INTRODUCTION
M eningioma is a primary brain tumor derived from arachnoid cells[1-3]. Meningiomas have slow growth properties that make them undetectable in causing symptoms of tumor compression[1]. Orbitocranial meningiomas can be classified by their location into primary, inferorbital and spheno-orbital optic meningiomas[4]. Rapid growth, infiltration to adjacent structures (connective tissue, bone, and brain tissue) can also occur in benign to malignant progression[2].
Progression of meningiomas has been known as the result of NF2 gene mutation[5]. NF2 gene analysis study has found that somatic mutation of meningioma (monosomy and chromosome deletion 22q) underlies and supports the hypothesis that NF2 serves as a tumor suppressor in meningioma tumorigenesis[6-7].Chromosomal instability in meningioma can be used as a marker for benign to malignant transformation[8]. NF2 gene mutation on chromosome 22 leads to phenotypic changes from arachnoid cell hyperplasia to benign meningiomas with 70%-80% fibroblastic (and transitional) histopathologic types and only 25% meningothelial type[9].
Possible role of steroid hormones and their receptors in the pathogenensis of meningiomas is remain unclear and reveals inconclusive conclusions regarding its developmental mechanisms and role in the risk of recurrent cases[10-12]. The progesterone receptor (PR) status has been known to be associated with a gene mutation on chromosome 22 (at a locus adjacent to the NF2 gene) which has been identified as the initial sign of meningioma tumorigenesis[13]. Those findings suggest that PR status could be used as clinical marker of genetic mutation of meningiomas which requires further study.Therefore, this study aimed to investigate the NF2 gene mutation at mRNA level and reveal the association between NF2 gene mutation and progesterone and estrogen receptors(ER) mRNA expression in meningioma. It might reveal whether orbitocranial meningioma is associated by intrinsic sporadic NF2 mutation or due to extrinsic factors (hormonal contraceptives exposure) as resembled by hormone receptors status (PR and ER).
SUBJECTS AND METHODS
Ethical Approval This study followed the tenets of the Declaration of Helsinki (2008) and was approved by Ethical Review Board of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada (Approval No: KE/FK/178/EC/2016). Written informed consent was obtained from all study participants.
Research Design and Subjects This study was an analytic observational study with case-control design. The tissue samples were taken consecutively from histopathologic specimen of meningioma tissue originating from patients diagnosed with sporadic orbitocranial meningioma (not associated with any familial neurofibromatosis 2) underwent lateral orbitotomy at Dr. Sardjito General Hospital and dr.Yap Eye Hospital, Yogyakarta, Indonesia from 2010 to 2016.Patients' basic characteristics were taken from the medical records which were corresponding to their histopathologic specimens. Patients whose tissues were included in the inclusion criteria were then contacted for interviews for their history of obstetric and gynecologic as well as their history of hormonal contraception using a questionnaire. Case group was the orbitocranial meningioma tumor tissue with mutations of the NF2 gene and the control group was without mutations of the NF2 gene. The inclusion criteria of this study were female patients, aged 20-69y at the time of diagnosis, orbitocranial meningioma diagnosis was established histopathologically,accurate medical record data, can be contacted for a structured interview and provide accurate data on appropriate questions in the questionnaire. Exclusion criteria of this study were patients who did not meet inclusion criteria, damaged paraffin blocks,insufficient cutting, incomplete medical record data and the patient cannot be contacted for interviews.
Quantitative Polymerase Chain Reaction Paraffin blocks of meningioma tissue were cut as thick as 4 µm. NF2 mutation was examined using quantitative PCR with Qbiomarker Somatic Mutation PCR Assay (4 cytoband coordinates:c.634C>T/p.Q212, c.655G>A/p.V219M, c.784C>T/p.R262,and c.1228C>T/p.Q410). Quantitative PCR examination was also performed to determine ER and PR expression after RNA extraction, amplification of cDNA was then performed for cDNA quantification (DTlite4, DNA-Technolog, Russia).Primer sets were GAPDH as a reference (GAPDH forward: 5'-GCA TCC TGG GCT ACA CTG AG-3'; GAPDH reverse: 5'-TCC ACC ACC CTG TTG CTG TA-3'), PR (forward: 5'-AGC TCA TCA AGG CAA TTG GTT T-3'; reverse: 5'-ACA AGA TCA TGC AAG TTA TCA AGA AGT T-3'), ERα (forward:5'-TCA CAT CTG TAT GCG GAA CC-3'; reverse: 5'- CGT AAC ACT TCC GAA GTC GG-3'), ERβ (forward: 5'-AGA CAT GAG AGC TGC CAA CC-3'; reverse: 5'-GCC AGG CAC ATT CTA GAA GG-3') and NF2 (forward: 5'-CCC CCA ACT CCC CTT TCC-3'; reverse: 5'-AGC CCT TTA GCC CCC CTG-3').
Statistical Analysis Continuous variables (e.g. age,gynecological history, and history of hormonal contraceptive use as well as PR and ER expression) were tested by Mann-Whitney test and categorical variables (sex, marital status, level of education, occupation, income, degree and histopathologic type of tumor) were tested using Fisher-Exact test. Odds ratio(OR) was obtained using univariate and multivariate logistic regression test. The P<0.05 was considered significant.
