotaxis neutrophil accumulation, destroy the host cell membrane and collagen fibers, which cause inflammation and coagulative necrosis in the corneal tissue. As we observed from the first day after infection, fungal hyphae infiltrated, a large number of inflammatory cells began to infiltrate from the limbus to the lesion, and the number of fungal hyphae was largely increased on the third day. A large number of inflammatory cells accumulated in the lesion and anterior chamber.Secondly, as a foreign substance, the fungus itself can be used as an antigen to induce the body's specific immune response.Both cellular and humoral immunity can play a role at this time. From the molecular level, various inflammatory factors began to be rapidly increased on the first day of infection,and inflammatory cells, mainly neutrophils and mononuclear macrophages, were infiltrated, leading to an inflammatory response of the cornea. In our experiment, we observed that inflammatory cytokines MCP-1 began to increase on 1d after infection, peaked at 3d, and then began to decline.
From our experiments, it can be seen that in the early stages of FK, the fungus proliferated in a large amount, the fungus load was the heaviest on the third day, and a large number of inflammatory cells began to infiltrate. Various inflammatory factors accumulated in large numbers and peaked on the third day. The lesions were the heaviest on the 5th day observed in the general observation. The direct destruction of the visible fungal was evident in the early stage, following secondary inflammation reaction of the cornea caused by inflammation of the body itself.
Role of Monocyte Chemotactic Protein-1 in Fungal Keratitis In the course of the development of inflammatory diseases, various chemokines play an important and even crucial role. Overactivated chemokines can raise excess leukocytes to the site of inflammation in the body. They can not only damage the body, but can also cause the development of chronic inflammatory diseases such as granulomas.Abnormal secretion of chemokines such as MCP-1 and abnormal expression of chemokine receptors can cause abnormal activation and proliferation of immune effector cells T and B lymphocytes, leading to autoimmune damage[14-18].
In our experiments, we found that after FK, the expression of the disease changed regularly with the expression of the disease. On the 1st day, the expression of MCP-1 began to increase, peaked at 3rd day, then began to decline, and the degree of corneal lesion peaked on 5th day. Whereas in the rapamycin-treated group, the expression of MCP-1 was suppressed, and the degree of corneal lesions was also relatively light. It can be seen that MCP-1 is positively correlated with the degree of corneal damage in FK, and proper inhibition of them can reduce the degree of corneal disease.
Therapeutic Effect of Rapamycin on Fungal Keratitis
Rapamycin was originally a low-toxicity macrolide antibiotic extracted from the water-absorbing Streptomyces fermentation broth. It is a safe, effective and low-toxic novel immunosuppressive agent[19-21].
Our experiments found that the extent of corneal lesions in the rapamycin treatment group was reduced, clinical scores were lower than those in the blank control group and the blank liposome control group, and the number of fungi was reduced.The expression of MCP-1 in rapamycin treatment group was significantly inhibited, and there was significant difference compared with FK control group and FK blank liposome control group (P<0.01). This is consistent with the results of Lin et al[22]. They found that rapamycin, an inhibitor of mammalian rapamycin target protein (mTOR), reduced the expression of several cytokines, including MCP-1. Since MCP-1 is an important inflammatory and immune factor in the FK process,it is believed that the therapeutic effect of rapamycin on FK, in addition to inhibiting its growth and reproduction incompetition with its own symbiotic fungal, also by inhibiting excessive inflammation and immunity reaction reaches the full course treatment of FK[22-25].
In summary, the keratopathy of FK is caused by the fungus itself reproducing and inflicting a certain level of damage.Afterwards, as the disease progresses, most of the fungus is eliminated, and the body itself becomes inflamed, resulting in further damage caused by excessive immunity. Rapamycin can inhibit the reproduction and infiltration of fungi at an early stage and inhibit the production and secretion of cytokines at the later stage, thereby reducing the body's excessive inflammation and immune response, restoring homeostasis as quickly as possible, promoting tissue repair and reconstruction,and thereby exerting a role in the treatment of FK effect.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zhang ZH, None; Teng F, None; Sun QX, None; Wang SZ, None; Liu C, None; Zhao GQ, None.
1 Mittal V, Jain R, Mittal R, Sangwan VS. Post-laser in situ keratomileusis interface fungal keratitis. Cornea 2014;33(10):1022-1030.
2 FlorCruz NV, Peczon IV, Evans JR. Medical interventions for fungal keratitis. Cochrane Database Syst Rev 2012(2):CD004241.
3 Yilmaz S, Maden A. Severe fungal keratitis treated with subconjunctival fluconazole. Am J Ophthalmol 2005;140(3):454-458.
4 Jurkunas UV, Langston DP, Colby K. Use of voriconazole in the treatment of fungal keratitis. Int Ophthalmol Clin 2007;47(2):47-59.
