INTRODUCTION
R etinal pigment epithelium (RPE) cells secrete a variety of cytokines, including vascular endothelial growth factor(VEGF) and pigment epithelium derived factor (PEDF), which are closely related to the development of intraocular angiogenic diseases. VEGF is a key factor to promote angiogenesis. It is a mitogen and chemokine of vascular endothelial cells that promotes cell division and proliferation, increases vascular permeability and induces angiogenesis. High affinity binding sites for VEGF were found on endothelial cells, which can directly affect vascular endothelial cells, significantly promoting mitosis[1]. In addition, VEGF can also increase the expression of plasminogen activator to cause extravasation of plasma protein, activation of polyols metabolic pathways,increase of glucose transport of the endothelial cells, and induction of diglyceride and protein kinase C mechanism,to finally promote the growth of blood vessels[2]. PEDF is an endogenous VEGF inhibitor with a variety of biological activities, including anti-oxidation, anti-neuronal apoptosis,anti-inflammation and inhibiting angiogenesis[3]. By inhibiting the mitosis of endothelial cells, PEDF can inhibit the proliferation and migration of endothelial cells to inhibit retinal neovascularization[4].
The increase in VEGF and the decrease in PEDF will significantly promote the formation of retinal neovascularization[4].Therefore, the increase of VEGF/PEDF ratio is an important factor in the development of retinal angiogenesis. Our previous studies demonstrated that autophagy activation was an important contributing factor to promote retinal angiogenesis under hypoxic condition, which was evidenced by significantly improved endothelial cell migration and tube formation[5]. Autophagy is a cellular degradation mechanism through which the lysosomal degradation of cytoplasmic components was carried out to meet the needs of metabolism of the cells themselves and of updates of some organelles, thus maintaining cellular homeostasis[6-7]. Like most other cells,RPE maintains a basic level of autophagy, which may change along with age and diseases. In some environment, such as lack of oxygen, oxidative stress, non-folding protein reactions or inflammation, autophagy of RPE can be activated[8-9].
Many factors in the body or the eyes can affect the process of angiogenesis. Among them, the expression of VEGF and PEDF by RPE cells has been recognized as the most important factor.However, the relationship between activation of autophagy in RPE cells and the levels of VEGF and PEDF expression is not clear yet. To clarify this, this study investigated the changes of autophagy level under hypoxic conditions and the effects of un-regulated autophagy on VEGF and PEDF expression in RPE cells. The results of this study shed new light on the roles of autophagy of RPE cells in retinal neovascularization.
MATERIALS AND METHODS
Cell Culture and Grouping The human RPE cell line,ARPE-19, was obtained from Procell Life Science &Technology Co., Ltd. China (CL-0026, Procell, China) and cultured in Dulbecco's modified Eagle's medium/F12 human amniotic membrane nutrient mixture containing penicillin and streptomycin (DMEM/F12; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, USA) at 37℃in a 5% CO2 humidified atmosphere. All experiments were performed based on ARPE-19 cells at passage 5-10. When ARPE-19 cells reached approximately 80% confluence, cells were harvested by digestion with 0.25% trypsin (Gibco,USA) and seeded at 5×105 per well on each well of 6 well plates. The cells were randomly classified into the following four groups: the control group, hypoxia group, hypoxia+3-MA group and hypoxia+CQ group. In the control group, the cells were routinely cultured in a normoxic incubator. In the hypoxia group, the cells were incubated in a hypoxic incubator(BioSpherix, USA) with 1% O2/5% CO2/94% N2 for 24h, to observe the effect of hypoxia on autophagy and the expression of VEGF and PEDF in RPE cells. In the hypoxia+3-MA group and hypoxia+CQ group, cells were pretreated with 10 mmol/L 3-MA (Selleck, USA) or 50 μmol/L CQ (Sigma,USA) for 1h and then placed in a hypoxic incubator for 24h,to observe the effect of hypoxia on the expression VEGF and PEDF after autophagy inhibition by one of the two autophagy inhibitors 3-MA or CQ in RPE cells. The morphology of ARPE-19 cells was observed under an inverted microscope and the cell specimens were prepared conventionally[10] and the ultrastructure of the cells was detected by TEM. Each experiment was repeated three times by 3 plates of cells from the same batch.