RESULTS
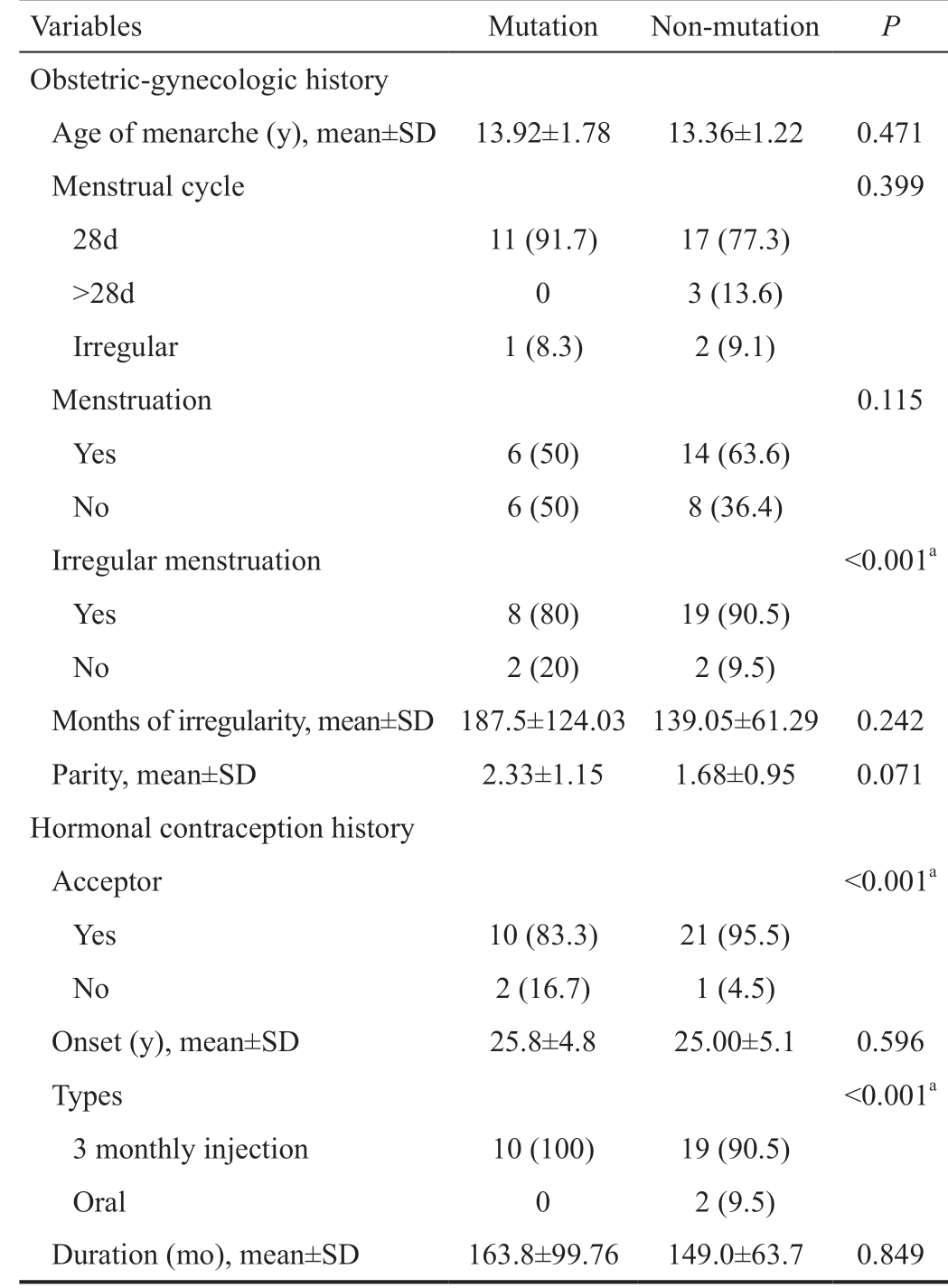
Thirty-four orbitocranial meningiomas tissues met the inclusion criteria. NF2 gene mutations were present in 35.29% (12 cases). The mean age of all subjects was 45.08±6.51y. There were no significant differences in age, education, occupation,family history of cancer, and eye complaints between the 2 groups (Table 1). The mean age of menarche of all subjects was 13.56±1.44y. There was no significant difference in age of menarche, menstrual cycle, menstrual period, and parity between 2 groups. There was a statistically significant difference in the proportion of irregular menstruation between 2 groups (P<0.001) as well as in the proportion of contraceptive acceptors between mutation and non-mutation groups [non-acceptor in the mutation group (16.7%) vs nonmutation (4.5%), P<0.001; Table 2]. There was statistically significant difference in the proportion of contraceptive use(the use of 3-monthly injections; mutation vs non-mutation:100% vs 90.5%, P<0.001). The mean duration of hormonal contraceptive use was 153.77±75.76mo or ±12y and mean onset of hormonal contraceptive use was 25.26±4.92y (no significant difference between 2 groups; Table 2).
