INTRODUCTION
R etinal degeneration diseases (RDDs), including retinitis pigmentosa, age-related macular degeneration,glaucoma, diabetic retinopathy and Stargardt disease, are leading causes of irreversible blindness worldwide. So far,the treatments available for RDDs showed limited efficacy.Because destruction of retinal pigment epithelium (RPE) cells are their common pathogenesis, recent studies have focused on RPE transplantation. Tremendous preclinical and clinical trials have demonstrated that RPE cell replacement therapy is a promising approach[1-2]. Human fetal retinal pigment epithelium (fRPE) cells seems to be the best bet for RPE cell replacement therapy, because they show full function of native RPE with no tumorigenicity[3-4]. However, traditional culture medium of fRPE contains fetal bovine serum (FBS)[4], which will not only bring about risk of zoonose and xenogeneic immune rejection after transplantation[5], but also lead to laboratory culture instability. Therefore, traditional cultured fRPE cells do not meet Good Manufacturing Practice (GMP)standard and cannot be used in clinical RPE transplantation.A propitiate animal-free culture system is needed for clinical application of fRPE cells transplantation.
Human AB serum is produced from blood bank-derived plasma,and is suggested to be an alternative to FBS in supporting mesenchymal stem/stromal cells (MSC) growth[6-7]. Knockout serum replacement (KSR) was originally developed to establish a feeder-free culture system of embryonic stem cells (ESCs)[8]. KSR is demonstrated to outperforms FBS as a growth media supplements for culturing immature spermatogonial tissue[9]. B27 has been reported to propagate and sustain human RPE in vitro[10]. Nowadays, B27, human AB serum and KSR has been put into mass production and can be easily purchased. So we wondered which is the best addictive in supporting fRPE propagation and differentiation in vitro.
In this study, an xeno-free culture system for human fRPE was established by replacing FBS-supplemented media with human
AB serum-supplemented media. fRPE cells which cultured in 10% human AB serum-supplemented medium exhibited morphology and function of native RPE. Our study provided a candidate cell source for clinical RPE replacement therapy.
Table 1 Information on reagents in xeno-free culture system
Name Vender, catalog Storage MEM α modification Sigma, M-4526 +4℃N1 supplement Sigma, N-6530 +4℃Glutamin-penicillin-streptomycin Sigma, G-1146 -20℃Non essential amino acids Sigma, M-7145 +4℃Taurine Sigma, T-0625 Room temperature Hydrocoortisone Sigma, H-0396 Dissolved in DPBS to 1000× solution and storage at -20℃Triiodo-thyronin Sigma, T-5516 Dissolved in DPBS (without Ca or Mg) to 10000× solution and storage at -20℃B27 Gibco, 12587-001 -20℃Fetal bovine serum, heat inactivated Thermo scientific, SH3007003HI -20℃Knock-out serum replacement Gibco, A3181501 -20℃Human AB serum Sigma, H6914 -20℃DPBS (with Ca and Mg) Gibco, 14040-133 +4℃Tryple select enzyme Gibco, A12859-01 +4℃Cell start Invitrogen, A10142-01 +4℃Cryo-SFM Promo cell, C-29912 +4℃
MATERIALS AND METHODS
Ethical Approval Approval of permission to work with human fRPE was granted from the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.This study has been registered with ClinicalTrials.gov (No.NCT02868424), and Chinese Clinical Trial Registry (No.ChiCTR-OPC-15006757). All the aborted fetus were 11th and 12th weeks of gestation. Informed consents were signed by donors to ensure that donations were voluntary with no commercial purpose.
Human Fetal Retinal Pigment Epithelium Cells Culture
Preparation and culture of human fRPEs cells were performed as described previously with modification[4]. In brief, the intact globes were curved out in Dulbecco's phosphate buffered saline (DPBS). Then eyes were dissected in a laminar flow hood with the aid of a microscope. The anterior segment,vitreous content and retina were removed. The RPE monolayer was carefully dissected and digested in tryple select enzyme for 7min before seeding. RPE cells were inoculated at the density of 3×105 per well in cell start-coated 6-well culture plates at 37℃ in a humidified atmosphere with 5% CO2. Media was changed every three days. fRPE cells were expanded,examined and frozen after reaching confluence. Detailed information of medium contents and culture related reagents is shown in Table 1.
Western Blot Human fRPE and human adult RPE cell line (ARPE-19) were lysed in RIPA buffer (Sigma-Aldrich,St.Louis, Mo, USA) containing a proteinase inhibitor cocktail(Roche, Indianapolis, IN, USA). Protein extracts (10-15 μg)were electrophorese using 4%-12% BisTris NuPAGE gel and blotted onto nitrocellulose membranes (Invitrogen, Carlsbad,CA, USA). The blots were incubated with antibodies against human RPE-specific 65-kDa protein (RPE65), pigment epithelium derived factor (PEDF), bestropohin 1 (BEST1)or cellular retinaldehyde-binding protein (CRALBP). β-actin were used as inner reference. A minimum of three experiments were performed.
