INTRODUCTION
F ungal keratitis is considered as a general cause of blindness and visual impairment worldwide[1]. Aspergillus fumigatus(A. fumigatus) is one of the causal pathogens of fungal keratitis.The corneal epithelium, containing five layers of epithelial cells, primarily serves as a physical barrier to prevent microbes from destructing the corneal stroma, thereby protecting the eye from fungal infections[2]. The corneal epithelial cells constitute the first-line protective barrier of the cornea against pathogenic microorganisms, which initiate the immune response inducing the production of cytokines and inflammatory factors[3].
Interleukin (IL)-33 is a new member of the IL-1 cytokines family. It is mainly produced by epithelial cells[4], fibroblasts[5],endothelial cells[6] and other cell types, indicating its likely function in the early immune responses against invasive pathogens[7]. Like IL-1α as well as high-mobility group protein B1 (HMGB-1), IL-33 has been reckoned to be a dualfunction cytokine, serving as both a conventional cytokine and a transcriptional regulation factor[8]. IL-33 has also been associated with the pathophysiology of numerous diseases,including asthma, arthritis, obesity, and atherosclerosis[9-12].In particular, epithelial-derived IL-33 plays a quintessential regulatory role in immune responses related to Th2 cytokinemediated inflammation[6,13]. Moreover, accumulating evidence points to a significant function of IL-33 in the mucosal epithelium in allergic inflammation[14]. Nevertheless, the expression of IL-33 and its potential role in the proinflammatory response of the corneal epithelium exposed to A. fumigatus remains unknown.
ST2 is a member of the IL-1 receptor superfamily which is firstly found in growth-stimulated fibroblasts[15]. Since recognition of IL-33 as the ligand for ST2[16], the function of IL-33/ST2 signaling has subsequently been investigated and confirmed in several studies in a wide variety of cells[17-18]. There are various forms of ST2, including a soluble secreted form of ST2, a transmembrane form of ST2L, and ST2V of undetermined localization[19]. Soluble ST2 is a decoy receptor for IL-33 since it is capable of binding and neutralizing IL-33, and it can inhibit the bound of IL-33 to ST2 competitively[19]. Excessive stimulation of ST2/IL-33 was associated with autoimmune diseases, including arthritis[17],allergic inflammation[20], and airway hyperactivity[21], pointing to a significant function of ST2 in the pathogenesis of inflammation-related diseases[18]. More recent studies also demonstrated that IL-33 was produced by dendritic cells through potential autocrine regulation to amplify the local allergic inflammatory response[22]. However, the role of IL-33 from corneal epithelial cells in immune-mediated inflammation of fungal keratitis has not yet been explored. Therefore, the purpose of the current study was to evaluate whether human corneal epithelial cells (HCECs) release IL-33 in response to infection with A. fumigatus, which may be a significant event in amplifying the inflammatory immune response in the corneal epithelium via the ST2 and p38 mitogen-activated protein kinase (MAPK) inflammatory signaling pathways. Toward this end, we examined the expression of IL-33 in mouse corneal tissues experimental infected with A. fumigatus and in human cornea samples from patients with fungal keratitis. We further detected the expression of IL-33 in HCECs and the influence of its overexpression on cell proliferation and inflammatory cytokine production. Together, these findings can help extend the immune and inflammation regulatory role of IL-33 towards gaining a new mechanistic understanding of fungal keratitis.
MATERIALS AND METHODS
Ethical Approval All mice were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The tissue samples were obtained under the approval from the Institutional Research Ethics Committee at the Affiliated Hospital of Qingdao University as well as agreements from patients and their families. The research conformed to the principles of the Declaration of Helsinki (as revised in Edinburgh 2008).
Animal Model of Keratitis Female 8-week-old C57BL/6 mice were obtained from Jinan Pengyue Laboratory Animal Co. Ltd. (Jinan, China). The disease was graded using the previous scale for statistical comparison of disease severity[23].Mice were anesthetized with 8% chloral hydrate and placed under a stereo microscope at 40× magnification. The central corneal epithelium (2-mm diameter range) of the left eye was removed, and 5 μL of 1×108 colony-forming unit (CFU)/mL A. fumigatus strain (Clinical Laboratory of Affiliated Hospital of Qingdao University, Qingdao, China) was applied to the corneal surface. Covered the ocular surface with a soft contact lens and sutured the eyelids. The mice corneas were removed for Western blot analysis at 1, 3, and 5d post-infection.
Preparation of Aspergillus fumigatus Hyphae The A.fumigatus strain was grown in Sabouraud liquid medium at 37℃ for 3d. The hyphae were collected, ground, washed twice in sterile phosphate buffer saline (PBS) and inactivated with 70% ethanol at 4℃ for 12h. Inactive hyphae were washed in PBS and stored at -20℃ with DMEM (5×106 CFU/mL)[24].
