INTRODUCTION
P erimetry, whether manual or automated, has remained a stable testing paradigm integral to the diagnosis and monitoring of glaucoma. The Heidelberg Edge Perimeter(HEP) is a newly developed modality for systematic visual field (VF) measurements, and has several novel features of note[1-2]. First, the HEP implements dynamic stimulus size variation during testing in order to accommodate glaucomatous subjects with loss of contrast sensitivity. Second, the HEP can integrate the results of the Spectralis optical coherence tomography (OCT) to detect clinically significant structurefunction correlations between anatomical assessments such as retinal nerve fiber layer (RNFL) thickness and minimum rim width and VF results[3]. In addition to standard automated perimetry (SAP), the HEP also offers a new stimulus in the Flicker Defined Form (FDF), which utilizes phase reversal of flickering black and white dots to generate a contour edge illusion. FDF has been found to be more sensitive than SAP in the detection of VF losses in early glaucoma, and demonstrated strong correlations with RNFL thickness and optic nerve structural measurements[4-8].
Due to the relative novelty of the HEP system, presently little is known about the ability of the HEP SAP to detect VF losses compared to SAP administered thorough validated systems.While a comparison between the HEP and the Humphrey Visual Field (HVF) Analyzer reported higher precision favoring the HEP, the HEP has not been directly compared to the Octopus Visual Field (OVF) Analyzer in a similar fashion[9]. The purpose of this study is to evaluate ability of the HEP to detect VF defects and to compare the test-retest repeatability of SAP using the HEP and OVF analyzers in subjects with glaucoma and unaffected controls. Validation against standardized VF analyzers like the Octopus is a pre-requisite for a new modality like the HEP before it can gain acceptance as a worthwhile investment for clinical practices and healthcare institutions.
SUBJECTS AND METHODS
Ethical Approval This prospective, cross-sectional study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the Institutional Review Board of the Wills Eye Hospital,Philadelphia, PA. Written informed consent was obtained from all subjects in accordance with the Health Insurance Portability and Accountability Act (HIPAA).
Study Subjects and Clinical Examination Subjects with glaucoma were recruited from the Wills Eye Hospital ophthalmology clinics between July 8, 2014 and May 15,2015. The diagnosis of glaucoma was made by glaucoma specialists during clinical examination based upon characteristic glaucomatous optic disc (e.g. high cup-to-disc ratio, vertical elongation of the optic cup, rim notching, cup-to-disc asymmetry between eyes, among others) and VF changes.Control subjects consisted of recruits from the Cataract and Primary Eye Care Service at the Wills Eye Hospital as well as volunteers with normal eye examinations. All subjects underwent an ophthalmic examination that included assessments of best-corrected visual acuity (BCVA), slit lamp biomicroscopy, Goldmann applanation tonometry, and fundoscopy.
Subjects were included if they were ≥18 years of age, with BCVA of 20/40 or better in the tested eye, spherical refraction within ±5 D, and cylinder correction within ±3 D. Subjects were excluded if they had any conditions preventing adequate examination of the pupil, active infection of the anterior or posterior segments of the eye, any intraocular surgical or laser procedure performed within 4wk prior to enrollment, use of medications known to affect VF sensitivity, or any coexisting intraocular diseases.
Visual Field Testing VF assessments were completed using the OVF analyzer 900 (Haag-Streit Diagnostics,Bern, Switzerland) and the HEP (Heidelberg Engineering,Heidelberg, Germany). Testing order was randomized. The G-TOP (Tendency Oriented Perimetry, 30-2) white-on-white strategy with a stimulus interval of 1s was used for OVF and the SAP III 30-2 Adaptive Staircase Thresholding Algorithm(ASTA) FAST strategy was used for HEP. FAST protocols have enjoyed a long history in HVF testing and have been shown to reduce testing times and test-retest variability without significantly affecting VF indices[10-13].