Table 1 Subject characteristics n (%)
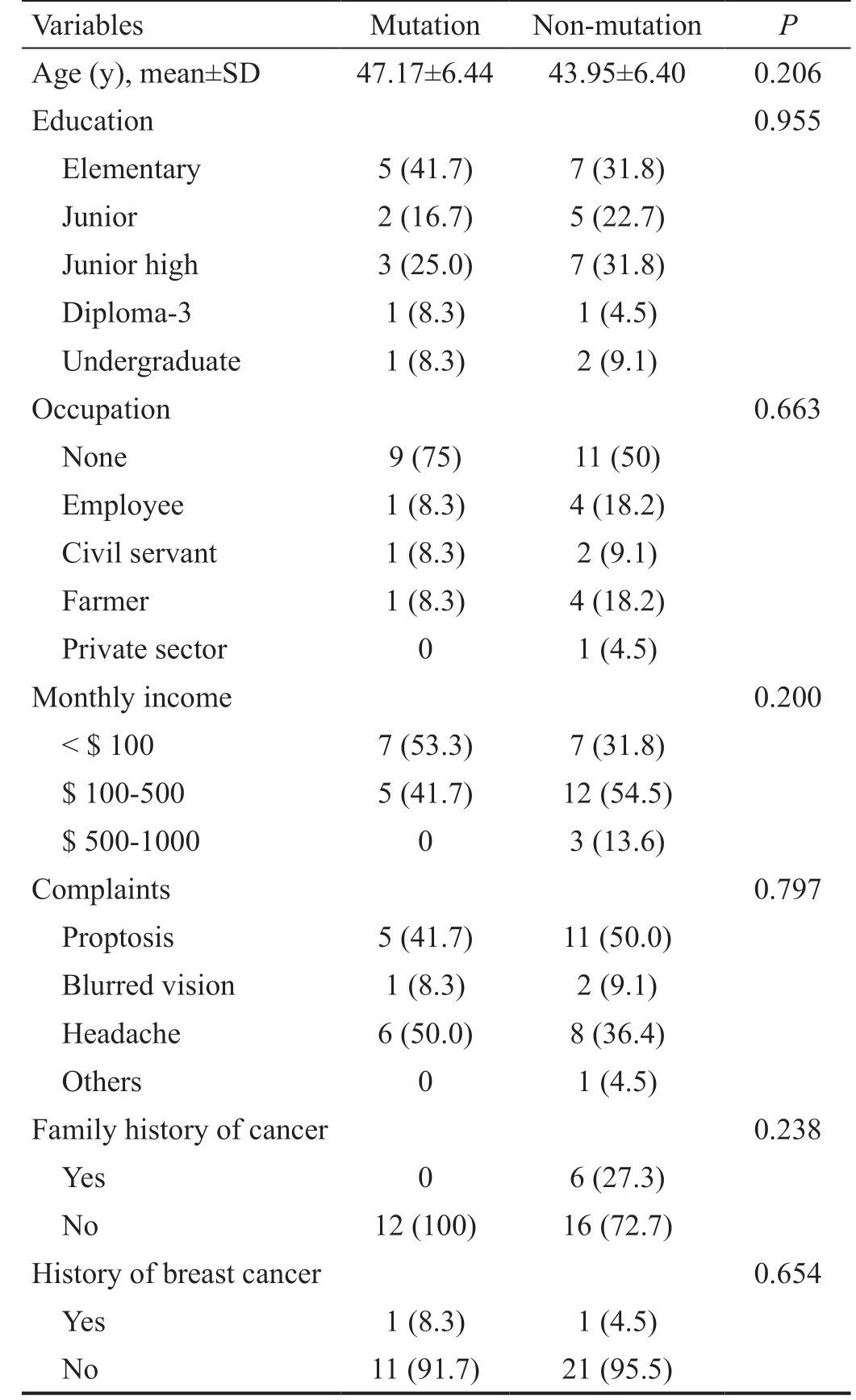
Variables Mutation Non-mutation P Age (y), mean±SD 47.17±6.44 43.95±6.40 0.206 Education 0.955 Elementary 5 (41.7) 7 (31.8)Junior 2 (16.7) 5 (22.7)Junior high 3 (25.0) 7 (31.8)Diploma-3 1 (8.3) 1 (4.5)Undergraduate 1 (8.3) 2 (9.1)Occupation 0.663 None 9 (75) 11 (50)Employee 1 (8.3) 4 (18.2)Civil servant 1 (8.3) 2 (9.1)Farmer 1 (8.3) 4 (18.2)Private sector 0 1 (4.5)Monthly income 0.200< $ 100 7 (53.3) 7 (31.8)$ 100-500 5 (41.7) 12 (54.5)$ 500-1000 0 3 (13.6)Complaints 0.797 Proptosis 5 (41.7) 11 (50.0)Blurred vision 1 (8.3) 2 (9.1)Headache 6 (50.0) 8 (36.4)Others 0 1 (4.5)Family history of cancer 0.238 Yes 0 6 (27.3)No 12 (100) 16 (72.7)History of breast cancer 0.654 Yes 1 (8.3) 1 (4.5)No 11 (91.7) 21 (95.5)
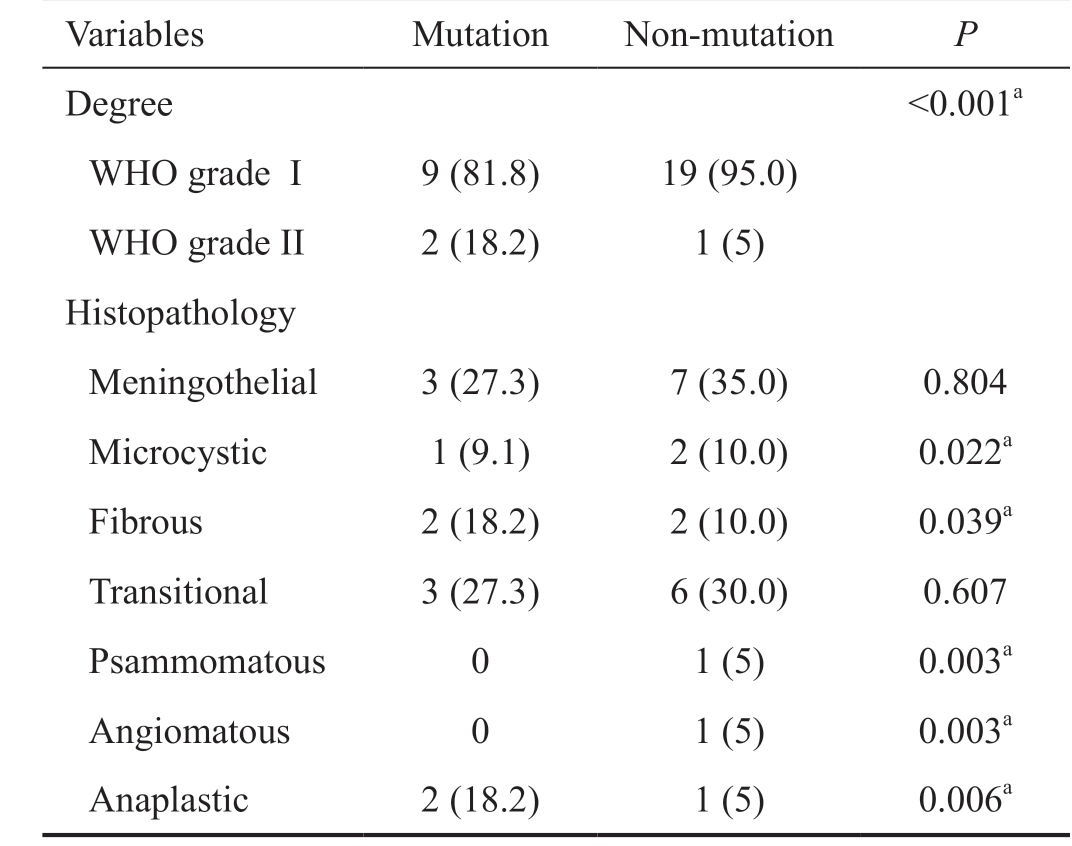
There was a statistically significant difference in meningioma histology degree between the 2 groups (P<0.001; Table 3).There was a trend (0.5<P<0.1) in serum and tumor ERα levels (serum and tumor: P=0.079 and P=0.060, respectively).Serum PR in the mutation group was significantly higher than the non-mutation group (P=0.033; Figure 1). There were no significant differences in serum ER mRNA between mutation and non-mutation groups (30.33±1.19 vs 29.92±0.99, P=0.357;Table 4).