Enzyme-Linked Immunosorbent Assay Three-day culture media were extracted when fRPE cells reaching confluence at P2. Protein levels of vascular endothelial growth factor(VEGF), PEDF, fibroblast growth factor-basic (FGF2),transforming growth factor beta (TGF-β) and beta nerve growth factor (β-NGF) were qualified by enzyme-linked immunosorbent assay (ELISA) kit (Qiaoyi, Anhui, China;USCN, Wuhan, China) using the manufacturer's protocol.Protein levels of cytokines in blank medium with no cells were also measured to exclude medium content influence. The plated RPE cells from each well were then dissociated and counted using a hemocytometer. A minimum of three assays were performed.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted using the Rneasy mini kit (Qiagen,Valencia, CA, USA) according to the manufacturer's specifications. The yield of RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific,USA), and the integrity was evaluated using agarose gel electrophoresis stained with ethidium bromide. To measure the expression of CRALBP, RPE65, BEST1 and PEDF,quantification was performed with a two-step reaction process:reverse transcription (RT) and polymerase chain reaction(PCR). Real-time PCR was performed using LightCycler®480 II real-time PCR instrument (Roche, Swiss). At the end of the PCR cycles, melting curve analysis was performed to validate the specific generation of the expected PCR product.The primer sequences were designed in the laboratory and synthesized by Generay Biotech (Generay, PRC) based on the mRNA sequences obtained from the NCBI database as shown in Table 2. The expression levels of mRNAs were normalized to GAPDH and were calculated using the 2-ΔΔCt method. Three samples were analyzed in each group.
Table 2 Primer information for quantitative real-time PCR
Gene Sequence from (5'-3') Product length (bp)RPE65 (H) Fw TACAGAAAGCACTGAGTTGAGC 154 RPE65 (H) Rev CCATTTAGTAAGTCCACATTCATTTCC CRALBP (H) Fw CAAAGCCATCCACTTCATCCACCA 162 CRALBP (H) Rev AAGTCAGAGGGCAGGATGTTCTCA PEDF/SERPINF1 (H) Fw TTATGAAGGCGAAGTCACCAAGTCCC 143 PEDF/SERPINF1 (H) Rev CATCCTCGTTCCACTCAAAGCCA BEST1 (H) Fw GTTTGCCAACCTGTCAATG 109 BEST1 (H) Rev GTGTCCACACTGAGTACG GAPDH (H) Fw GGAAGCTTGTCATCAATGGAAATC 168 GAPDH (H) Rev TGATGACCCTTTTGGCTCCC
Table 3 Information on the first fluorescent antibody
Antibody Vender, Catalog Concentration City, State, Country Host RPE65 LifeSpan BioSciences, LS-C671938 1/100 Seattle, WA, USA Rabbit CRALBP Abcam, ab15051 1/250 Cambridge, MA, USA Mouse BEST1 Abcam, ab2182 1/500 Cambridge, MA, USA Mouse MERTK Abcam, ab5110108 1/100 Cambridge, MA, USA Goat
Fluorescence-Activated Cell Sorting Approximate 2×105 of human fRPE cells were digested with tryple select enzyme.Then cells were fixed and permeated with Cytofix/Cytoperm Fixation/Permeabilization solution kit (BD, Franklin Lakes,NJ, USA) following the manufacturer's instructions. Next,cells were stained with KI67 anti-bodies (BioLegend, San Diego, CA, USA). Negative control was set up by staining corresponding isotype control. The cells were washed twice before being analyzed on a fluorescence-activated cell sorter(FACS; Beckman, S.Kraemer Boulevard Brea, CA,USA).Fluorescence intensity was expressed as arbitrary units on a log scale. Histogram overlays were made with the use of the analyze software Flow Jo. Cell cycle analysis was conducted with a cell cycle assay kit (Keygen Biotech, Nanjing, Jiangsu,China). Cell apoptosis assay using Annexin V/PI staining(BD, Franklin Lakes, NJ, USA) was conducted following kit protocols. A minimum of three assays were performed.
Immunocytochemistry The expression of CRALBP, MER proto-oncogene tyrosine kinase (MERTK), BEST1, RPE65 were determined by indirect immunofluorescence. Briefly,cells were cultured on cell start-coated cell chamber slides(Thermo Fisher Scientific, Lab-Tek, Waltham, Massachusetts,USA) at the density of 3×104 per well for more than three weeks until pigment was shown. Then slides were washed with phosphate buffer solution (PBS) for three times and fixed in 4% paraformaldehyde at room temperature for 30min. After being washed with PBS three times again, slides were treated with PBST (PBS with 0.1% tween) on ice for 10min. Then slides were blocked in 5% bull serum albumin (BSA) for 1h at room temperature. Incubate in first antibody for 2-3h at 37℃.Wash thoroughly with PBS then incubate in second antibody for 1h at room temperature, followed by the addition of DAPIFluoromount-G (Southern Biotech, Birmingham, Alabama,USA) to preserve immunofluorescent labels. Information on the primary antibody is provided in Table 3.