Subjects were tested with the appropriate refraction. Trial lenses were substituted when the subjects' own prescription glasses or contact lenses were unavailable, or when the spectacles were judged to impede VF testing. Testing commenced after a brief tutorial to familiarize the subject with the examination. OVF and HEP VFs with fixation losses (FL),false positive errors (FP), and false negative errors (FN) less than 33% were included for analysis. Mean deviation (MD),pattern standard deviation (PSD), FL, FN, FP, and test duration were recorded. The square root of loss of variance (sLV), a comparable parameter to PSD, was recorded for OVF. A selfselected subset of glaucoma subjects returned for retesting 3-6mo after the initial visit to determine test-retest repeatability for both machines. Each subject was given a survey to assess testing method preference post-examination.
Statistical Analysis Because the HEP and OVF parameters are scaled differently, we first explored utilizing an algorithm developed to convert HEP to OVF values[14]. Agreement analyses of the converted HEP and OVF values indicated that the algorithm may not be optimal for use in HEP (Figure 1).
As such, in lieu of Bland-Altman plots, Pearson's correlation coefficient was utilized to assess consistency between the HEP and OVF for the unconverted MD and PSD/sLV values.Receiver operating characteristic (ROC) curves were used to determine the ability of the HEP and OVF to differentiate between control and glaucoma subjects. The area under the ROC curve (AUC) was calculated for each measure. To assess testing repeatability for the MD and PSD/sLV parameters,intraclass correlation coefficients (ICCs) were computed. Intereye correlation was accounted for by using the mixed effect model. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
A total of 79 glaucoma and 36 control subjects were enrolled.A total of 109 glaucomatous and 65 control eyes receiving both HEP and OVF testing. Mean SD BCVA was 0.057 (0.106;Snellen converted to logMAR) and 0.063 (0.085), respectively.In the glaucoma group, primary open angle glaucoma was the predominant diagnosis, the mean age was 70.1 (9.0)y(range 48-90y), and subjects were 58% female (n=46). In the control group, the mean age was 67.9 (9.2)y (range 47-87y)and subjects were 64% female (n=23). The two groups were comparable with respect to age, sex, and ethnicity (Table 1).

Figure 1 Comparisons between the converted HEP and the OVF analyzer for MD and PSD/sLV Bland-Altman plots with the representations of the limits of agreement for MD (A) and PSD/sLV (B) suggested that the conversion algorithm may not be optimal for use in the HEP device. Horizontal lines represent -1.96s (bottom line), mean (middle line), and +1.96s (top line).

Figure 2 Areas under the ROC curve (AUC) for the HEP and the OVF demonstrated fair to good diagnostic accuracy for both devices Comparisons were made between devices for MD (A) and PSD/sLV (B).
Pearson's correlation coefficients between HEP and OVF were strong at -0.84 for MD and 0.79 for PSD/sLV (P<0.01 for both).Among glaucoma patients, correlation remained strong between machines at -0.86 for MD and 0.83 for PSD/sLV (P<0.01 for both).AUC was used to compare the predictive accuracy of the HEP and OVF devices in differentiating between glaucoma and controls. The MD AUCs were both fair and similar between the HEP (0.74) and the OVF (0.79; P=0.26). Likewise, the PSD/sLV AUCs were fair to good and comparable between the HEP (0.74) and the OVF (0.82; P=0.08, Figure 2).
Thirty-five randomly selected subjects with glaucoma returned for repeat testing. ICC was used to assess test-retest repeatability of SAP administered through the OVF and the HEP (Table 2). The test-retest repeatability was high for both HEP (ICCs of 0.96 for MD and 0.95 for PSD/sLV) and OVF(ICCs of 0.82 and 0.88, respectively).
The mean OVF testing time of 2.63 (0.55)min was significantly shorter than that of the HEP testing time of 5.15 (2.25)min(P<0.01). Post-testing satisfaction surveys administered to all subjects showed a trend towards preferring the HEP over the OVF for both glaucoma (33% vs 23%) and controls (44% vs 14%) without reaching statistical significance (P=0.18). The other glaucoma (44%) and control (42%) subjects expressed no distinct preference for testing modality.