The present study was limited to detecting the presence or absence of NF2 mutations (qualitatively) from the 4 cytoband coordinates for nucleotide change (c.634C>T/p.Q212,c.655G>A/p.V219M, c.784C>T/p.R262, and c.1228C>T/p.Q410). NF2 mutations were found in c.634C>T/p.Q212(50%) and c.1228C>T/p.Q410 (41.67%). NF2 expression was 30.33±1.19 and was not associated with NF2 mutation(P=0.294; OR=0.697, 95%CI: 0.355-1.368). NF2 mutations in cytoband c.655G>A/p.V219M and c.784C>T/p.R262 were significantly associated with meningothelial and transitional histopathology type (Table 5). In this study, there were no significant confounding factors statistically interfering with the results of the study (Table 6).
Table 2 Obstetric-gynecologic and hormonal contraception history n (%)
aSignificant statistically.
Variables Mutation Non-mutation P Obstetric-gynecologic history Age of menarche (y), mean±SD 13.92±1.78 13.36±1.22 0.471 Menstrual cycle 0.399 28d 11 (91.7) 17 (77.3)>28d 0 3 (13.6)Irregular 1 (8.3) 2 (9.1)Menstruation 0.115 Yes 6 (50) 14 (63.6)No 6 (50) 8 (36.4)Irregular menstruation <0.001a Yes 8 (80) 19 (90.5)No 2 (20) 2 (9.5)Months of irregularity, mean±SD 187.5±124.03 139.05±61.29 0.242 Parity, mean±SD 2.33±1.15 1.68±0.95 0.071 Hormonal contraception history Acceptor <0.001a Yes 10 (83.3) 21 (95.5)No 2 (16.7) 1 (4.5)Onset (y), mean±SD 25.8±4.8 25.00±5.1 0.596 Types <0.001a 3 monthly injection 10 (100) 19 (90.5)Oral 0 2 (9.5)Duration (mo), mean±SD 163.8±99.76 149.0±63.7 0.849
Table 3 Degree and type of histopathology of tumor n (%)
aSignificant statistically.