Transmission Electronic Microscope Cells were fixed in 2.5% glutaraldehyde solution, then epoxy resin-embedded after dehydration. Samples were sliced before examined by a transmission electronic microscope (JEOL, Japan). Selected areas were trimmed for ultrathin sectioning and stained with uranyl acetate before electron microscopy observation.
Statistical Analyses Statistical analyses were performed using GraphPad Prism 7 software (GraphPad Software Inc, La Jolla,CA, USA). The values are expressed as the mean±standard deviation (SD), with P<0.05 being considered statistically significant. Significance was assessed using the paired and unpaired Student's t-test (Prism 7.0). One-way ANOVA followed by Scheffe's test was performed.
RESULTS
Optimum Concentration of B27, KSR and Human AB Serum fRPE cells were cultured in 10% FBS from passage 0 (P0). Culture mediums were changed into mediums supplemented with different concentrations of B27, human AB serum and KSR after seeding at P2. The optimum working concentration of B27, human AB serum and KSR was determined by cell cycle, apoptosis and the expression of KI67 (Figure 1). Our results showed that the percentage of KI67+ cells was significantly increased in the 15% KSR group (13.6%) 10% AB serum group (25.7%), and 2%B27 group (7.95%). The percentage of KI67+ cells was significantly reduced in 15% KSR group and 2% B27 group when compared to 10% FBS group (26.3%). There was no significant difference in the percentage of KI67+ cells between 10% AB serum group and 10% FBS group (Figure 1A). The percentage of cells in S phase was significantly increased in the 15% KSR group (7.2%), 10% AB serum group (14.2%), and 2% B27 group (5.17%). The cells in S phase was significantly reduced in 2% B27 group and significantly increased in 10%AB serum group compared to 10% FBS group (9.06%). There was no significant difference in S phase cells between 15%KSR group and 10% FBS group (Figure 1B). FACS results indicated that the proportion of apoptotic cells which positively stained with Annexin and PI was significantly decreased in 10% AB serum group (1.34%), and 2% B27 group (1.71%).Additionally, the apoptotic cells was significantly increased in 10% KSR group (2.4%), 20% KSR group (2.21%), 15%AB serum group, 1% B27 group and 4% B27 group (3.67%)compared to 10% FBS group (0.92%; Figure 1C).
Taken together, the optimum concentration of KSR was 15%,the optimum concentration of human AB serum was 10%, and the optimum concentration of B27 was 2%. What's more, the proliferation ability of fRPE cells was elevated in 10% AB serum group and repressed in 15% KSR and 2% B27 groups when compared to 10% FBS group.
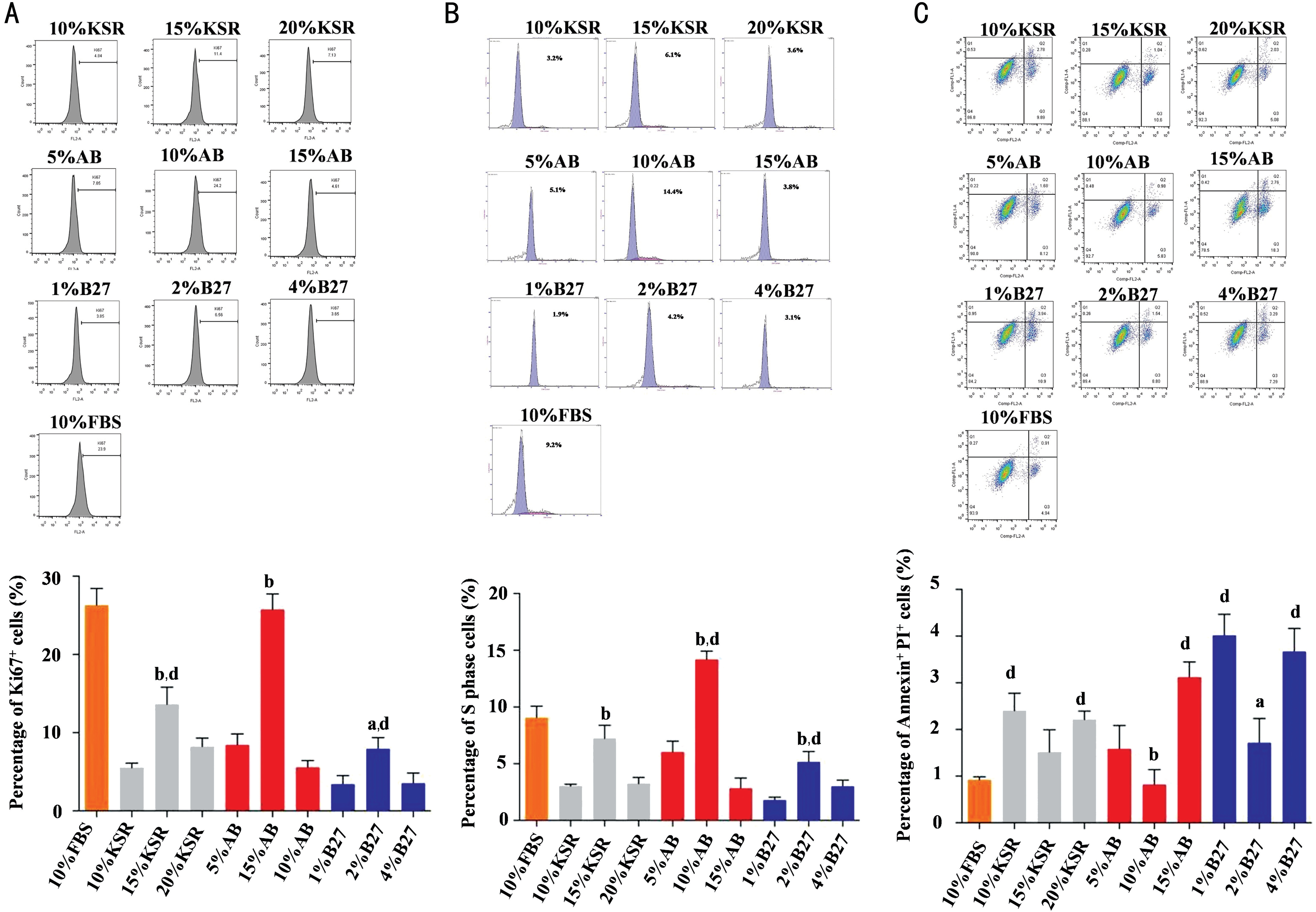