Table 1 Demographics and clinical characteristics of study subjects who underwent SAP testing using the HEP and OVF analyzers
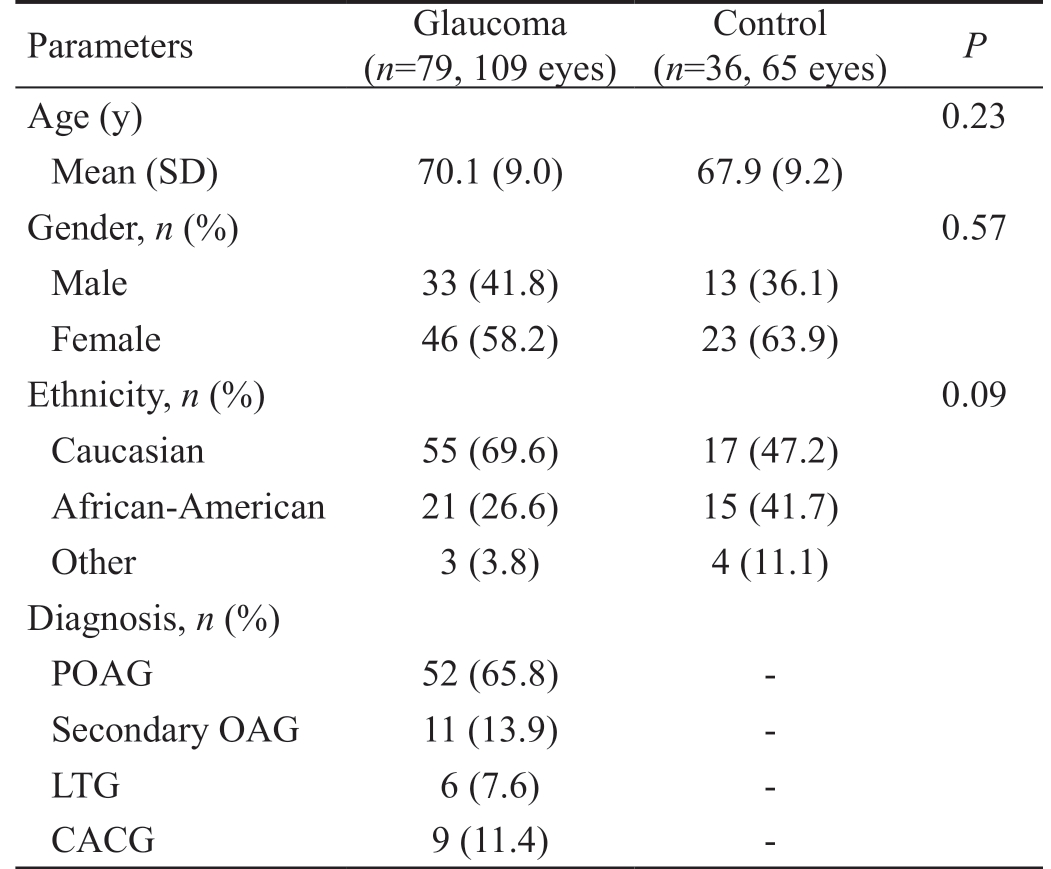
Two-sample t-test was used to compare means, and Fisher's exact test was used to compare proportions. POAG: Primary open angle glaucoma; OAG: Open angle glaucoma; LTG: Low tension glaucoma;CACG: Chronic angle-closure glaucoma.
Parameters Glaucoma(n=79, 109 eyes)Control(n=36, 65 eyes) P Age (y) 0.23 Mean (SD) 70.1 (9.0) 67.9 (9.2)Gender, n (%) 0.57 Male 33 (41.8) 13 (36.1)Female 46 (58.2) 23 (63.9)Ethnicity, n (%) 0.09 Caucasian 55 (69.6) 17 (47.2)African-American 21 (26.6) 15 (41.7)Other 3 (3.8) 4 (11.1)Diagnosis, n (%)POAG 52 (65.8) -Secondary OAG 11 (13.9) -LTG 6 (7.6) -CACG 9 (11.4) -
DISCUSSION
The HEP is a novel VF testing device with several innovative features. In particular, it provides stimulus size variation for
testing VF areas with more severe sensitivity loss. The aims of this study were to compare the diagnostic ability of HEP SAP with that of the OVF and to evaluate the test-retest repeatability of both modalities. This is one of the first reports examining HEP in a clinical ophthalmic practice and to the best of our knowledge the first report directly comparing the HEP and the OVF. Results demonstrated strong consistency between the two devices for both MD and PSD/sLV in the glaucoma group. The AUCs were comparable between HEP and OVF, indicating similar efficacy in determining VF losses.The ICCs demonstrated high test-retest repeatability for both HEP (0.96 for MD and 0.95 for PSD) and OVF (0.82 for MD and 0.88 for PSD).