Western Blot for Autophagic Markers RPE cells were harvested by trypsinization and then lysed in RIPA buffer(Beyotime, China), and the cell lysates were centrifuged at 15 000 rpm for 15min at 4℃. Equal amounts of proteins were separated by 10% SDS-PAGE and then electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes(Amersham, Little Chalfont, UK). The membranes were blocked with 5% non-fat milk for 2h at room temperature and incubated overnight at 4℃ with the following primary antibodies: rabbit anti-LC3B (1:1000, #2775, Cell signaling Technology, USA), rabbit anti-Beclin-1 (1:1000, #3738, Cell signaling Technology, USA), rabbit anti-Atg5 (1:500, 10181-2-AP, Proteintech Group, Inc, China), rabbit anti-p62 (1:1000,#5114, Cell signaling Technology, USA), and rabbit anti-GAPDH (1:1000, AB-P-R 001, Hangzhou Xianzhi biology Co., LTD, China). The membranes were then incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000, BA1054, Boster, China) for 2h at room temperature. Then the membranes were incubated with ECL Western Blotting Substrate (Thermo Fisher Scientific, USA) to visualize the protein bands and the density of each band was analyzed with BandScan software (Glyko Inc., USA).
Quantification of VEGF and PEDF Release by ELISA The supernatants of ARPE-19 cells were collected for cytokine measurements. The concentrations of VEGF (pg/mL) and PEDF (pg/mL) in the collected culture media were measured with Human VEGF ELISA kits (EHC108.48 Neobioscience Technology Company, China) and Human PEDF ELISA kits(E-EL-H1875c, Elabscience Biotechnology Co., Ltd, China),respectively, according to the manufacturer's instructions.
Statistical Analysis Data analysis was performed by using SPSS software (SPSS, version 17.0; SPSS Science, Chicago,IL, USA). All data were presented as means±standard deviation(SD) and tested for the normality of distribution. The one-way ANOVA followed by LSD post hoc analysis was carried out to compare means of three or more groups. P-values <0.05 were considered as statistically significant.
RESULTS
Morphology and Autophagosome of ARPE-19 Cells Under an inverted microscope, the morphology of ARPE-19 cell was different. It was mainly polygonal and spindle cells were less without obvious melanin particles in the cytoplasm (Figure 1).There were no significant differences in cell morphology among different treatment groups. In addition, autolysosomes could be seen in the cytoplasm in all groups. Formation of autophagic vacuoles was significantly increased in the hypoxia group compared with the control group and the hypoxia+3-MA or CQ group (Figure 2).
Expression Levels of LC3B-II/I, Beclin-1, Atg5 and p62
We then measured the expression levels of several key factors in the autophagic flux with Western blot. The results showed that the LC3B-II/I ratio and Beclin-1 and Atg5 protein levels were significantly up-regulated in the hypoxia group compared to the control group (all P<0.05), while the expression of p62,as substrate of autophagy, was decreased in the hypoxia group(P<0.05), suggesting that autophagy was activated in RPE cells on exposure to hypoxia. When the cells were pre-treated with 3-MA in the hypoxic environment, significantly reduced LC3B-II/I ratio, Beclin-1 and Atg5 levels as well as increased expression of p62 were detected compared to the hypoxic cells(all P<0.05, Figures 3 and 4). When the cells were pre-treated with CQ in the hypoxic environment, significantly increased expression of LC3B-II/I ratio and p62 and reduced Beclin-1 and Atg5 levels were detected compared to the hypoxic cells(all P<0.05, Figures 3 and 4).
Expression of VEGF and PEDF by RPE Cells in Response to Hypoxia The VEGF and VEGF concentrations in the supernatant of different groups of cells were then determined by ELISA. As shown in Figure 5, VEGF concentration (pg/mL)of the control group, hypoxia group, hypoxia+3-MA group and hypoxia+CQ group was 305.48±26.90, 623.72±27.35,479.19±21.82 and 396.53±31.96, respectively. This result indicated that exposure to hypoxia led to significantly increased secretion of VEGF to the culture medium (P<0.01 vs control cells), while pre-treatment with 3-MA and CQ significantly attenuated the hypoxia-induced secretion of VEGF in RPE cells (both P<0.01). The PEDF concentration (pg/mL) of the control group was significantly higher than that of the hypoxia group (815.58±19.49 vs 367.84±22.16, P<0.001), and the hypoxia+3-MA (489.24±29.60 vs 367.84±22.16, P<0.001)and hypoxia+CQ group (643.56±18.87 vs 367.84±22.16,P<0.001) both had attenuated secretion of PEDF than the hypoxia group (Figure 5). In addition, the ratio of VEGF/PEDF in the supernatant of RPE cells was calculated. VEGF/PEDF of the control group, hypoxia group, hypoxia+3-MA group and hypoxia+CQ group was 0.38±0.04, 1.70±0.17, 0.98±0.10 and 0.62±0.06, respectively (Figure 5). This result suggested that inhibition of autophagy reduced the expression of VEGF/PEDF in RPE cells on exposure to hypoxia.