Human Corneal Tissue for Ex Vivo Immunohistochemical Staining Paraffin sections of corneal tissues were collected from patients suffering from fungal keratitis who received corneal transplantation (n=20) from the Ophthalmic Pathology Department of the Affiliated Hospital of Qingdao University. Healthy control corneal tissues were collected from donors recruited at the Affiliated Hospital of Qingdao University. Paraffin sections (4-mm thickness) were immunohistochemically stained with primary antibody against IL-33 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) using the streptavidin-peroxidase method as previously reported[25].
Cells Culture and Stimulation Fresh human corneoscleral tissues were obtained from donors at the Affiliated Hospital of Qingdao University. HCECs were cultured in 12-well plates with explants of corneal limbal rims in a supplemented hormonal epidermal medium (SHEM) containing 5% fetal bovine serum (Invitrogen-GIBCO BRL, Grand Island, NY,USA). HCECs that grew well and were not contaminated with fibroblasts were used for this study. Cells grown to a density of 80% were switched to serum-free SHEM and treated with inactive A. fumigatus hyphae (60 μL/mL) at various concentrations. For evaluate of IL-33/ST2/p38 signaling pathway, HCECs were pretreated with 10 ng/mL soluble recombinant human ST2 (Abbiotec, San Diego,CA, USA), 5 μg/mL specific ST2 neutralizing antibody(Abbiotec), 10 ng/mL recombinant human IL-33 (Abbiotec), or 250 µmol/L of the p38-MAPK pathway inhibitors SB203580(Selleckchem, Houston, USA) for 1h before the addition of A. fumigatus, respectively. After 1-24h of A. fumigatus treatment, the cells were scraped from the dish, lysed, and mRNA and protein expression were detected by quantitative real-time reverse transcription polymerase chain reaction(qRT-PCR) and Western blot. The supernatant of the cultured cells was collected after 24-48h of infection for Enzymelinked immunosorbent assay (ELISA). Cells incubated with soluble recombinant ST2 or recombinant human IL-33 for 24-72h were used for Cell Counting Kit-8 (CCK8) assay. All the experiments were repeated at least in triplicates.
Quantitative Real-time Reverse Transcription Polymerase Chain Reaction Total RNA was isolated from cells with the Qiagen RNeasy® Mini kit and then quantified on a NanoDrop®ND-1000 spectrophotometer. Synthesis of the first-strand cDNA was carried out by reverse transcription of 1 µg of total RNA with Ready-To-Go You-Prime First-Strand Beads as described previously[26]. Quantitative PCR was executed in an Mx3005PQ PCR system (Stratagene) with a 20-µL reaction volume containing 5 µL of cDNA, 1 µL of TaqMan®Gene Expression Assay for IL-33, IL-6, IL-1β (TaqMan Assay Hs00369211_m1, Hs00174131_m1, Hs01555413_m1) or GAPDH (Hs99999905_m1), and 10 µL Master Mix (Applied Biosystems, Foster City, CA, USA). The thermocycler parameters were 50℃ for 2min, 95℃ for 10min, followed by 40 cycles of 95℃ for 15s and 60℃ for 1min. DNA contamination was evaluated using a non-template control run simultaneously. Relative mRNA expression levels of the target genes were normalized to GAPDH.
Enzyme-linked Immunosorbent Assay Double-sandwich ELISA was carried out for human IL-6 and IL-1β protein level determination in conditioned media and culture cell lysates from diverse treatments using ELISA DuoSet kits (BioLegend,San Diego, CA, USA). The absorbance was read at 450 nm using a VERSAmax plate reader (Molecular Devices, Sunnyvale,CA, USA) with a reference wavelength of 570 nm[26].
Western Blotting Total cellular proteins were extracted from mice corneas and HCECs with RIPA lysis buffer and polymethanesulfonyl fluoride (100:1), and the lysate was centrifuged at 12 000 g for 15min at 4℃. The supernatant was collected, and after 150min of incubation on ice, the extracts were centrifuged at 12 000 g for 10min at 4℃. The protein concentration of the supernatant was determined[27].Post nuclear lysates were mixed with loading buffer, boiled for 10min, and resolved by 8% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore). Non-specific binding sites were inhibited with Western blocking buffer(Beyotime, Jiangsu, China) at room temperature for 2-3h. The membrane was then incubated with rabbit anti-IL-33(Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:1000 followed by 1:5000 diluted goat anti-rabbit secondary antibody(Elabscience, Wuhan, China), or incubated with 1:1000 diluted rabbit anti-human poly-clonal antibodies against human phospho-p38 protein and total p38 (Elabscience),followed by incubation with 1:5000 diluted goat anti-rabbit IgG (Elabscience). The membrane was also blotted with a monoclonal anti-GAPDH antibody (Elabscience) as a loading control. BeyoECL Plus reagents (Beyotime) were used for the development of colorimetric signals on the membrane. The band image was visualized with chemiluminescence (ECL;Thermo Fisher Scientific, Waltham, MA, USA).