For any HEP testing session, a stimulus of Goldmann size III is presented to detect sensitivities between 40 and 16 dB.For sensitivities below 16 dB, stimulus size increases in a linear fashion following a published Goldmann table while luminance remains the same. Although SAP testing exhibits high variability for VF locations of reduced sensitivity,increasing the target size in response to decreased sensitivity allows for greater test-retest repeatability on the HEP[15].Indeed, HEP SAP has been shown to provide equivalent testretest repeatability compared to the HVF, while this study demonstrated the superior test-retest repeatability of HEP relative to OVF[16].
The OVF has become a widely-utilized modality due its ability to perform rapid threshold testing (approximately 2.5min) resulting in increased subject comfort and diminished fatigue without a significant loss in sensitivity and precision compared to the HVF[17-20]. Previous studies have shown that longer testing times result in a decreased mean sensitivity and an increased mean defect, presumably related to fatigue artifacts[11,21-23]. The SAP III 30-2 ASTA FAST testing strategy was used in this study to mitigate the effect of fatigue. While shorter testing strategies have been linked to higher variability in areas of low sensitivity, this association was not apparent in this study as evidenced by the high repeatability score on the HEP SAP protocol[24]. Interestingly, despite the fact thatthe HEP SAP had a significantly longer testing time compared to the OVF, subject preferences were comparable between testing modalities. Subjects who preferred HEP testing cited OVF background noise, the need for a patch during OVF testing, a lack of clicker noise in OVF, the larger screen and better contrast in HEP, as well as higher overall comfort level during HEP testing as reasons for their selection. Subjects who preferred OVF indicated the shorter duration and higher comfort level of OVF testing as factors.
Table 2 Test-retest repeatability (assessed using ICCs) of SAP administered to a random subset of study subjects using the HEP and OVF analyzers
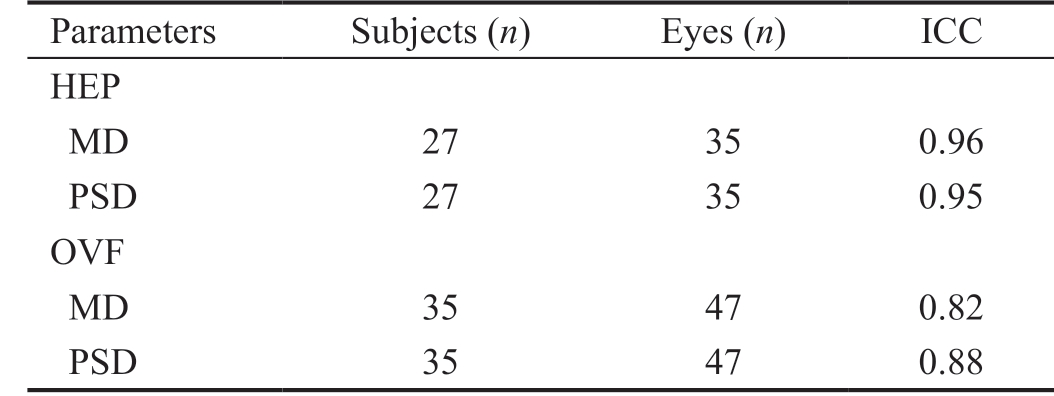
ICCs: Intraclass correlation coefficients; SAP: Standard automated perimetry.