Figure 1 Morphology of ARPE-19 cells after culture for 24h under an inverted microscope Bar=100 μm.
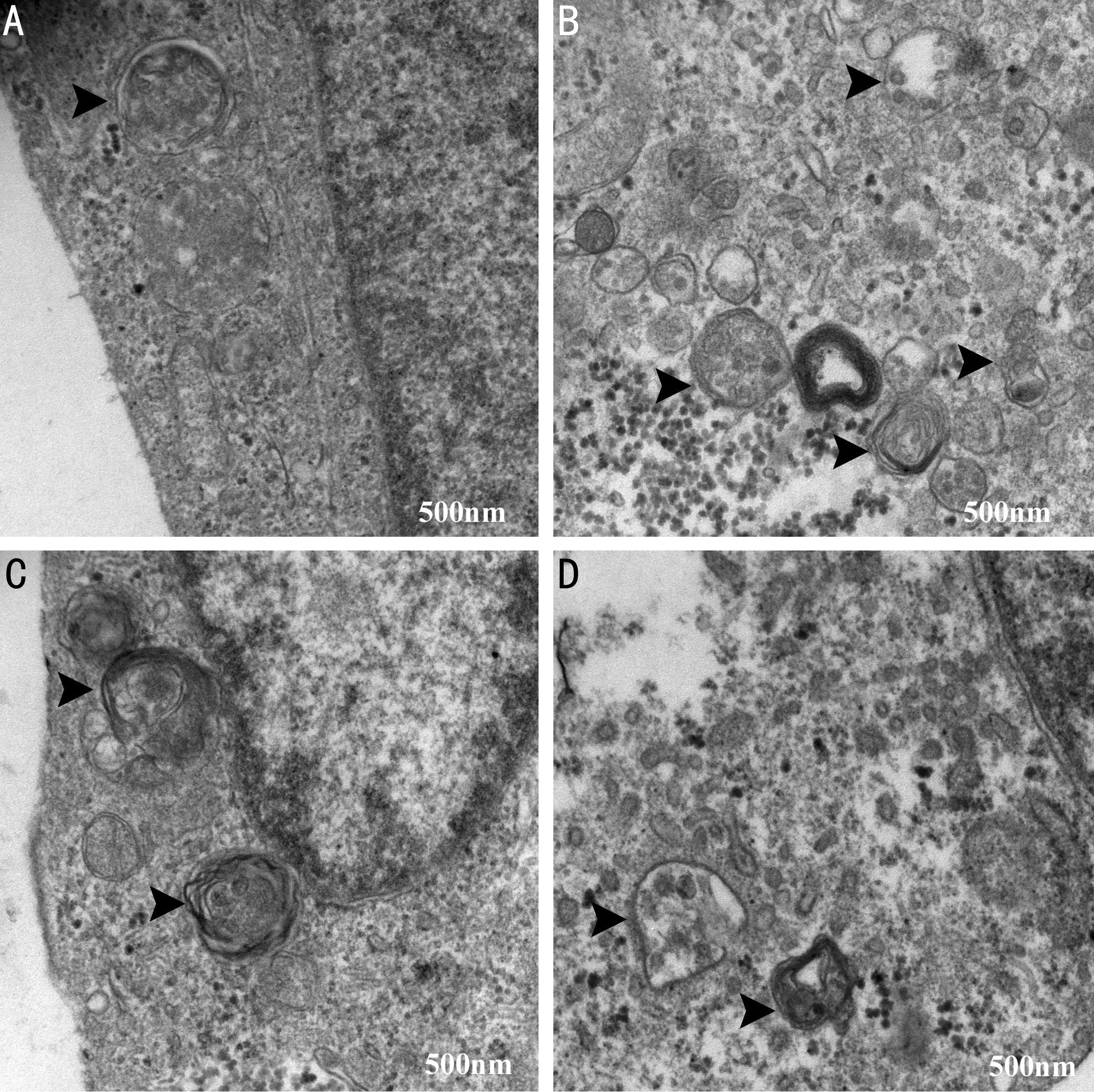
Figure 2 Representative images of autophagosomes in ARPE-19 cells under TEM A: The control group; B: The hypoxia group;C: The hypoxia+3-MA group; D: The hypoxia+CQ group. The arrows indicated the double-membrane bounded vacuoles digesting organelles or cytosolic contents. Bar=500 nm.