5 Sivashanmugam M, Nagarajan H, Vetrivel U, Ramasubban G, Therese KL, Narahari MH4. In silico analysis and prioritization of drug targets in Fusarium solani. Med Hypotheses 2015;84(2):81-84.
6 Das M, Murthy SI, Dikshit S. Natamycin and voriconazole in fungal keratitis. Arch Ophthalmol 2011;129(6):814.
7 Sánchez Romano J, Mørk T, Laaksonen S, Ågren E, Nymo IH, Sunde M, Tryland M. Infectious keratoconjunctivitis in semi-domesticated Eurasian tundra reindeer (Rangifer tarandus tarandus): microbiological study of clinically affected and unaffected animals with special reference to cervid herpesvirus 2. BMC Vet Res 2018;14:15.
8 O'Callaghan RJ. The pathogenesis of staphylococcus aureus eye infections. Pathogens 2018;7(1):E9.
9 Kasparova EA, Sobkova OI, Yang B. Corneal collagen cross-linking in the treatment of infectious keratitis and corneal ulcers. Vestn Oftalmol 2017;133(6):113-119.
10 Rathi A, Chakrabarti A, Agarwal T, Pushker N, Patil M, Kamble H,Titiyal JS, Mohan RS, Kashyap S, Sharma S, Sen S, Satpathy G, Sharma N. Pythium keratitis leading to fatal cavernous sinus thrombophlebitis.Cornea 2018;37(4):519-522.
11 Ramakrishnan S, Mandlik K, Sathe TS, Gubert J, Krishnan T, Baskaran P. Ocular infections caused by Scedosporium apiospermum: a case series.
Indian J Ophthalmol 2018;66(1):137-140.
12 Garduño E, Hidalgo R, Bigorra L, Torres JP. Fungal keratitis in an immunocompetent patient. Enferm Infecc Microbiol Clin 2011;29(2):154-155.
13 Behrens-Baumann W. Diagnosis and therapy of fungal keratitis: a review. Klin Monbl Augenheilkd 1997;210(5):aA10-aA13.
14 Chen MH, Wang QF, Chen LG, Shee JJ, Chen JC, Chen KY, Chen SH,Su JG, Liu YW. The inhibitory effect of Gynostemma pentaphyllum on MCP-1 and type I procollagen expression in rat hepatic stellate cells. J Ethnopharmacol 2009;126(1):42-49.
15 Liu Y, Li J, Liu Y, Wang P, Jia H. Inhibition of zymosan-induced cytokine and chemokine expression in human corneal fibroblasts by triptolide. Int J Ophthalmol 2016;9(1):9-14.
16 Xu Q, Zhao GQ, Lin J, Wang Q, Hu LT, Jiang Z. Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus. BMC Ophthalmol 2015;15:126.
17 Zhang HB, Li HX, Li YY, Zou YL, Dong XM, Song WG, Jia CK,Li SY, Xi HJ, Liu DM, Wang YQ. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur J Immunol 2013;43(10):2671-2682.
18 Bryant-Hudson KM, Carr DJ. CXCL1-deficient mice are highly sensitive to pseudomonas aeruginosa but not herpes simplex virus type 1 corneal infection. Invest Ophthalmol Vis Sci 2012;53(11):6785-6792.
19 Kimura K, Orita T, Nomi N, Fujitsu Y, Nishida T, Sonoda KH.Identification of common secreted factors in human corneal fibroblasts exposed to LPS, poly(I: C), or zymosan. Exp Eye Res 2012;96(1):157-162.
20 Xue ML, Thakur A, Cole N, Lloyd A, Stapleton F, Wakefield D,Willcox MD. A critical role for CCL2 and CCL3 chemokines in the regulation of polymorphonuclear neutrophils recruitment during corneal infection in mice. Immunol Cell Biol 2007;85(7):525-531.
21 Chodosh J. Human adenovirus type 37 and the BALB/c mouse:progress toward a restricted adenovirus keratitis model (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:346-365.
22 Lin HY, Chang KT, Hung CC, Kuo CH, Hwang SJ, Chen HC, Hung CH, Lin SF. Effects of the mTOR inhibitor rapamycin on monocytesecreted chemokines. BMC Immunol 2014;15:37.
23 Brothers KM, Kowalski RP, Tian SH, Kinchington PR, Shanks RMQ.Bacteria induce autophagy in a human ocular surface cell line. Exp Eye Res 2018;168:12-18.
24 Hsu CM, Chiang ST, Chang YY, Chen YC, Yang DJ, Chen YY,Lin HW, Tseng JK. Lychee flower extract inhibits proliferation and viral replication of HSV-1-infected corneal epithelial cells. Mol Vis 2016;22:129-137.
25 Jiang XY, McClellan SA, Barrett R, Foldenauer M, Hazlett LD. HGF signaling impacts severity of Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci 2014;55(4):2180-2190.