Figure 1 Optimum concentration of B27, KSR and human AB serum A: FACS outcomes showed the KI67+ cells was significantly increased in the 10% AB serum group 15% KSR group, and 2% B27 group. The KI67+ cells was significantly reduced in the 15% KSR group and 2% B27 group compared to 10% FBS group. B: The S phase cells was significantly increased in the 10% AB serum group, 15% KSR group, and 2%B27 group. Besides, the S phase cells was significantly increased in 10% AB serum group and significantly reduced in 2% B27 group compared to 10% FBS group. C: The apoptotic cells was significantly reduced in the 10% AB serum group, and 2% B27 group. The apoptotic cells was significantly elevated in the 10% KSR, 20% KSR, 15% AB serum, 1% B27 and 4% B27 groups when compared to 10% FBS group. aP<0.01,bP<0.001 vs corresponding concentration of KSR, B27 and AB serum groups respectively; dP<0.001 vs 10% FBS group. All experiments were repeated three times; error bars indicate SD.
Differentiation and Function of fRPE Cells in B27, KSR and Human AB Serum fRPE mediums were changed into 10% FBS, 10% AB serum, 15% KSR and 2% B27-supplemented mediums respectively at P1 to investigate the differentiation potential of fRPE cells. Light microscope images showed fully differentiated fRPE cells with typical cobblestone morphology and pigmentation in all groups at P2. However, many cells in 15% KSR group stretched and dedifferentiated into fibroblast-like cells. Many cells in 2% B27 group did not show hexagon morphology and pigmentation.Additionally, fRPE cells in 10% AB serum group packed more closely and the morphology of fRPE cells in 10% AB serum group were more uniform than in 10% FBS group. At P3,fRPE cells in 15% KSR group were detached and fRPE cells in 2% B27 serum group failed reaching confluence. While fRPE cells in 10% AB serum group and 10% FBS group exhibited a typical epithelial morphology with pigmentation (Figure 2A).We examined the paracrine function of fRPE cells in all groups by detecting the protein level of RPE-secreted growth factors(VEGF, PEDF, FGF2, TGF-β and β-NGF) in culture mediums at P2. ARPE19 was examined as control. ELISA results showed that the secretion of FGF2, TGFβ, β-NGF, PEDF and VEGF were significantly reduced in 15% KSR group and 2%B27 group compared to 10% FBS group (P<0.05) as well as 10% AB serum group (P<0.05). There was no significant difference of FGF2, TGFβ, β-NGF, PEDF and VEGF between 10% FBS group and 10% AB serum group (Figure 2B). Then we examined fRPE cell function by detecting the expression level of RPE signature markers (CRALBP, BEST1, PEDF and RPE65). The expression of CRALBP, BEST1, PEDF and RPE65 were significantly elevated in 10% FBS group and 10% AB serum group compared to 15% KSR group (P<0.05)as well as 2% B27 group (P<0.05). Besides, the expression of CRALBP, BEST1, PEDF and RPE65 were slightly increased in 10% AB serum group than 10% FBS group. ARPE19 cells were examined as control (Figure 2C, 2D).