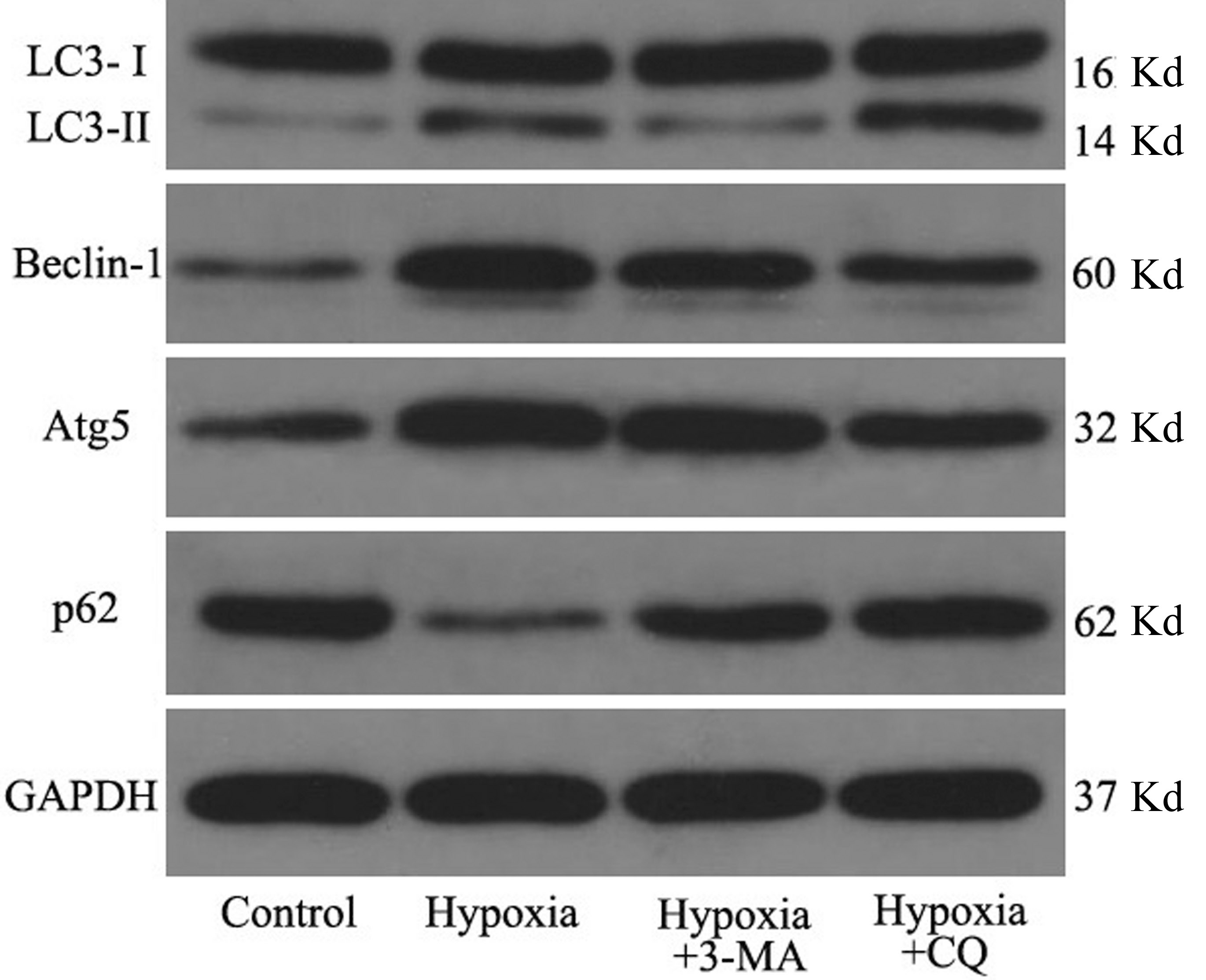
Figure 3 Expression levels of LC3B-II/I, Beclin-1, Atg5 and p62 in RPE cells in different groups by Western blot with GAPDH as loading control.