Cell Counting Kit-8 Assay The CCK8 assay (Solarbio,Beijing, China) was performed to examine the proliferation of HCECs after treatment with different concentrations of recombinant IL-33 (5, 10 ng/mL) or soluble recombinant human ST2 (10 ng/mL). Almost 8000 cells were inoculated into 96-well plates and cultured for 48 and 72h in the presence of IL-33 or solute ST2. Then CCK8 solution was added for 2-4h for absorbance measurement at a wavelength of 490 nm using a microplate reader (Molecular Devices).
Cell Count Almost 8×105 HCECs were seeded into 12-well plates and treated with or without different concentrations of recombinant IL-33 (5 and 10 ng/mL) and soluble recombinant human ST2 (10 ng/mL) for 48 and 72h. Cells are digested with trypsin to single cell suspension and stained by 4% trypan blue solution. Viable cells were counted under microscope by trypan blue exclusion at 48 and 72h.
Statistical Analysis All data are presented as mean±SD.The difference between groups was evaluated by analysis of variance and an unpaired two-tailed Student's t-test. It is considered to be significant when P<0.05.
RESULTS
A. fumigatus Increased Expression of IL-33 in the Cornea and HCECs Evaluation of the clinical disease score (Figure 1A) and slit-lamp photography (Figure 1B-1D) showed that disease severity reached a peak at 3d in A. fumigatus-infected C57BL/6 mice corneas (P<0.05, P<0.01). Western blot analysis further demonstrated that the protein level of IL-33 in the mice corneas was elevated and peaked at 3d after infection (Figure 1E). In addition, immunohistochemistry showed that IL-33 protein was primarily localized in the cytoplasm of normal corneal epithelial tissue, whereas stronger staining of the multilayer corneal epithelium was found in the tissues of patients with fungal keratitis (Figure 1F). In HCECs, immunohistochemical staining of IL-33 was chiefly distributed in the cytoplasm, and stronger cytoplasmic along with more nuclear staining was found in HCECs stimulated by A. fumigatus (Figure 1G). Moreover, IL-33 mRNA expression was induced by A. fumigatus in HCECs, reaching a maximal level at 8 h of stimulation (Figure 1H) with a 6-fold increase compared to the control (P<0.01).
Effect of IL-33 on Cytokines Production in HCECs Stimulated with A. fumigatus Significant induction of IL-6 mRNA (up to 4-5 fold) was detected in HCECs stimulated by A. fumigatus for 8 h (Figure 2A), while the expression of IL-1β mRNA peaked at 4h (up to 4-5 fold; Figure 2C). Such stimulatory effect of A. fumigatus on these inflammatory cytokines was verified at the protein levels, and the effect was shown to be dose-dependent with the treatment of
2-10×106 CFU/mL of A. fumigatus hyphae (Figure 2B, 2D).Treatment with recombinant human IL-33 further upregulated the production of these cytokines at both the mRNA (Figure 2A, 2C) and protein (Figure 2B, 2D) levels compared with that of the A. fumigatus-stimulated groups, indicating a possible function of IL-33 in promoting the production of inflammatory cytokines of HCECs stimulated by A. fumigatus.
IL-33 Upregulated A. fumigatus -Induced Inflammatory Cytokines via ST2 Signaling in HCECs Since we found that HCECs generated IL-33 in response to A. fumigatus, which promoted subsequent inflammatory cytokines production,we next investigated whether this effect is mediated by ST2 signaling. After stimulation of cells or the culture supernatant with 5×106 CFU/mL A. fumigatus, remarkable elevation of IL-6 and IL-1β mRNA (Figure 3A, 3C) and protein (Figure 3B,3D) was detected. In addition, pre-treatment with recombinant human IL-33 (10 ng/mL) 1h prior to A. fumigatus stimulation significantly stimulated the IL-6 and IL-1β expression induced by A. fumigatus at both the mRNA (Figure 3A, 3C) and protein(Figure 3B, 3D) levels, whereas pre-treatment with 10 ng/mL of soluble recombinant human ST2 protein or 5 μg/mL ST2neutralizing antibody 1h prior to A. fumigatus stimulation naturally suppressed the expression of IL-6 and IL-1β at the mRNA (Figure 3A, 3C; P<0.01, n=4) and protein (Figure 3B,3D; P<0.01, n=4) levels, compared with the A. fumigatusalone treated group. Compared with the recombinant IL-33 and A. fumigatus treated group, soluble ST2 protein or ST2 neutralizing antibody together with recombinant IL-33 pretreatment prior to A. fumigatus also downregulated the expression of these cytokines at mRNA (Figure 3A, 3C;P<0.01, n=4) and protein (Figure 3B, 3D; P<0.01, n=4) levels.