In summary, fRPE cells were fully differentiated and showed native RPE morphology and function in 10% human AB serum-supplemented medium at P2 and P3. While fRPE cells in 15% KSR medium and 2% B27 medium gradually lost RPE morphology and function, and could only be passaged to P2.
Xeno-free Culture System-Generated fRPE Cells In order to verify the efficacy of 10% human AB serum culture system,we cultured fPRE cells in 10% AB serum-supplemented xeno-free culture medium from P0. Pigmented cobblestonelike fRPE cells can be produced from P0 to P3. At P4, fRPE cells dedifferentiation into spindle-shaped cells resembling fibroblasts and failure to reach confluency (Figure 3A).Electron micrograph of xeno-free culture system-generated fRPE cells showed RPE-specific structures at P3: apical microvilli, melanosomes and tight junctions (Figure 3B).Immunocytochemical staining demonstrated that fRPE cells can fully differentiated and expressed RPE-specific markers CRALBP, RPE65, MERTK and BEST1 at P3 (Figure 3C).
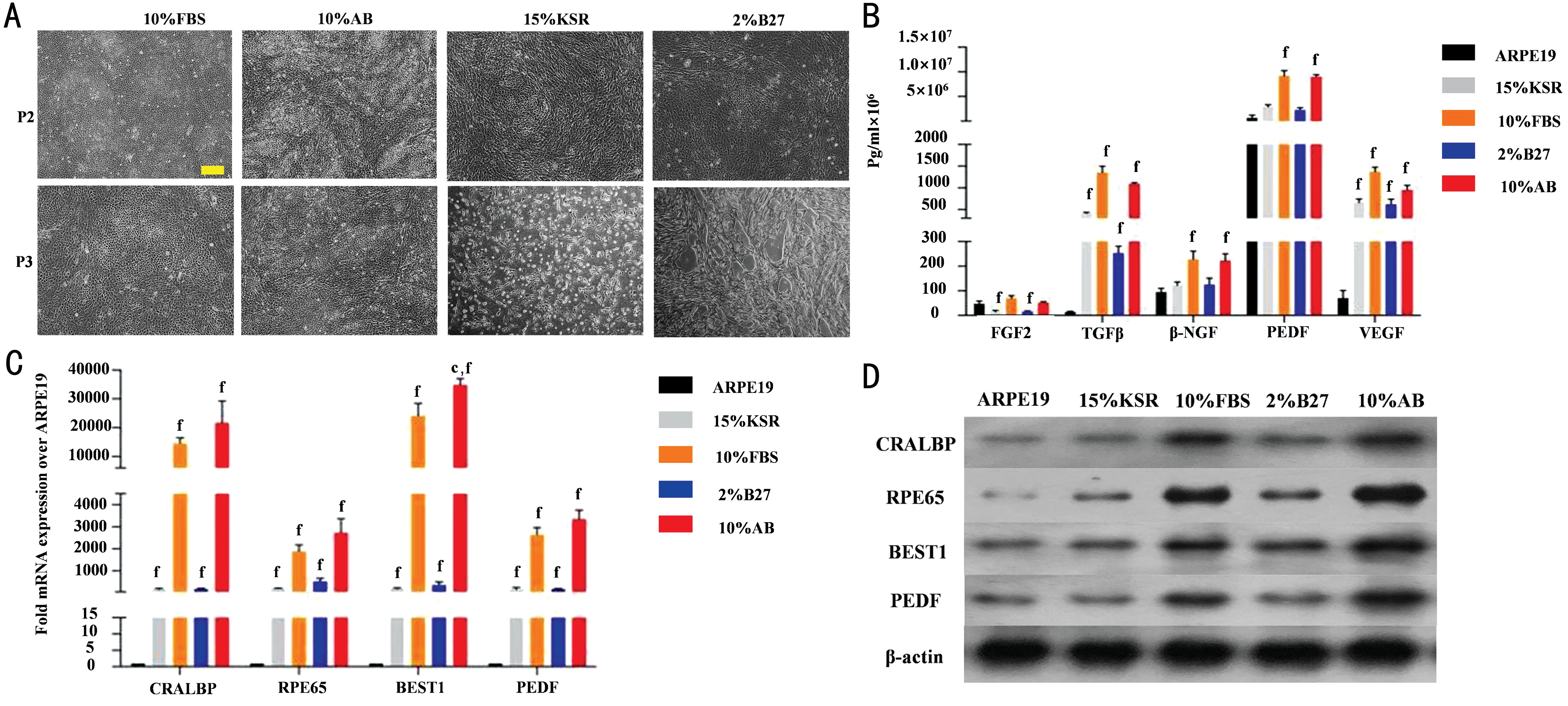
Figure 2 Differentiation and function of fRPE cells in B27, KSR and human AB serum A: Light microscope images of fRPE cells cultured in 2% B27, 15% KSR, 10% human AB serum and 10% FBS-supplemented mediums at P2 and P3. Scale bar=20 μm. B: ELISA results showing the secreted protein level of fRPE-derived trophic factors (FGF2, TGFβ, β-NGF, PEDF and VEGF). C, D: qPCR and Western blot analyses of RPE-specific markers (CRALBP, RPE65, BEST1, PEDF) in ARPE19 cells as well as fRPE cells cultured in 2% B27, 15% KSR, 10% human AB serum and 10% FBS-supplemented mediums at P2. fP<0.001 vs ARPE19 cells; cP<0.01 vs 10% FBS group. All experiments were repeated three times; error bars indicate SD.