DISCUSSION
Our study showed that exposure to hypoxia significantly promoted the activation of autophagy and secretion of VEGF and led to decreased secretion of PEDF in RPE cells. When autophagy was blocked by 3-MA or CQ, the level of VEGF was reduced, while PEDF level was increased. These results suggested that expression of VEGF and PEDF was potentially regulated by autophagy to further participate in retinal neovascularization. Therefore, regulating the secretion of key cytokines, VEGF and PEDF, in RPE cells might represent another important mechanism of autophagy for promoting retinal angiogenesis under hypoxic conditions. Together with our previously published results[5], the results of this study suggested that activation of autophagy could promote endothelial cell migration and lumen formation, increase VEGF and decrease PEDF levels and thereby promote retinal neovascularization. Therefore, inhibition of autophagy is expected to be a novel target to prevent or halt retinal neovascularization in various ways.
RPE cells, located between the neuroepithelial layer of retina and choroid, have a variety of complex physiological and biochemical functions, such as barrier function, phagocytosis,participation in the circulation metabolism, antioxidant function and secretion of many growth factors[11-12]. One of the most important physiological functions of RPE cells is to influence the physiological characteristics of the neural retinal cells and RPE cells themselves by secreting growth factors.Some of these growth factors are involved in regulation of the function of RPE cells, and others, such as VEGF and PEDF, are closely associated with the occurrence of many eye diseases[13-14], including retinal neovascularization.
Hypoxia is the most widely studied factor to regulate VEGF mRNA and protein expression. In a variety of ischemic retinopathy, damage of the blood-retinal barrier after ischemiahypoxia caused entering of some cytokines into the eye, to stimulate the expression of VEGF by the retina, and meanwhile the content of VEGF in intraocular fluid was also increased[15].These changes can also increase the number of VEGF receptors on endothelial cells and increase the affinity of the receptors.VEGF binds to the receptors to mediate the proliferation of vascular endothelial cells, resulting in the formation of new blood vessels[16]. On the contrary to VEGF, PEDF is considered to be the most effective natural vascular inhibitor[17]. In the aqueous humor and vitreous cavity of normal people, a high concentration of PEDF is kept to maintain the non-vascular structure of the vitreous body and cornea. PEDF was found to significantly inhibit neovascularization of the cornea and retina in the animal models[18-19]. The mechanism of PEDF to prevent the proliferation of endothelial cells in the retinal angiogenesis was through inhibiting VEGF-sourced MAPK activity[20].
Under normal circumstances, the influence of endogenous vascular inhibitors is dominant and there is no pathologic angiogenesis. In pathological conditions, such as long-term hypoxia and hyperglycemia, increased expression of angiogenic factors and (or) decreased expression of angiogenic inhibitors result in retinal neovascularization. Current research suggests that expression of VEGF is increased and PEDF is reduced in eyes of patients with AMD, proliferative diabetic retinopathy(PDR) and other ocular diseases, and the increase of VEGF/PEDF ratio may be the main reasons for the formation of retinal neovascularization[21-24]. This study used ARPE-19 cells(a widely used cell line to study retinal diseases such as AMD)as the model and found that secretion of VEGF was increased while secretion of PEDF was decreased (the VEGF/PEDF ratio was accordingly increased) in RPE cells under hypoxic conditions, which was in line with previous research results.