Variables Mutation Non-mutation P Degree <0.001a WHO grade I 9 (81.8) 19 (95.0)WHO grade II 2 (18.2) 1 (5)Histopathology Meningothelial 3 (27.3) 7 (35.0) 0.804 Microcystic 1 (9.1) 2 (10.0) 0.022a Fibrous 2 (18.2) 2 (10.0) 0.039a Transitional 3 (27.3) 6 (30.0) 0.607 Psammomatous 0 1 (5) 0.003a Angiomatous 0 1 (5) 0.003a Anaplastic 2 (18.2) 1 (5) 0.006a
Table 4 ER serum and tissue expression
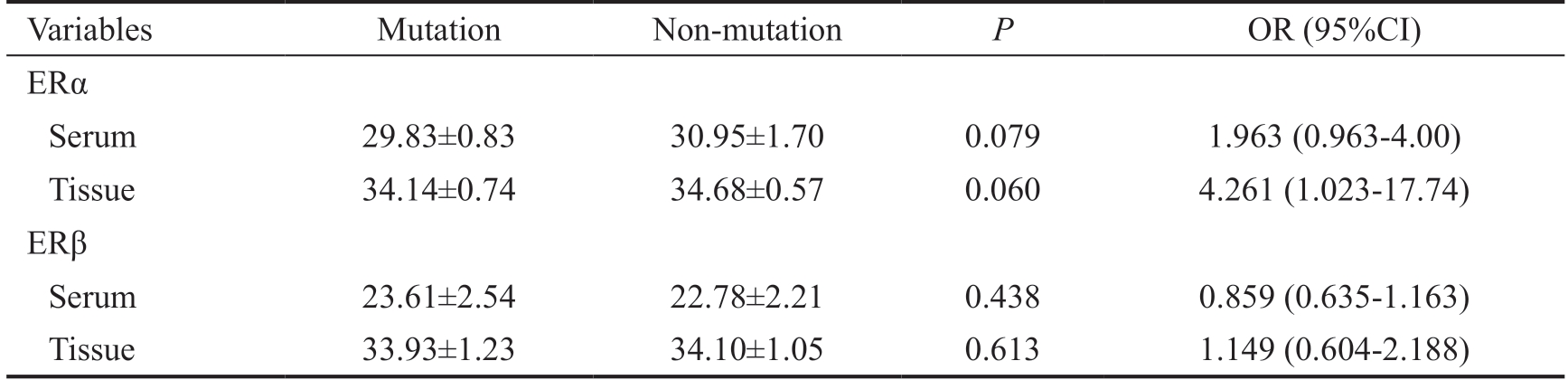
Variables Mutation Non-mutation P OR (95%CI)ERα Serum 29.83±0.83 30.95±1.70 0.079 1.963 (0.963-4.00)Tissue 34.14±0.74 34.68±0.57 0.060 4.261 (1.023-17.74)ERβ Serum 23.61±2.54 22.78±2.21 0.438 0.859 (0.635-1.163)Tissue 33.93±1.23 34.10±1.05 0.613 1.149 (0.604-2.188)
Table 5 Histopathology of meningioma and cytoband coordinates for nucleotide change of NF2 mutation

a Statistically signifciant.
Histopathology c.634C>T/p.Q212 c.655G>A/p.V219M c.784C>T/p.R262 c.1228C>T/p.Q410 Meningothelial 0.388 0.012a 0.039a 0.267 Microcystic 0.508 0,625 1.000 0.727 Fibrous 