Parameters Subjects (n) Eyes (n) ICC HEP MD 27 35 0.96 PSD 27 35 0.95 OVF MD 35 47 0.82 PSD 35 47 0.88
Study limitations included small sample size and self-selection of the glaucoma subjects who returned for repeat testing.The sample size of our 79 patients with glaucoma and 36 controls provided 80% power to detect an AUC difference of 0.11 (e.g. 0.74 vs 0.85) between HEP and OVF for detecting glaucoma. VF testing was one component of the glaucoma diagnosis which might have introduced a bias in evaluating the diagnostic ability of HEP and OVF. Subjects with advanced glaucoma were more likely to have greater inherent test-retest variability on SAP and this may have influenced the results,and test-retest repeatability was not evaluated for control subjects. Other potential limitations of this study include the lack of familiarity with VF testing for the control group.The presence of a learning curve for inexperienced subjects in VF testing is a well-known phenomenon, with multiple studies demonstrating score improvements upon repeat testing[25-26]. While we did not include an evaluation of lens status in our subjects, since the same eyes received both HEP and OVF testing, it is unlikely that the presence of cataracts or pseudophakia would have affected comparisons between testing modalities. Finally, while the 30-2 is routinely used in glaucoma screening, 24-2 strategies are more commonly used in evaluations of progression. As such, it would be interesting to see how 24-2 strategies compared between HEP and OVF.In conclusion, the strong correlations between the HEP and the OVF, in addition to the favorable ICC values for HEP relative to OVF, suggest that the HEP may be useful in detecting VF loss with a higher degree of reliability compared to OVF.In particular, the automatic increase in stimulus size upon a reduction in sensitivity may be useful in testing subjects with advanced glaucoma. Although HEP testing lasted longer than OVF, subject preferences were not affected. Future studies,including those comparing the HEP to the HVF, are necessary before the utility of the HEP in ophthalmic clinical practice can be clearly defined.
ACKNOWLEDGEMENTS
Foundation: Supported by Wills Eye Innovation Grant#WEF15064. Heidelberg Engineering Inc. (California,USA) provided the Heidelberg Edge Perimeter used in this study. The sponsor had no role in the design or conduct of this research.
Conflicts of Interest: Cui QN, None; Gogte P, None; Lam JM, None; Siraj S, None; Hark LA, None; Myers JS, None;Katz LJ, None; Waisbourd M, None.
1 Mulak M, Szumny D, Si eja-Bujewska A, Kubrak M. Heidelberg edge perimeter employment in glaucoma diagnosis: preliminary report. Adv Clin Exp Med 2012;21(5):665-670.
2 Kaczorowski K, Mulak M, Szumny D, Misiuk-Hojło M. Heidelberg edge perimeter: the new method of perimetry. Adv Clin Exp Med 2015;24(6):1105-1112.
3 Cui Q, Fudemberg SJ, Resende AF, Vu TA, Zhou C, Rahmatnejad K,Hark LA, Myers JS, Katz LJ, Waisbourd M. Validation of the structurefunction correlation report from the heidelberg edge perimeter and spectral-domain optical coherence tomography. Int Ophthalmol 2018.
4 Medeiros FA, Sample PA, Weinreb RN. Frequency doubling technology perimetry abnormalities as predictors of glaucomatous visual field loss.Am J Ophthalmol 2004;137(5):863-871.
5 Soliman MA, de Jong LA, Ismaeil AA, van den Berg TJ, de Smet MD.Standard achromatic perimetry, short wavelength automated perimetry,and frequency doubling technology for detection of glaucoma damage.Ophthalmology 2002;109(3):444-454.
6 Ichhpujani P, Lo DC, Cvintal V, Waisbourd M, Averbuch A, Leiby BE,Myers JS, Spaeth GL, Katz LJ. Flicker defined form, standard perimetry and Heidelberg retinal tomography: structure-function relationships. Can J Ophthalmol 2015;50(4):290-296.
7 Horn FK, Scharch V, Mardin CY, Lämmer R, Kremers J. Comparison of frequency doubling and flicker defnied form perimetry in early glaucoma.
Graefes Arch Clin Exp Ophthalmol 2016;254(5):937-946.
8 Reznicek L, Muth D, Vogel M, Hirneiß C. Structure-function relationship between flicker-defined form perimetry and spectral-domain optical coherence tomography in glaucoma suspects. Curr Eye Res 2017;42(3):418-423.