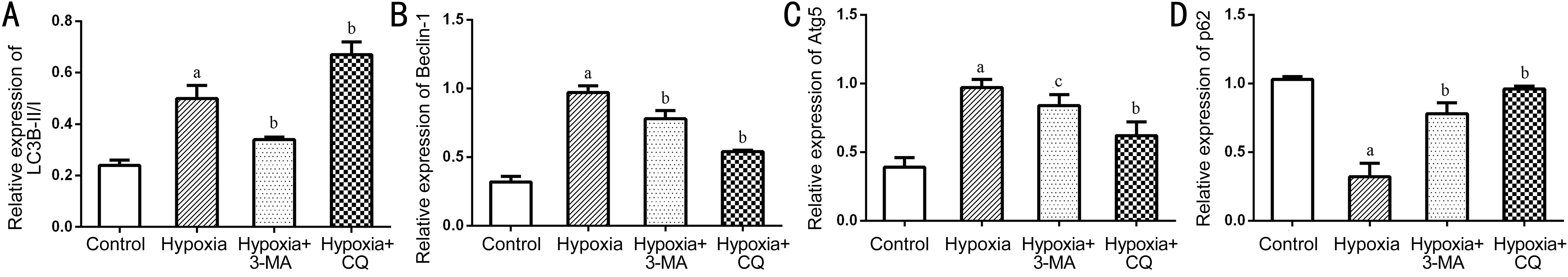
Figure 4 Quantification of the Western blots data The levels of LC3B (A), Beclin-1(B), Atg5 (C) and p62 (D) were normalized to that of GAPDH. The histograms represented the results of 3 independent experiments aP<0.01 vs Control; bP<0.01 vs Hypoxia; c P<0.05 vs Hypoxia.
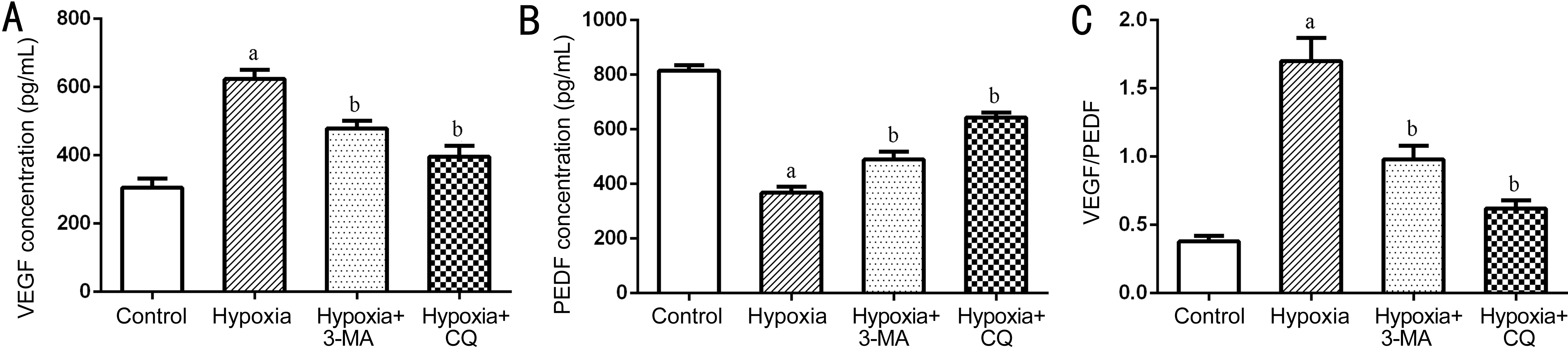
Figure 5 Quantification of the concentration of VEGF (A), PEDF (B) and VEGF/PEDF (C) in the supernatant of RPE cells in different groups measured by ELISA The histograms represented the results of 3 independent experiments aP<0.01 vs Control; bP<0.01 vs Hypoxia.
Autophagy maintains self-sustainment and plays an important role in many physiological activities, such as cell growth,development and self-protection. In general, a certain degree of autophagy exists in the cells under physiological and pathological conditions, and the autophagy level within the normal range can provide protection and repair for the cells[25-26]. More than 30 specific genes related to autophagy(ATG) have been identified, and the proteins encoded by them were involved in various stages of autophagy formation in a coordinated manner. Among them, LC3, Beclin-1, Atg5,etc. are widely used as autophagy marker proteins. LC3, the homologous protein of Atg8 in mammalian cells, exists in both LC3-I and LC3-II, and participates in the formation of autophagosome. When autophagy occurs, the cytoplasmic form of LC3 (LC3-I) converts to (autophagic) membrane-type(LC3-II) by enzymolysis of a small amount of polypeptide[27].Beclin-1, a homologous gene of yeast Atg6, is a necessary component of other autophagy genes involved in autophagy formation[28]. P62 is another common autophagosomelysosome membrane-associated autophagy marker protein.When autophagy is induced, autophagosomes combine with lysosomes to absorb and degrade p62, and this process will reduce its expression level. Therefore, the level of p62 is negatively related to autophagy[29]. Regularly, the ratio of LC3-II/LC3-I is combined with the level of p62 as an indicator of the level of autophagy.