DISCUSSION
Our findings provided a novel xeno-free culture system for human fRPE cells to meet GMP standard, so that fRPE cells can be transplanted to treat RDDs patients. To replace FBS,B27, human AB serum and KSR were added into culture medium as substitutes. We first screened out the optimum working concentration of B27, AB serum and KSR by examining proliferation ability and apoptosis of fRPE cells(Figure 1). Then we compared morphology and function of fRPE cells between FBS group and B27, AB serum, KSR groups. Paracrine function is known as one of RPE critical functions, because many functions of RPE cells attribute to RPE trophic factors secretion: TGF-β plays a pivotal role in immune privilege[11], PEDF, β-NGF and FGF2 promote RPE proliferation and protect RPE from oxidative stress[12-14], PEDF,TGF-β and VEGF regulate angiogenesis[15], PEDF, FGF2 and β-NGF support photoreceptor[16-17]. What's more, studies have demonstrated photoreceptor rescue effect by exogenous trophic factor administration in RDDs animal models: FGF2 injection preserved degenerated photoreceptors[18]; NGF regressed retinal degeneration by stimulation of other endogenous biological mediators[19]; PEDF showed antiapoptotic effect on photoreceptors[20]. Other functions of fRPE cells were detected by examining the expression of typical RPE markers: the expression of RPE65 and CRALBP associates with retinoid cycle, the expression of BEST1 associates with chloride channels, the expression of PEDF associates neurotrophic and anti-angiogenic effect. Our results revealed that fRPE cells cultured in 15% KSR and 2% B27 media showed repressed propagation and differentiation ability. These fRPE cells gradually lost epithelial morphology and RPE function, and could only survive to P2 (Figure 2).
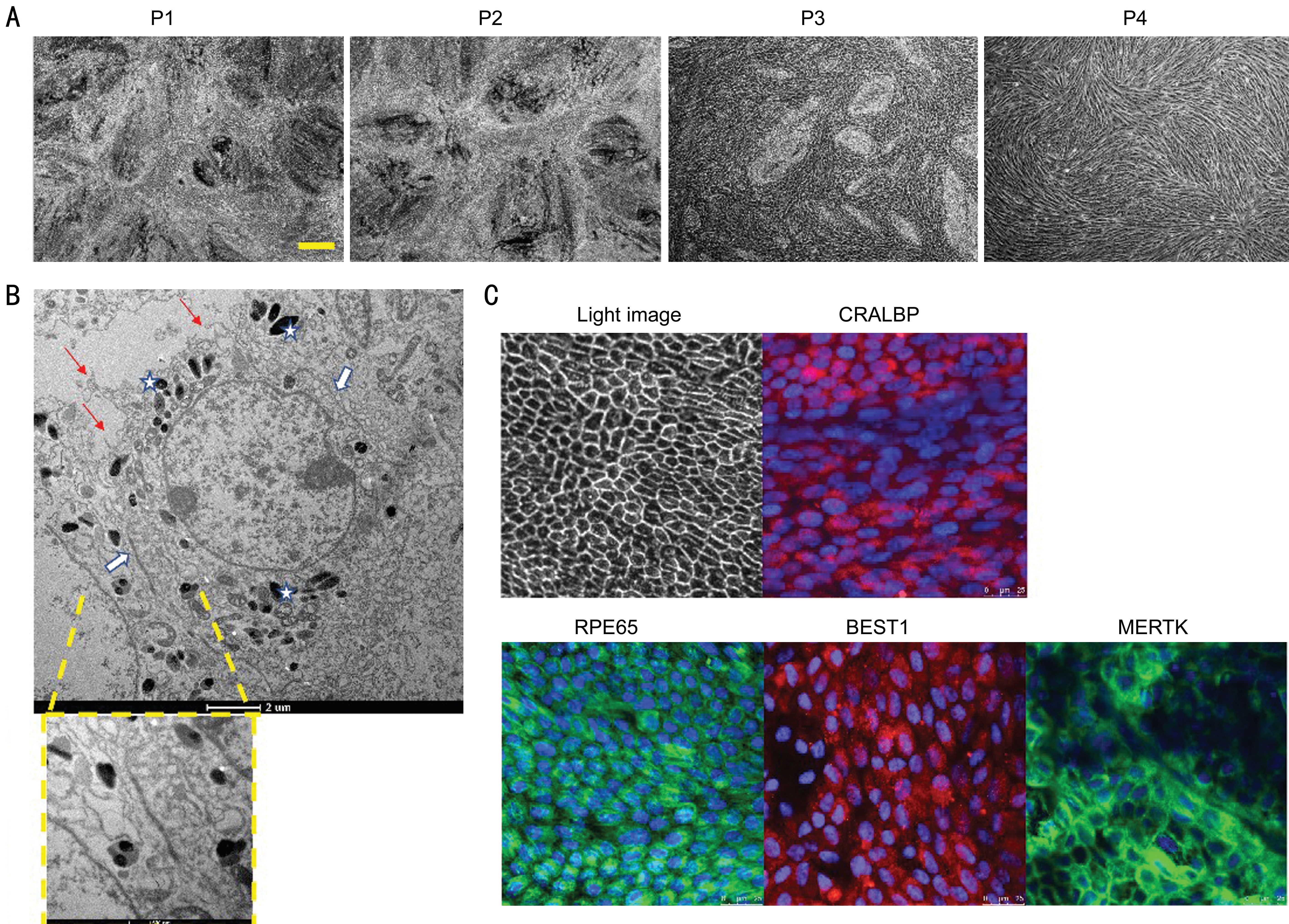
Figure 3 Characterization of xeno-free culture system-generated fRPE cells A: Light microscope images showing the morphology and density of fRPE cells cultured in 10% AB serum mediums for three weeks at P2, P3 and P4. Scale bar=20 μm. B: Electron micrograph showing cell morphology and structure of xeno-free culture system-generated fRPE cells. RPE-specific structures are highlighted: apical microvilli (red arrows), melanosomes (white stars) and tight junctions (white arrows). Scale bars represent 2 μm and 500 nm respectively. C: Fluorescence microscope view showing the expression of RPE-specific markers: CRALBP, RPE65, BEST1 and MERTK. Scale bar=25 μm.