0.727 0,375 0.687 1.000 Transitional 0.549 0.008a 0.039a 0.388 Psammomatous 0.125 1.000 1.000 0.219 Angiomatous 0.125 1.000 1.000 0.219 Anaplastic 0.219 1.000 1.000 0.453
Table 6 Confounding analysis
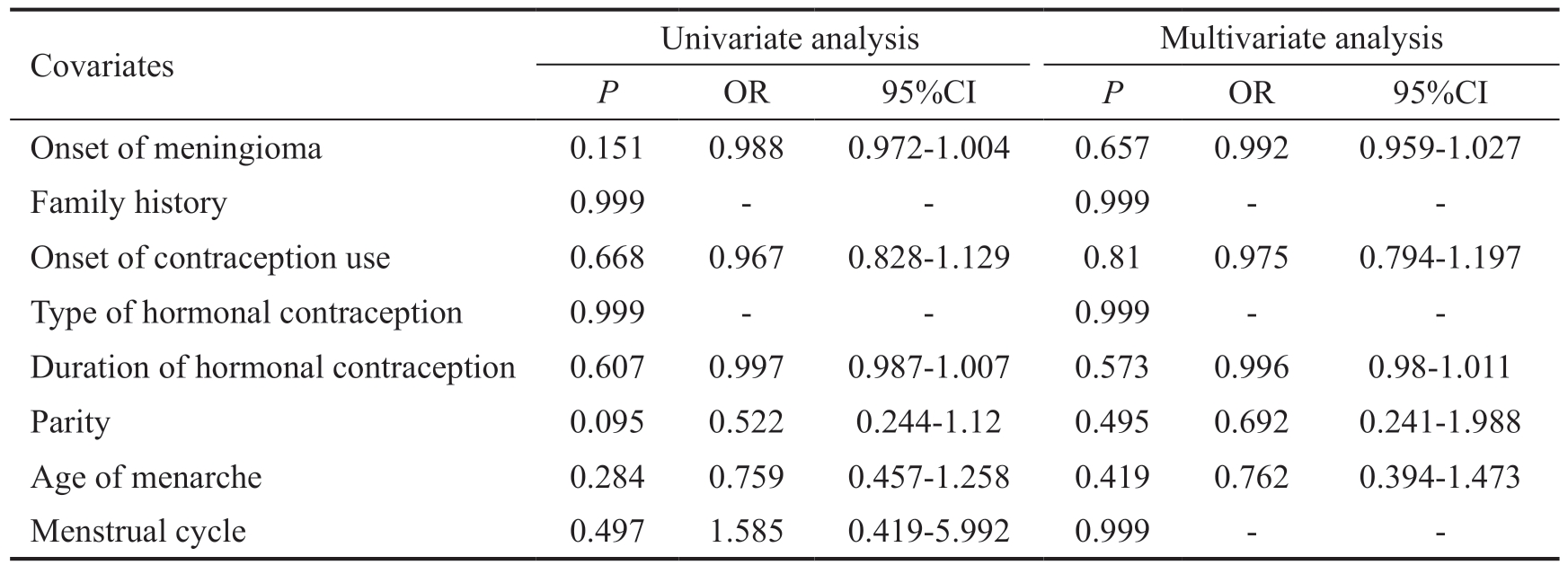
Covariates Univariate analysis Multivariate analysis P OR 95%CI P OR 95%CI Onset of meningioma 0.151 0.988 0.972-1.004 0.657 0.992 0.959-1.027 Family history 0.999 - - 0.999 - -Onset of contraception use 0.668 0.967 0.828-1.129 0.81 0.975 0.794-1.197 Type of hormonal contraception 0.999 - - 0.999 - -Duration of hormonal contraception 0.607 0.997 0.987-1.007 0.573 0.996 0.98-1.011 Parity 0.095 0.522 0.244-1.12 0.495 0.692 0.241-1.988 Age of menarche 0.284 0.759 0.457-1.258 0.419 0.762 0.394-1.473 Menstrual cycle 0.497 1.585 0.419-5.992 0.999 - -
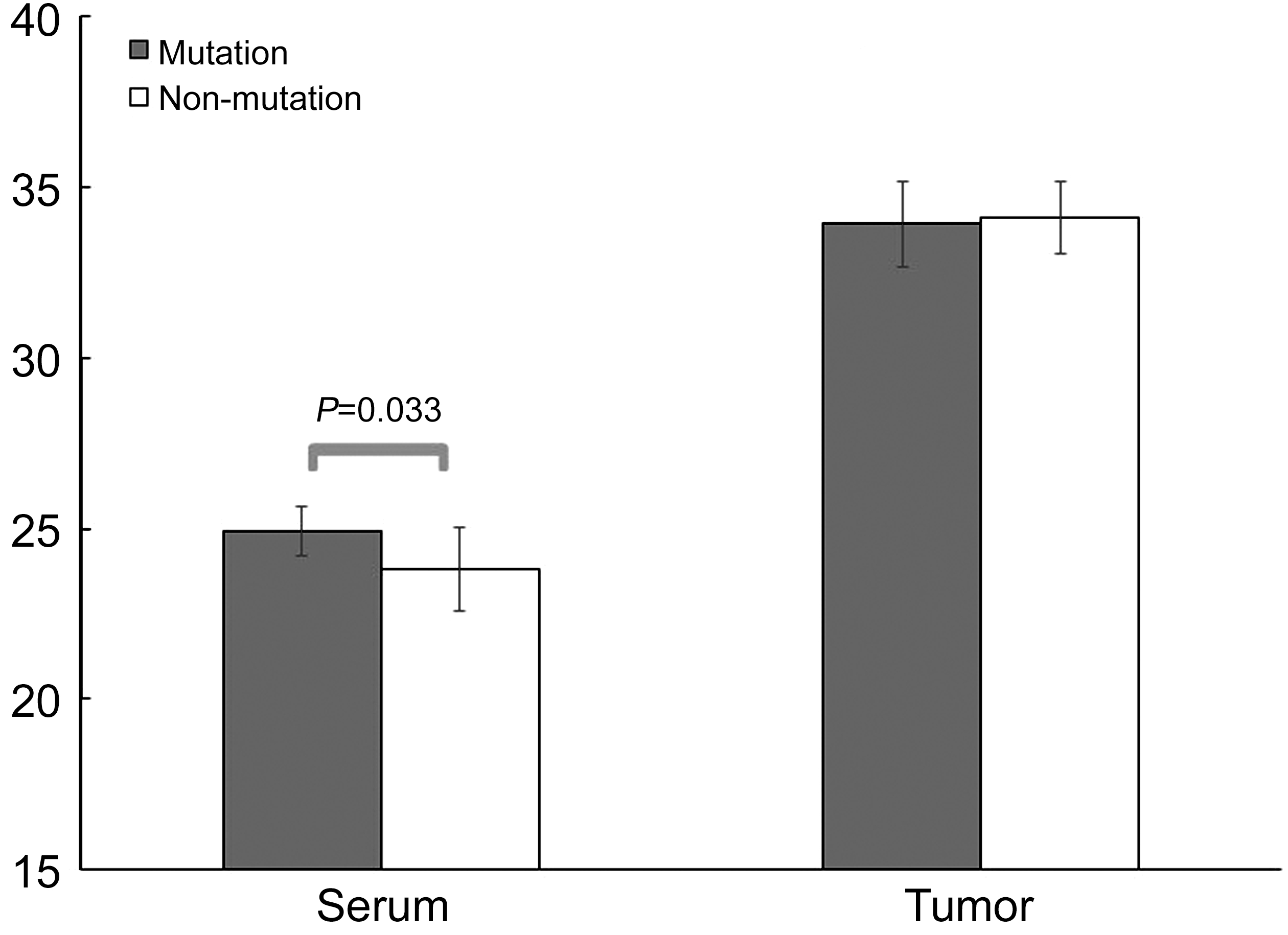
Figure 1 PR mRNA expression in NF2 mutation and non NF2 mutation meningioma Significant mean difference of serum NF2 between mutation and non-mutation groups (P=0.033).