In this study, we used two autophagy inhibitors, 3-MA and CQ, to block autophagy in RPE cells under hypoxic condition.3-MA is an inhibitor of class III PI3K pathway and has been widely used as an autophagy inhibitor. 3-MA acts on the early autophagy induction stage, which can specifically block the formation of autophagosome, reduce the ratio of LC3-II/I, and inhibit the expression of Beclin-1 and Atg5. CQ is a lysosomal autophagy inhibitor, which can destroy the acid and alkaline environments of lysosomes and prevent the combination of autophagosomes with lysosomes to inhibit the last process of autophagy[30]. In view of the different mechanisms of autophagy inhibitors, we included both early inhibitor (3-MA)and late inhibitor (CQ) in this study to avoid the off-target effect of these two inhibitors on interpreting the data. The concentration of 3-MA (10 mmol/L) and CQ (50 μmol/L)adopted in this study was based on the reference of related reports[31-34]. We then measured the protein expressions of LC3-II/I, Beclin-1, Atg5 and p62 as indicators for autophagic levels of RPE cells under different conditions. This study confirmed that autophagy was activated in RPE cells under the hypoxic environment, showing an increase in the protein expression of LC3-II/I, Beclin-1 and Atg5, and a decrease in p62 expression.In addition, 3-MA inhibited the formation of autophagosome,leading to decrease of LC3-II/I and increase of p62. After CQ treatment, the combination of autophagosomes and lysosomes was prevented, so that LC3 and p62 could not be degraded,resulting in an accumulation of LC3 in the cytoplasm.Therefore, LC3-II/I and p62 level was increased.
This study also found that hypoxia can promote the expression of VEGF and inhibit the expression of PEDF in RPE cells,and the overexpression of VEGF was reduced and the downregulation of PEDF was rescued following the pre-treatment with 3-MA or CQ. Since 3-MA and CQ were both autophagy inhibitors and the results obtained after their treatment were consistent, the results strongly suggested that inhibition of autophagy led to decreased expression of VEGF and increased PEDF level in RPE cells. Our previous research showed that inhibition of autophagy could inhibit the proliferation,migration and tube formation of vascular endothelial cells to inhibit angiogenesis[5,35]. In combination with the results of this study, in addition to the direct effect on angiogenesis,regulating the secretion of VEGF and PEDF in RPE cells might represent another important way in which autophagy promotes retinal angiogenesis. However, how does autophagy regulate the secretion of angiogenic factors in RPE cells needs further exploration, and the influence of autophagy in the pathological process of retinal neovascularization still needs to be investigated in vivo models.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81500726); the Medical Research Project of Xi'an Science Technology Bureau[No.201805097YX5SF31(4)]; the Health Research Foundation of Shaanxi Province (No.2018D074); the Outstanding Youth Talent Support Plan of Shaanxi Ordinary University.
Conflicts of Interest: Li R, None; Du JH, None; Yao GM,None; Yao Y, None; Zhang J, None.
1 Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol 1997;273(5 Pt 2):H2091-H2104.
2 Wu YD, Zhang QZ, Ann DK, Akhondzadeh A, Duong HS, Messadi DV, Le AD. Increased vascular endothelial growth factor may account for elevated level of plasminogen activator inhibitor-1 via activating ERK1/2 in keloid fibroblasts. Am J Physiol , Cell Physiol 2004;286(4):C905-C912.
3 Elahy M, Baindur-Hudson S, Cruzat VF, Newsholme P, Dass CR. Mechanisms of PEDF-mediated protection against reactive oxygen species damage in diabetic retinopathy and neuropathy. J Endocrinol 2014;222(3):R129-R139.
4 Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett 2001;489(2-3):270-276.
5 Li R, Du JH, Chang Y. Role of autophagy in hypoxia-induced angiogenesis of RF/6A cells in vitro. Curr Eye Res 2016;41(12):1566-1570.
6 Clark SL Jr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol 1957;3(3):349-362.
7 Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol 1962;12:198-202.