Figure 1 A. fumigatus increased expression of IL-33 in the cornea and HCECs A: Clinical disease score; B-D: Representative images from C57BL/6 mice corneas infected with A. fumigatus (AF) at 1, 3, and 5d; E: Protein expression of IL-33 in mice corneas infected with A.fumigatus. F: Representative images showing IL-33 localization in ex vivo donor corneal tissues or the corneal epithelium of fungal keratitis patients by immunohistochemical staining with isotype IgG as a negative control; G: Immunohistochemical images showing IL-33 protein in primary HCECs with or without exposure to A. fumigatus, with isotype IgG as a negative control. Magnification 400×; H: mRNA expression of IL-33 in HCECs exposed to A. fumigatus hyphae (5×106) over time. aP<0.05; bP<0.01; cP<0.001; n=4.
IL-33/ST2 Signaling Upregulated A. fumigatus-Induced Inflammatory Responses via p38 Activation in HCECs
We further explored whether the p38 signaling pathway is involved in the IL-33/ST2 regulatory mechanism in HCECs.Western blot analysis (Figure 4A) showed an increase in p38 phosphorylation (indicating its activation) in HCECs stimulated with A. fumigatus hyphae (5×106 CFU/mL).Compared with the A. fumigatus infection group, preincubation with recombinant human IL-33 for 1h before A. fumigatus stimulation significantly promoted p38 phosphorylation, which was blocked by treatment with soluble ST2 protein (10 ng/mL)or the MAPK p38 inhibitor SB203580 (Figure 4A). In addition, pre-treatment of the cells with SB203580 (10 μmol/L)inhibited the mRNA (Figure 4B, 4D) and protein production(Figure 4C, 4E) of IL-6 and IL-1β induced by A. fumigatus in HCECs.
IL-33 Promoted Cell Proliferation of HCECs via its Receptor ST2 The results of the CCK8 assay showed that IL-33 administration (5 and 10 ng/mL) for 48 and 72h significantly promoted the proliferation of HCECs compared with that of untreated control cells, and treatment with soluble recombinant human ST2 protein attenuated this effect (Figure 5A, 5B). Cell count results also demonstrated that IL-33 administration for 48 and 72h promoted the proliferation of HCECs, and this effect was suppressed with soluble ST2 protein (Figure 5C, 5D).
DISCUSSION
Corneal epithelial cells act as the principal barrier in protecting the cornea from infections by pathogenic microorganisms.Since the cornea does not contain blood vessels and has few Langerhans cells and other full-time immune cells[28-29], the local immune response of corneal epithelial cells to pathogenic microorganisms is of great significance for the occurrence,development, and prognosis of corneal inflammation.
IL-33 is a recently identified secretory protein that participates in various chronic inflammation reactions and autoimmune diseases[9,30]. For example, IL-33 has been proven as a proinflammatory mediator for allergic inflammation[31].Through a heterodimeric membrane receptor comprising ST2 and an IL-1R1 auxiliary protein, IL-33 activates Th2 lymphocytes, mast cells, eosinophils, basophils, macrophages,natural killer (NK) cells, and NK T cells[7,32-33]. Indeed, IL-33 is also considered to play a significant role in infectious diseases,showing involvement in response to bacterial agents such as the lipopolysaccharide-induced inflammatory response[34].However, the function of IL-33/ST2 in the local corneal epithelium of fungal keratitis has not been explored in detail until now. The present study demonstrated that the production of IL-33 and other pro-inflammatory cytokines was driven by HCECs in response to A. fumigatus, and that IL-33/ST2 likely tunes this inflammatory response to the fungus via the p38/MAPK signaling pathway. The HCECs-produced IL-33 is likely to amplify the local inflammatory response via an autocrine mechanism and ST2 signaling.

Figure 2 Effect of IL-33 on cytokines production in HCECs stimulated with A. fumigatus HCECs were exposed to A. fumigatus hyphae(2, 5, or 10×106) for 0, 4, 8, and 16h with or without prior incubation of recombinant human IL-33 (10 ng/mL). A, C: mRNA expression levels of inflammatory cytokines (IL-1β and IL-6) measured by qRT-PCR; B, D: Protein expression levels of inflammatory cytokines determined by ELISA in culture supernatants. Results shown are mean±SD of four independent experiments. aP<0.05; bP<0.01, cP<0.001, n=4.