DISCUSSION
This study was the first study investigating NF2 mutation at the level of mRNA and its association with mRNA PR which might become an important method for future investigation using serum (“liquid biopsy”). The result of NF2 mutation in the present study was in line with a previous study in which the mutation of NF2 gene (on chromosome 22) was found in 30% to 70% of sporadic meningiomas[8]. The most common genetic alteration found in meningiomas involving NF2 gene on the 22q chromosome where the incidence of NF2 mutations can in the form of insertion, deletion, or nonsense mutation[14].The loss of the NF2 gene product (merlin) is considered to be an early event in the pathogenesis of sporadic meningiomas.Tumor suppressor gene inactivation might need bi-allelic NF2 inactivation that is NF2 intrinsic mutation and exogenous events[14] such as chronic exposure to exogenous progesterone.The orbitocranial meningioma is arising from the skull base, so called sphenoid-orbital meningiomas, which is more associated with the incidence of chromosome 22q mutations and has more aggressive clinical course than meningiomas on the cerebrum,cerebellum and lateral skull surfaces[4]. However, intracranial meningiomas might exhibit more heterogeneous and complex chromosome abnormalities associated with more than one tumor cell clone[15].
Gynecological history is a marker of endogenous hormonal exposure and the use of hormonal contraceptives is a marker of exogenous hormonal exposure. However, in this study it was found that endogenous hormonal factors (age of menarche,menstrual cycle and menstrual period, and parity) were not related to NF2 mutation. A study by Claus et al[12] revealed that age of menarche is not associated with the risk of meningioma.The evidence related to the role of endogenous progesterone in the meningioma has been revealed by the finding of association of PR+ cases with the incidence of meningioma[16]. There is a sign of the role of endogenous progesterone hormone that is the worsening symptoms during the luteal phase of the menstrual cycle and during pregnancy. However, previous research had shown the associated risk of meningioma in patients with hormonal contraceptive use (oral, sub-dermal implants, injection, and intrauterine devices with hormones)[17]as well as in users of hormone replacement therapy.
The role of sex hormones was revealed by the finding that 88% of primary meningiomas are positive PR and 40%positive ER[18]. The hormone receptor association with NF2 gene mutation in the tumorigenesis of meningioma was known from the presence of a chromosome 22q mutation with the condition of ER-/PR- or ER+/PR+[13]. Negative or low PR expression might be associated with a history of chronic exposure to exogenous progesterone. Therefore, meningioma tumorigenesis in the non-mutation group might be assumed to be due to a chronic use of exogenous progesterone (hormonal contraceptives). While in the tumorigenesis in mutation group might be underlined merely on intrinsic factor (intrinsic NF2 gene mutation). The loss of PR is associated with a higher degree of meningioma which can be identified by MIB-1/Ki-67[19]. Increased MIB-1 was also associated with an increased recurrence risk in individuals who had previously been performed tumor resection[19].
NF2 mutations has been known to be associated with a higher degree of meningioma and increased risk of recurrence rate[20].In the previous study, NF2 mutation was found in 17% to 38% grade II meningiomas and characterized by mutations not only on the 22q chromosome but also at 1p, 6q, and 14q[8].Atypical meningiomas are associated with loss of 1p, 6q,10q, 14q, 17p and 18q and gains at 20q, 12q, 15q, 1q, 9q and 17q[16]. The most common types of histopathologic (subtypes)in meningiomas are fibrous, transitional, and meningotelial.NF2 mutation was associated with fibroblastic and transitional histopathologic types[20]. In this study the highest proportion of meningothelial and transitional is 27.3%, followed by fibrous and anaplastic as much as 18.2%. NF2 mutations are known to be a result in increased production of collagen and extracellular matrix causing a histopathologic type associated with collagencontaining meningiomas such as fibrous and transitional[13].