8 Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration(AMD). Ageing Res Rev 2009;8(4):349-358.
9 Kaarniranta K, Salminen A, Eskelinen EL, Kopitz J. Heat shock proteins as gatekeepers of proteolytic pathways-Implications for agerelated macular degeneration (AMD). Ageing Res Rev 2009;8(2):128-139.
10 Li R, Wang LZ, Du JH, Zhao L, Yao Y. Autophagy activation and the mechanism of retinal microvascular endothelial cells in hypoxia. Int J Ophthalmol 2018;11(4):567-574.
11 Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nat Rev Mol Cell Biol 2001;2(10):738-748.
12 Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M.The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier: implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol 2010;2010:190724.
13 Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K.Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem 2009;284(44):30177-30186.
14 He SK, Kumar SR, Zhou P, Krasnoperov V, Ryan SJ, Gill PS, Hinton DR. Soluble EphB4 inhibition of PDGF-induced RPE migration in vitro.
Invest Ophthalmol Vis Sci 2010;51(1):543-552.
15 Shimada K, Baba T, Neugebauer S, Onozaki A, Yamada D,Midorikawa S, Sato W, Watanabe T. Plasma vascular endothelial growth factor in Japanese type 2 diabetic patients with and without nephropathy.
J Diabetes Complicat 2002;16(6):386-390.
16 Koch S, Tugues S, Li XJ, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011;437(2):169-183.
17 Ogata N, Tombran-Tink J, Nishikawa M, Nishimura T, Mitsuma Y,Sakamoto T, Matsumura M. Pigment epithelium-derived factor in the vitreous is low in diabetic retinopathy and high in rhegmatogenous retinal detachment. Am J Ophthalmol 2001;132(3):378-382.
18 Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGFinduced migration and growth. Invest Ophthalmol Vis Sci 2002;43(3):821-829.
19 Huang Q, Wang SJ, Sorenson CM, Sheibani N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp Eye Res 2008;87(3):226-241.
20 Ren JG, Jie CF, Talbot C. How PEDF prevents angiogenesis: a hypothesized pathway. Med Hypotheses 2005;64(1):74-78.
21 Matsunaga N, Chikaraishi Y, Izuta H, Ogata N, Shimazawa M,Matsumura M, Hara H. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy.Ophthalmology 2008;115(11):1916-1922.
22 Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales' disease. Retina 2008;28(6):817-824.
23 Pons M, Marin-Castaño ME. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS One 2011;6(2):e16722.
24 Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA.Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 2009;116(3):488-496.e2.
25 Abounit K, Scarabelli TM, McCauley RB. Autophagy in mammalian cells. World J Biol Chem 2012;3(1):1-6.
26 Wu WH, Zhang MP, Zhang F, Liu F, Hu ZX, Hu QD, Yan XY, Huang SM. The role of programmed cell death in streptozotocin-induced early diabetic nephropathy. J Endocrinol Invest 2011;34(9):e296-e301.
27 Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell 2010;140(3):313-326.
28 Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P,Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA,Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J 2007;26(10):2527-2539.29 Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012;8(4):445-544.
30 Mitter SK, Rao HV, Qi XP, Cai J, Sugrue A, Dunn WA Jr, Grant MB,Boulton ME. Autophagy in the retina: a potential role in age-related macular degeneration. Adv Exp Med Biol 2012;723:83-90.
31 Szatmári-Tóth M, Kristóf E, Veréb Z, Akhtar S, Facskó A, Fésüs L, Kauppinen A, Kaarniranta K, Petrovski G. Clearance of autophagyassociated dying retinal pigment epithelial cells - a possible source for inflammation in age-related macular degeneration. Cell Death Dis 2016;7(9):e2367.
32 Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Mádi A, Fésüs L.Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1β release in human macrophages. Autophagy 2011;7(3):321-330.
33 Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA, Koh JY. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci 2010;51(11):6030-6037.34 Chen PM, Gombart ZJ, Chen JW. Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation:possible implications of lysosomal dysfunction in macular degeneration.Cell Biosci 2011;1(1):10.
35 Li R, Tian J, Du JH, Zhao L, Yao Y, Yu ZX, Chang WP, Shi R, Li J.Manipulation of autophagy: a novelly potential therapeutic strategy for retinal neovascularization. BMC Ophthalmol 2018;18(1):110.