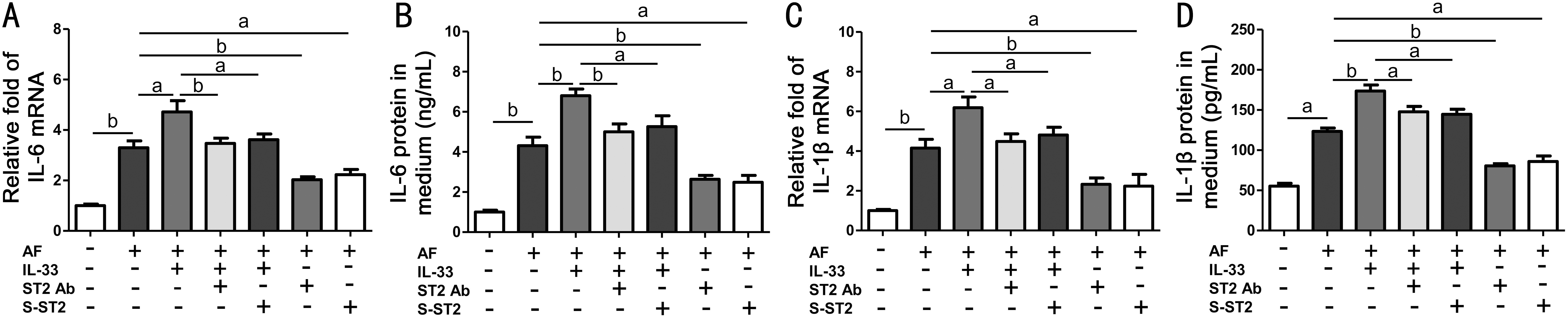
Figure 3 IL-33 upregulated A. fumigatus -induced inflammatory cytokines via ST2 signaling in HCECs The HCECs were exposed to A. fumigatus hyphae (5×106) with or without prior incubation with recombinant human IL-33 (10 ng/mL), soluble ST2 protein (10 ng/mL) or ST2 neutralizing antibody (5 μg/mL) for 1h. A, C: Cytokine (IL-6 and IL-1β) mRNA levels in cells treated with A. fumigatus hyphae (5×106) for 4h by qRT-PCR; B, D: Cytokine (IL-6 and IL-1β) levels from culture supernatants after treatment of cells with A. fumigatus hyphae for 48h by ELISA. Results are the mean±SD of four independent experiments. aP<0.01; bP<0.001, n=4.
IL-33 is chiefly generated by epithelial cells via Toll-like receptor-mediated inflammatory response[22], which is assumed to contribute to the basal production of IL-33 by HCECs in response to A. fumigatus or another fungus. After HCECs were incubated with A. fumigatus, IL-33 expression was markedly provoked. Moreover, using paraffin sections acquired from patients with fungal keratitis after corneal transplantation, the HCECs were also confirmed to generate IL-33 in vivo. This production of IL-33 was observed in the multiple layers of the human corneal epithelium based on immunohistochemistry staining.
IL-33 is known to be the ligand of ST2, the widely accepted receptor of Th2 cells[22]. Upon binding to ST2, IL-33 promotes the generation of Th2 inflammatory cytokines (including IL-4 and IL-13) by Th2 cells in allergic disease[31]. Since soluble ST2 can competitively bind to IL-33, it has been regarded as an antagonist of IL-33[19]. ST2 was confirmed to be expressed in HCECs, and ST2 signaling was found to be essential for the IL-33-stimulated generation of proinflammatory cytokines by HCECs[26]. The present study further showed that the mRNA and protein expression of IL-6 and IL-1β was significantly stimulated after pre-treatment with exogenous recombinant human IL-33 1h prior to A. fumigatus stimulation, which was significantly suppressed by pretreatment with soluble ST2 protein or ST2 neutralizing antibody. Overall, these findings suggest that HCECs-generated IL-33 is likely a source potentially able to amplify inflammation through ST2 signaling. Thus, we concluded that IL-33 upregulated A. fumigatus induced inflammatory cytokines via ST2 signaling in HCECs. We further demonstrated that p38 was remarkably activated in HCECs exposed to A. fumigatus but was suppressed by ST2 or the MAPK p38 inhibitor SB203580,which also restrained the induction of inflammatory factors(IL-6 and IL-1β) by A. fumigatus. The p38 MAPK pathway is activated in several cell types[35] and can mediate mucosal epithelial inflammation[36]. Therefore, the present findings extend the critical function of the p38/MAPK signaling pathway to the A. fumigatus-induced inflammation of HCECs in which IL-33/ST2 signaling regulates the A. fumigatusinduced inflammatory responses in HCECs via p38 activation.It has been proved that IL-33 can regulate cell proliferation and differentiation of renal cell carcinoma by binding to ST2 and inducing the downstream transcription factor NF-κB[37].We also showed that administration of recombinant human IL-33 for 48 and 72h promoted the proliferation of HCECs,which was attenuated by recombinant ST2 protein. Thus,IL-33 promoted the proliferation of HCECs via its receptor ST2 alike that reported for renal cell carcinoma; however, the role and specific mechanism of IL-33/ST2 in the proliferation of HCECs remains to be further studied.