In the present study the mutation characteristics such as homozygous deletions or neutral copies of LOH (loss of heterozygosity) were not investigated. The loss of allele loss on chromosome 22q12.2 (which includes the NF2 gene) is found in 40%-70% of sporadic meningiomas and most of the NF2-associated meningiomas[14]. In a previous study, it was found that only 15% meningiomas without LOH chromosome 22 had NF2 mutations[21]. Meningioma pathogenesis was not only independent of NF2 mutation, but also strongly associated with exogenous progesterone exposure. Benign to malignant meningioma progression was found to be due to NF2 mutation. Mutation analysis at mRNA level was a simple and reliable method to investigate mutation using patients'serum and promising method for so called a “liquid biopsy”.NF2 mutations can be used for early identification of tumor progression, planning of surgical intervention to prevent the development of meningiomas thereby reducing morbidity and mortality.
ACKNOWLEDGEMENTS
Conflicts of Interest: Supartoto A, None; Mahayana IT,None; Heriyanto DS, None; Sasongko MB, None; Respatika HD, None; Sakti DH, None; Nurlaila PS, None; Kusnanto H, None; Pawiroranu S, None; Haryana SM, None.
1 Olar A, Wani KM, Wilson CD, Zadeh G, DeMonte F, Jones DT, Pfister SM, Sulman EP, Aldape KD. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol 2017;133(3):431-444.
2 Yuzawa S, Nishihara H, Tanaka S. Genetic landscape of meningioma.Brain Tumor Pathol 2016;33(4):237-247.
3 Benson VS, Pirie K, Green J, Casabonne D, Beral V, Million Women Study Collaborators. Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. Br J Cancer 2008;99(1):185-190.
4 Ho CY, Mosier S, Safneck J, Salomao DR, Miller NR, Eberhart CG,Gocke CD, Batista DA, Rodriguez FJ. Genetic profiling by singlenucleotide polymorphism-based array analysis defines three distinct subtypes of orbital meningioma. Brain Pathol 2015;25(2):193-201.
5 Begnami MD, Palau M, Rushing EJ, Santi M, Quezado M. Evaluation of NF2 gene deletion in sporadic schwannomas, meningiomas, and ependymomas by chromogenic in situ hybridization. Hum Pathol 2007;38(9):1345-1350.
6 Torres-Martín M, Kusak ME, Isla A, Burbano RR, Pinto GR, Melendez B, Castresana JS, Rey JA. Whole exome sequencing in a case of sporadic multiple meningioma reveals shared NF2, FAM109B, and TPRXL mutations, together with unique SMARCB1 alterations in a subset of tumor nodules. Cancer Genet 2015;208(6):327-332.
7 Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cell Biol 2009;29(6):1472-1486.
8 Goutagny S, Yang HW, Zucman-Rossi J, Chan J, Dreyfuss JM, Park PJ, Black PM, Giovannini M, Carroll RS, Kalamarides M. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res 2010;16(16):4155-4164.
9 Choy W, Kim W, Nagasawa D, Stramotas S, Yew A, Gopen Q, Parsa AT,Yang I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus 2011;30(5):E6.
10 Cea-Soriano L, Blenk T, Wallander MA, Rodríguez LA. Hormonal therapies and meningioma: is there a link? Cancer Epidemiol 2012;36(2):198-205.
11 Claus EB, Black PM, Bondy ML, Calvocoressi L, Schildkraut JM,Wiemels JL, Wrensch M. Exogenous hormone use and meningioma risk:what do we tell our patients? Cancer 2007;110(3):471-476.
12 Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL,Schildkraut JM. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg 2013;118(3):649-656.
13 Claus EB, Park PJ, Carroll R, Chan J, Black PM. Specific genes expressed in association with progesterone receptors in meningioma.Cancer Res 2008;68(1):314-322.
14 Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol 2010;99(3):379-391.
15 Sayagués JM, Tabernero MD, Maíllo A, Trelles O, Espinosa AB,Sarasquete ME, Merino M, Rasillo A, Vera JF, Santos-Briz A, de Alava E, Garcia-Macias MC, Orfao A. Microarray-based analysis of spinal versus intracranial meningiomas: different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol 2006;65(5):445-454.
16 Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016;17(9):e383-e391.
17 Korhonen K, Auvinen A, Lyytinen H, Ylikorkala O, Pukkala E. A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol 2012;175(4):309-314.
18 Korhonen K, Salminen T, Raitanen J, Auvinen A, Isola J, Haapasalo H. Female predominance in meningiomas can not be explained by differences in progesterone, estrogen, or androgen receptor expression. J Neurooncol 2006;80(1):1-7.
19 Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol 2006;5(12):1045-1054.
20 Ragel BT, Jensen RL. Molecular genetics of meningiomas. Neurosurg Focus 2005;19(5):E9.
21 Chowdhury T, Yoo Y, Seo Y, Dho YS, Kim S, Choi A, Choi M,Park SH, Park CK, Lee SH, Lee JY. Genomic analysis of synchronous intracranial meningiomas with different histological grades. J Neurooncol 2018;138(1):41-48.