9 Kaczorowski K, Mulak M, Szumny D, Baranowska M, Jakubaszko-Jabłońska J, Misiuk-Hojło M. Comparison of visual field measurement with heidelberg edge perimeter and humphrey visual field analyzer in patients with ocular hypertension. Adv Clin Exp Med 2016;25(5):937-944.10 Nordmann JP, Denis P, Nguer Y, Mouton-Chopin D, Saraux H. Static threshold visual field in glaucoma with the Fastpac algorithm of the Humphrey Field Analyser. Is the gain in examination time offset by any loss of information? Eur J Ophthalmol 1994;4(2):105-110.
11 Bengtsson B, Heijl A. SITA Fast, a new rapid perimetric threshold test. Description of methods and evaluation in patients with manifest and suspect glaucoma. Acta Ophthalmol Scand 1998;76(4):431-437.
12 Bengtsson B, Heijl A, Olsson J. Evaluation of a new threshold visual field strategy, SITA, in normal subjects. Swedish Interactive Thresholding Algorithm. Acta Ophthalmol Scand 1998;76(2):165-169.
13 Bengtsson B, Heijl A. Comparing significance and magnitude of glaucomatous visual field defects using the SITA and Full Threshold strategies. Acta Ophthalmol Scand 1999;77(2):143-146.
14 Zeyen T, Roche M, Brigatti L, Caprioli J. Formulas for conversion between Octopus and Humphrey threshold values and indices. Graefes Arch Clin Exp Ophthalmol 1995;233(10):627-634.
15 Wall M, Kutzko KE, Chauhan BC. Variability in patients with glaucomatous visual field damage is reduced using size V stimuli. Invest Ophthalmol Vis Sci 1997;38(2):426-435.
16 Goren D, Ho YH, Schuelein E, Flanagan JG. A comparison of standard automated perimetry on the Heidelberg Edge Perimeter and the Humphrey Field Analyzer. Invest Ophthalmol Vis Sci 2010;51(13):4335.
17 King AJ, Taguri A, Wadood AC, Azuara-Blanco A. Comparison of two fast strategies, SITA Fast and TOP, for the assessment of visual fields in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 2002;240(6):481-487.18 Scuderi GL, Cesareo M, Perdicchi A, Recupero SM. Standard automated perimetry and algorithms for monitoring glaucoma progression.Prog Brain Res 2008;173:77-99.
19 Monsalve B, Ferreras A, Calvo P, Urcola JA, Figus M, Monsalve J,Frezzotti P. Diagnostic ability of Humphrey perimetry, Octopus perimetry,and optical coherence tomography for glaucomatous optic neuropathy.Eye (Lond) 2017;31(3):443-451.
20 Rajalakshmi AR, Suma E, Prabhu DR. Comparative analysis of visual field plotting by octopus interzeag 1-2-3, humphrey field analyser II and frequency doubling perimetry in glaucoma patients in south indian population. J Clin Diagn Res 2015;9(7):NC01-NC03.
21 Heijl A, Drance SM. Changes in differential threshold in patients with glaucoma during prolonged perimetry. Br J Ophthalmol 1983;67(8):512-516.
22 O'Brien C, Poinoosawmy D, Wu J, Hitchings R. Evaluation of the Humphrey FASTPAC threshold program in glaucoma. Br J Ophthalmol 1994;78(7):516-519.
23 Flanagan JG, Wild JM, Trope GE. Evaluation of FASTPAC, a new strategy for threshold estimation with the Humphrey Field Analyzer, in a glaucomatous population. Ophthalmology 1993;100(6):949-954.
24 Artes PH, Iwase A, Ohno Y, Kitazawa Y, Chauhan BC. Properties of perimetric threshold estimates from full threshold, SITA standard, and SITA fast strategies. Invest Ophthalmol Vis Sci 2002;43(8):2654-2659.
25 Hirasawa K, Shoji N. Learning effect and repeatability of automated kinetic perimetry in healthy participants. Curr Eye Res 2014;39(9):928-937.26 Heijl A, Lindgren G, Olsson J. The effect of perimetric experience in normal subjects. Arch Ophthalmol 1989;107(1):81-86.