Figure 4 IL-33/ST2 signaling upregulated A. fumigatus-induced inflammatory responses via p38 activation in HCECs HCECs were exposed to A. fumigatus hyphae (5×106) with or without prior incubation of recombinant human IL-33 (10 ng/mL), soluble ST2 protein (10 ng/mL), or the MAPK p38 inhibitor SB203580 for 1h. A:Western blots for human phospho-p38 protein and total p38 protein.B, D: Cytokine (IL-6 and IL-1β) mRNA levels of cells treated with A. fumigatus hyphae (5×106) for 4h determined by qRT-PCR. C, E:Cytokine (IL-6 and IL-1β) protein levels from the culture supernatants of cells treated with A. fumigatus hyphae for 48h determined by ELISA. aP<0.01; bP<0.001.
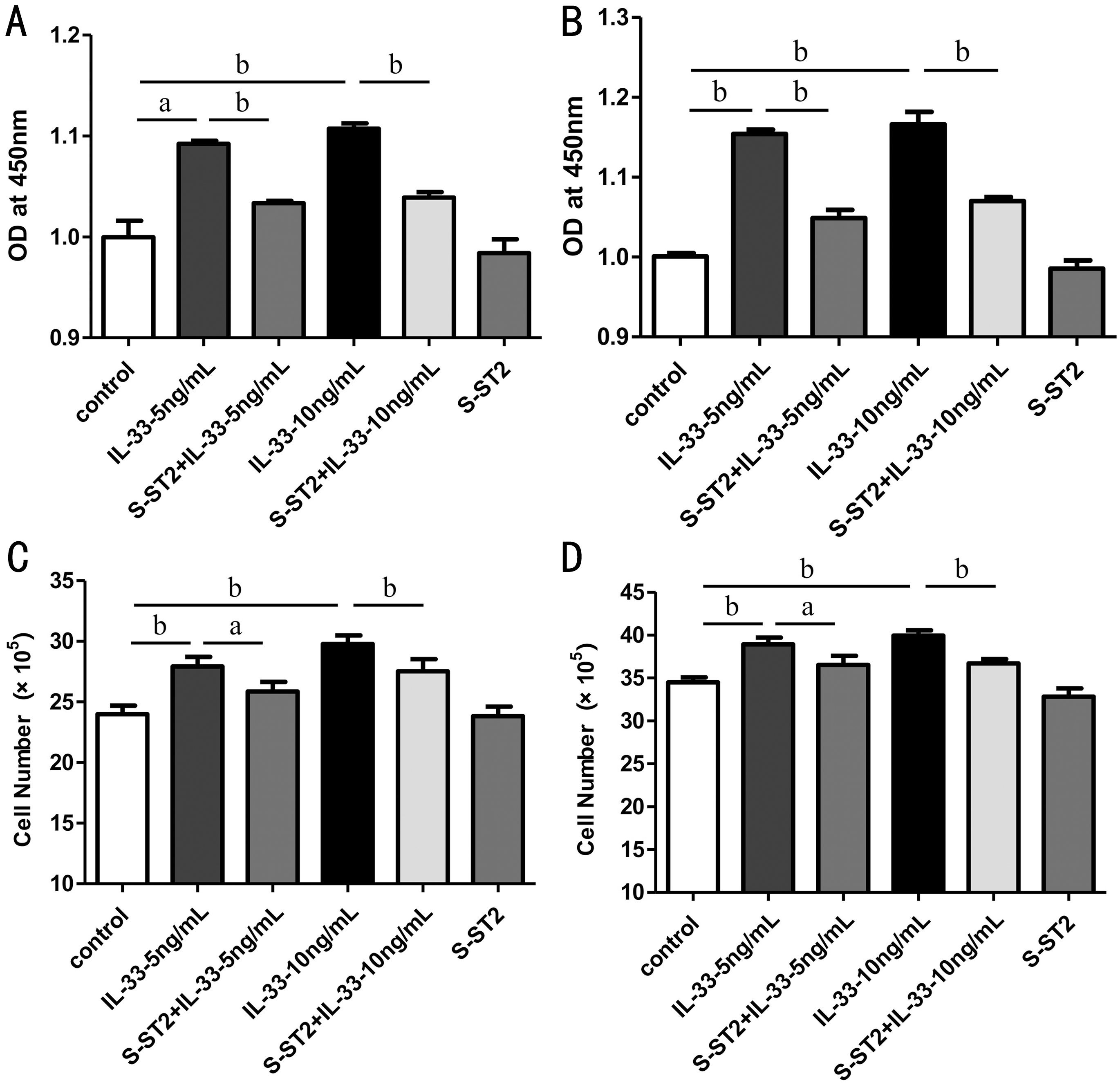
Figure 5 IL-33 promoted the proliferation of HCECs via its receptor ST2 A, B: Cell proliferation of HCECs treated with or without recombinant IL-33 protein (5 and 10 ng/mL) and soluble ST2 protein (10 ng/mL) for 48 and 72h was measured by CCK8 assay; C,D: Cell viability of HCECs treated with or without recombinant IL-33 protein and soluble ST2 protein for 48 and 72h was examined by cell count. aP<0.01; bP<0.001.