Finally, we generated fRPE cells using 10% human AB serumsupplemented culture medium from P0. A typical cobblestone morphology with pigment was shown in fRPE cells from P0 to P3. At P4, fRPE cells underwent epithelial to mesenchymal transition (EMT) by dedifferentiation into spindle-shaped cells resembling fibroblasts and failure to reach confluency. EMT can also be observed in intraocular fibrotic diseases (such as epiretinal membrane), traditional cultured fRPE cells and RPE cells generated from pluripotent sources (including embryonic stem cells and pluripotent stem cell)[21-23]. This transition is result from wound-healing response and higher oxidative stress in vitro culture progress, as well as unknown hormones,cytokines and growth factors in culture mediums[24-25]. Studies have elucidated that epithelial-mesenchymal transition (EMT)can be repressed by adding TGF-β receptor inhibitor and Rhoassociated and coiled-coil protein kinase (ROCK) inhibitor into RPE culture medium[22,26]. Additionally, the expression of RPE-specific markers was demonstrated and functional RPE structure can be observed: apical microvilli help phagocytosing shed photoreceptor outer segments, tight junctions help building blood-ocular barrier (Figure 3).
In summary, our study indicated that culturing fRPE cells in 10% human AB serum medium was more favorable compared with KSR medium, B27 and traditional FBS medium when fRPE cells are to be applied in cell-based therapy. Further study will be needed to confirm the safety and effectivity of xeno-free culture system-generated fRPE cell transplantation in treating RDDs.
ACKNOWLEDGEMENTS
We thank colleagues from Ethics Committee of Jiangsu Province Hospital and National Stem Cell Clinical Trial Base in Jiangsu Province Hospital, for their supervision and administration. We thank medical workers in the department of Obstetrics and Gynecology in Jiangsu Province Hospital, for doctor-patient communication and specimens conservation.
Foundation: Supported by National Key Research and Development Program of China (No.2017YFA0104101).
Conflicts of Interest: Shen H, None; Wang M, None; Li D,
None; Yuan ST, None; Liu QH, None.
1 Ben M'Barek K, Habeler W, Plancheron A, Jarraya M, Regent F, Terray A, Yang Y, Chatrousse L, Domingues S, Masson Y, Sahel JA, Peschanski M, Goureau O, Monville C. Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med 2017;9(421). pii: eaai7471.
2 Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R,Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV,Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015;385(9967):509-516.
3 Liao JL, Yu JH, Huang K, Hu J, Diemer T, Ma ZC, Dvash T, Yang XJ,Travis GH, Williams DS, Bok D, Fan GP. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet 2010;19(21):4229-4238.
4 Maminishkis A, Chen S, Jalickee S, Banzon T, Shi GP, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci 2006;47(8):3612-3624.