In summary, we have clarified that A. fumigatus induces the expression and generation of IL-33 in the cornea and HCECs and that the IL-33/ST2/p38 signal pathway plays an essential role in amplifying the inflammatory response of HCECs to A. fumigatus infection. IL-33 also promoted the cell proliferation of HCECs via its receptor ST2. These results suggest a new mechanism by which the A. fumigatus-induced inflammatory response may be amplified by IL-33 through a potential autocrine mechanism, highlighting a new therapeutic target for fungal keratitis via the local blockade of IL-33 generated by HCECs.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81700800;No.81870632; No.81800800); Natural Science Foundation of Shandong Province (No.ZR2013HQ007; No.ZR2017MH008;No.ZR2017BH025); the Youth National Natural Science Foundation of China (No.81500695).
Conflicts of Interest: You J, None; Lin J, None; Zhou YF,
None; Peng XD, None; He H, None; Li C, None; Zhu GQ,None; Zhao XQ, None; Zhao GQ, None.
1 Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull 2010;81(2-3):219-228.
2 Guo H, Wu XY. Innate responses of corneal epithelial cells against Aspergillus fumigatus challenge. FEMS Immunol Med Microbiol 2009;56(1):88-93.
3 Xu Q, Zhao GQ, Lin J, Wang Q, Hu LT, Jiang Z. Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus. BMC Ophthalmol 2015;15:126.
4 Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N,Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol 2012;188(7):3488-3495.
5 Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang GY,Lehtimäki S, Karisola P, Reunala T, Wolff H, Lauerma A, Alenius H. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 2012;132(5):1392-1400.
6 Aoki S, Hayakawa M, Ozaki H, Takezako N, Obata H, Ibaraki N, Tsuru T, Tominaga S, Yanagisawa K. ST2 gene expression is proliferationdependent and its ligand, IL-33, induces inflammatory reaction in endothelial cells. Mol Cell Biochem 2010;335(1-2):75-81.
7 Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res 2012;52(1-2):89-99.
8 Choi YS, Park JA, Kim J, Rho SS, Park H, Kim YM, Kwon YG.Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochemical and Biophysical Research Communications 2012;421(2):305-311.
9 Ravanetti L, Dijkhuis A, Dekker T, Sabogal Pineros YS, Ravi A,Dierdorp BS, Erjefält JS, Mori M, Pavlidis S, Adcock IM, Rao NL,Lutter R. IL-33 drives influenza-induced asthma exacerbations by halting innate and adaptive antiviral immunity. Journal of Allergy and Clinical Immunology 2018.
10 Staurengo-Ferrari L, Trevelin SC, Fattori V, Nascimento DC, de Lima KA, Pelayo JS, Figueiredo F, Casagrande R, Fukada SY, Teixeira MM,Cunha TM, Liew FY, Oliveira RD, Louzada-Junior P, Cunha FQ, Alves-Filho JC, Verri WA. Interleukin-33 Receptor (ST2) Deficiency Improves the Outcome of Staphylococcus aureus-Induced Septic Arthritis. Front Immunol 2018;9:962.
11 Duffen J, Zhang M, Masek-Hammerman K, Nunez A, Brennan A,Jones JEC, Morin J, Nocka K, Kasaian M. Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Rα2. J Immunol 2018;200(4):1347-1359.
12 Wasserman A, BenShoshan J, EntinMeer M, MayselAuslender S,GuznerGur H. Interleukin-33 augments Treg cell levels: a flaw mechanism in atherosclerosis. Israel Medical Association Journal 2012;14(10):620-623.
13 Christianson CA, Goplen NP, Zafar I, Irvin C, Good JT Jr, Rollins DR, Gorentla B, Liu WM, Gorska MM, Chu HW, Martin RJ, Alam R.Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol 2015;136(1):59-68.e14.
14 Zhang LL, Lu R, Zhao GQ, Pflugfelder SC, Li DQ. TLR-mediated induction of pro-allergic cytokine IL-33 in ocular mucosal epithelium.
The International Journal of Biochemistry & Cell Biology 2011;43(9):1383-1391.
15 Tominaga S. ST2 gene: a gene that is induced by growth stimulation and encoding a product highly similar to the interleukin 1 receptors.Seikagaku 1995;67(5):356-364.
16 Schmitz J, Owyang A, Oldham E, Song YL, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin JZ, Li XX, Gorman DM, Bazan JF,Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005;23(5):479-490.
17 Chen S, Chen BN, Wen ZY, Huang Z, Ye L. IL-33/ST2-mediated inflammation in macrophages is directly abrogated by IL-10 during rheumatoid arthritis. Oncotarget 2017;8(20):32407-32418.
18 Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 2017;8:475.
19 Tago K, Noda T, Hayakawa M, Iwahana H, Yanagisawa K, Yashiro T,Tominaga S. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun 2001;285(5):1377-1383.
20 Takatori H, Makita S, Ito T, Matsuki A, Nakajima H. Regulatory mechanisms of IL-33-ST2-mediated allergic inflammation. Front Immunol 2018;9:2004.
21 Araujo ES, de Jesus Pereira CA, de Moura Pereira AT, Moreira JM, de Rezende MC, Rodrigues JL, Teixeira MM, Negrão-Corrêa D. The role of IL-33/ST2, IL-4, and eosinophils on the airway hyperresponsiveness induced by Strongyloides venezuelensis in BALB/c mice. Parasitol Res 2016;115(8):3107-3117.
22 Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB, de Paiva CS,Pflugfelder SC, Li DQ. Potential autocrine regulation of interleukin-33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol 2013;6(5):921-930.
23 Li C, McClellan SA, Barrett R, Hazlett LD. Interleukin 17 regulates mer tyrosine kinase-positive cells in pseudomonas aeruginosa keratitis.Invest Ophthalmol Vis Sci 2014;55(10):6886-6900.
24 Liu Y, Zhao GQ, Lin J, Li C, Li Q, Che CY, Wang Q, Hu LT. The role of Syk signaling in antifungal innate immunity of human corneal epithelial cells. BMC Ophthalmol 2015;15:55.
25 Zhang J, Zhao GQ, Lin J, Che CY, Li C, Jiang N, Hu LT, Wang Q.Role of PTX3 in corneal epithelial innate immunity against Aspergillus fumigatus infection. Exp Eye Res 2018;167:152-162.
26 Lin J, Zhang LL, Zhao GQ, Su ZT, Deng RZ, Pflugfelder SC, Li DQ.A novel interleukin 33/ST2 signaling regulates inflammatory response in human corneal epithelium. PLoS One 2013;8(4):e60963.
27 Niu YW, Zhao GQ, Li C, Lin J, Jiang N, Che CY, Zhang J, Xu Q. Aspergillus fumigatus increased PAR-2 expression and elevated proinflammatory cytokines expression through the pathway of PAR-2/ERK1/2 in cornea. Invest Ophthalmol Vis Sci 2018;59(1):166-175.
28 Cursiefen C, Rummelt C, Jünemann A, Vorwerk C, Neuhuber W,Kruse FE, Schroedl F. Absence of blood and lymphatic vessels in the developing human cornea. Cornea 2006;25(6):722-726.
29 Perez VL. Visualization of immune responses in the cornea. Cornea 2017;36:S5-S8.
30 Zhang L, Jiang LL, Cao ZW. Interleukin-33 promotes the inflammatory reaction in chronic rhinosinusitis with nasal polyps by NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 2017;21(20):4501-4508.
31 Chia N, Kumar RK, Foster PS, Herbert C. Enhanced pro-inflammatory response of macrophages to interleukin-33 in an allergic environment. Int Arch Allergy Immunol 2018;176(1):74-82.
32 Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R,Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 2008;20(8):1019-1030.
33 Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010;10(2):103-110.
34 Zhang YP, Lv R, Hu XM, Jiang L, Xiao DJ, Sun Y, Zhao JN, Bao Q, Xie JR. The role of IL-33 on LPS-induced acute lung injury in mice.Inflammation 2017;40(1):285-294.
35 Ma XY, Zou J, He LP, Zhang Y. Dry eye management in a Sjögren's syndrome mouse model by inhibition of p38-MAPK pathway. Diagnostic Pathology 2014;9(1):5.
36 Yang JB, Quan JH, Kim YE, Rhee YE, Kang BH, Choi IW, Cha GH,Yuk JM, Lee YH. Involvement of PI3K/AKT and MAPK pathways for TNF-α production in SiHa cervical mucosal epithelial cells infected with trichomonas Vaginalis. Korean J Parasitol 2015;53(4):371-377.
37 Wu CW, Wu YG, Cheng C, Hong ZD, Shi ZM, Lin SQ, Li J, He XY,Zhu AY. Interleukin-33 predicts poor prognosis and promotes renal cell carcinoma cell growth through its receptor ST2 and the JNK signaling pathway. Cell Physiol Biochem 2018;47(1):191-200.