5 Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schäfer R,Sella S, Rodeghiero F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther 2016;7(1):93.
6 Cánovas D, Bird N. Human AB serum as an alternative to fetal bovine serum for endothelial and cancer cell culture. ALTEX 2012;29(4):426-428.
7 dos Santos VTM, Mizukami A, Orellana MD, Caruso SR, da Silva FB,Traina F, de Lima Prata K, Covas DT, Swiech K. Characterization of human AB serum for mesenchymal stromal cell expansion. Transfus Med Hemother 2017;44(1):11-21.
8 Garcia-Gonzalo FR, Izpisúa Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One 2008;3(1):e1384.
9 Liu F, Cai CH, Wu X, Cheng YX, Lin T, Wei GH, He DW. Effect of KnockOut serum replacement on germ cell development of immature testis tissue culture. Theriogenology 2016;85(2):193-199.
10 Gamm DM, Melvan JN, Shearer RL, Pinilla I, Sabat G, Svendsen CN, Wright LS. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2008;49(2):788-799.
11 Hirsch L, Nazari H, Sreekumar PG, Kannan R, Dustin L, Zhu DH,Barron E, Hinton DR. TGF-β2 secretion from RPE decreases with polarization and becomes apically oriented. Cytokine 2015;71(2):394-396.
12 Wang X, Liu X, Ren Y, Liu Y, Han SY, Zhao JK, Gou XC, He Y. PEDF protects human retinal pigment epithelial cells against oxidative stress via upregulation of UCP2 expression. Mol Med Rep 2019;19(1):59-74.
13 Li WS, Wen J, Jiang DY, Ding JG. Effects of nerve growth factor on proliferation and DNA synthesis of cultured human fetal retinal pigment epithelium cells. Yan ke xue bao 2002;18(1):45-48,29.
14 Tsao YP, Ho TC, Chen SL, Cheng HC. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci 2006;79(6):545-550.
15 Jeong HS, Yun JH, Lee DH, Lee EH, Cho CH. Retinal pigment epithelium-derived transforming growth factor-β2 inhibits the angiogenic response of endothelial cells by decreasing vascular endothelial growth factor receptor-2 expression. J Cell Physiol 2019;234(4):3837-3849.
16 Di Pierdomenico J, Scholz R, Valiente-Soriano FJ, Sánchez-Migallón MC, Vidal-Sanz M, Langmann T, Agudo-Barriuso M, García-Ayuso D,Villegas-Pérez MP. Neuroprotective effects of FGF2 and minocycline in two animal models of inherited retinal degeneration. Invest Ophthalmol Vis Sci 2018;59(11):4392-4403.
17 Polato F, Becerra SP. Pigment epithelium-derived factor, a protective factor for photoreceptors in vivo. Adv Exp Med Biol 2016;854:699-706.
18 Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 1990;347(6288):83-86.
19 Lenzi L, Coassin M, Lambiase A, Bonini S, Amendola T, Aloe L.Effect of exogenous administration of nerve growth factor in the retina of rats with inherited retinitis pigmentosa. Vision Res 2005;45(12):1491-1500.
20 Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Sakamoto T, Tabata T, Ueda Y, Hasegawa M, Tobimatsu S, Ishibashi T, Sueishi K. Simian lentiviral vector-mediated retinal gene transfer of pigment epitheliumderived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther 2003;10(17):1503-1511.
21 Shih YH, Radeke MJ, Radeke CM, Coffey PJ. Restoration of mesenchymal RPE by transcription factor-mediated reprogramming.
Invest Ophthalmol Vis Sci 2017;58(1):430-441.
22 Radeke MJ, Radeke CM, Shih YH, Hu J, Bok D, Johnson LV,Coffey PJ. Restoration of mesenchymal retinal pigmented epithelial cells by TGFβ pathway inhibitors: implications for age-related macular degeneration. Genome Med 2015;7(1):58.
23 Kole C, Klipfel L, Yang Y, Ferracane V, Blond F, Reichman S, Millet-Puel G, Clérin E, Aït-Ali N, Pagan D, Camara H, Delyfer MN, Nandrot EF, Sahel JA, Goureau O, Léveillard T. Otx2-genetically modified retinal pigment epithelial cells rescue photoreceptors after transplantation. Mol Ther 2018;26(1):219-237.
24 Tian HB, Xu JY, Tian Y, Cao YQ, Lian CP, Ou QJ, Wu BX, Jin CX,Gao FR, Wang J, Zhang JP, Zhang JF, Li WY, Lu LX, Xu GT. A cell culture condition that induces the mesenchymal-epithelial transition of dedifferentiated porcine retinal pigment epithelial cells. Exp Eye Res 2018;177:160-172.
25 Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelialmesenchymal transition. Nat Rev Mol Cell Biol 2014;15(3):178-196.
26 Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y. Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells. Curr Mol Med 2014;14(4):523-534.