INTRODUCTION
C yclodestructive procedures had been widely considered as the last option for management of uncontrolled glaucoma after failure of all standard lines[1]. These modalities had proved to achieve encouraging intraocular pressure (IOP)lowering abilities over the past decades. However, concerns regarding their probable side effects as their non-selective action over the target tissues and the arbitrary dose-effect correlation had often compelled surgeons to save them for eyes with advanced and end-stage glaucoma[2].
High-intensity focused ultrasound technology (HIFU)had been advocated formerly in 1980s as a relatively safe alternative modality for ciliary body ablation[3]. However, it was abandoned owing to its complexity and the drawbacks encountered by the procedure[4]. Several technical adjustments with substantial modifications in the procedure had reintroduced it as a novel approach for cyclodestruction[5].
Ultrasound ciliary plasty (UCP) permits a precise thermal action over the target tissues causing less collateral damage to adjacent tissues while treating non-optically transparent structures[6]. Recently, published studies reported encouraging results of UCP in patients with refractory glaucoma regarding both safety and efficacy[7-9]. These results had favored the extension of UCP indication to include glaucoma patients who were not operated before[10].
Though proposed, the use of UCP as a primary treatment of glaucoma is still not widely studied. The main objective of the current study was to evaluate the safety and efficacy of UCP in patients with medically uncontrolled glaucoma with different etiologies.
SUBJECTS AND METHODS
Ethical Approval A retrospective study involved revising the case records of patients who had undergone UCP at Dar AlShifa Hospital, Kuwait during the period from January 2017 to June 2018. The study followed the tenets of the Declaration of Helsinki and was approved by local Institutional Review Board. Written informed consent was obtained from all the enrolled patients.
Adult patients (>18y) with moderate to advanced primary or secondary glaucoma without previous incisional glaucoma surgery were included in this study. Patients were enrolled if they had uncontrolled IOP [defined as IOP >21 mm Hg despite maximal tolerated antiglaucomatous drugs (AGD)] and presented with a contraindication to glaucoma invasive surgery.Only records of patients who completed the 12mo' follow-up period were counted in. Patients were excluded from the study if they were diagnosed with normal tension glaucoma or had intraocular surgery (apart from cataract extraction) or laser treatment within 3mo before UCP therapy, or had previous cyclo-destructive procedure as well as patients diagnosed with ocular infection within 2wk before UCP. Records of patients with glaucoma surgical intervention within less than 12mo after UCP treatment were not included in the study.
Data pertaining to patient demographics as well as glaucoma diagnosis were retrieved. In addition, the following data were recorded before and after UCP therapy: best-corrected visual acuity using (BCVA) Snellen's chart converted to logMAR for statistical analysis, slit-lamp biomicroscopy with gonioscopy,dilated fundus examination and Goldmann applanation tonometry (3 readings). Pretreatment investigations comprised:white-to-white measurement of the corneal diameter and axial length measurements by IOL Master 500 (Carl Zeiss Meditec AG., Germany), ultrasound pachymetry with Tomey SP-100 (Tomey Corp. Nagoya, Japan) and visual fields using Humphrey Field Analyzer (24-2, SITA, standard program Carl Zeiss Meditec AG., Germany).
Procedure High-intensity focused ultrasound procedures were performed by 2 authors under peribulbar anesthesia. After lying in supine position; the coupling cone was first adjusted on the center of the patient's eye and kept in place via low vacuum suction. The treatment probe was then introduced inside the cone, and the transducers were consecutively automatically being activated by constant pressing over the foot switch.In the current study, the 2nd generation probe was used in all the cases (EyeOP1, Eye Tech care; France). The following parameters were used for all treatments: operating frequency,21 MHz; number of sectors activated, 6; acoustic power, 2.45 W; duration of each shot, 8s; and time between each shot, 20s.Choice of the probe diameter (11, 12 or 13 mm) was based on the patient's eye biometric readings. More details about the procedure are available in the video article by Giannaccare et al[11].Follow-up visits were scheduled at 1d, 1wk, 1, 3, 6, 9 and 12mo. Preoperative AGD were maintained unchanged during the first month after treatment after which it was adapted
whenever necessary. In addition to the pressure-lowering drugs; postoperative combination of dexamethasone and tobramycin (Tobradex; Alcon Laboratories, Inc., Fort Worth,TX, USA) was given 4 times daily for 1mo followed by gradual withdrawal.
Outcome Measures Primary outcomes were the reduction of the IOP and success rates at the end of the follow-up period,while the secondary outcomes were the report of intra- or postoperative complications and visual acuity.
Success and failure rates: qualified success was defined as IOP>5 mm Hg and reduction by ≥30% from baseline values with or without AGD. Failure was considered whenever IOP reduction was <30% despite the use of AGD or the development of any devastating complications or the need of other glaucoma surgeries.
Statistical Analysis Data were analyzed by Statistical Package of Social Science (SPSS; Chicago, USA, version 16).Normalization of data was tested with Shapiro-Wilk test.Categorical variables were represented with number and percent and compared with Chi-square test. Continuous variables were represented in mean±SD for parametric data and median (range) for non-parametric variables. Paired t-test was used to compare parametric data while Wilcoxon Signed-Rank test was used to compare non-parametric ones.
RESULTS
Demographics A total of 78 records for 74 patients were retrieved and evaluated. The records of 62 eyes of 62 patients fulfilled the inclusion criteria and were included in the study. The mean age was 63.8y with 67.7% males. All patients had at least 12mo follow up period with mean of 13.6±1.4mo. Neovascular glaucoma was the most frequent diagnosis accounted for 24.2% of the treated eyes followed by primary open angle glaucoma (POAG) representing 21%.All the patients were receiving the maximal tolerated AGD(mean=3.2). Patient's demographic and baseline clinical data are given in details in Table 1.
Efficacy For the entire included eyes, the mean IOP showed statistically significant reduction from 35.2±8.3 mm Hg before treatment to 20.6±8.7 mm Hg at 12th month (P<0.0005)with a mean percentage IOP reduction of 42.3% (SD=16.7)corresponding to mean IOP reduction of 14.5±6.6 mm Hg(range 3-28 mm Hg). Figure 1 illustrates the scatter-gram plot for the pretreatment versus post-treatment IOP at 12mo.
Table 2 summarizes the mean IOP reductions from baseline values along the follow-up points with the percent of qualified success at each point. There was statistically significant reduction in the IOP all over the follow-up points (P<0.0005).Qualified success was achieved in 77.4% of eyes (48/62) at the last follow-up.
The curve displayed in Figure 2 represents the mean IOP in all patients over the follow-up time. There was significant
Table 1 Demographic and baseline clinical characteristics of the studied patients mean±SD (range)

SD: Standard deviation; POAG: Primary open angle glaucoma; NVG:Neovascular glaucoma; CACG: Chronic angle-closure glaucoma;IOP: Intraocular pressure; AGD: Anti-glaucomatous drugs; BCVA:Best corrected visual acuity.
Parameter Value Age (y) 63.8±10.4 (42-82)Gender, n (%)Male 42 (67.7)Female 20 (32.3)Type of glaucoma, n (%)Primary glaucoma POAG 13 (21)CACG 10 (16.1)Secondary glaucoma NVG 15 (24.2)Uveitic 11 (17.7)Pseudoexfoliative 4 (6.5)Others 9 (14.5)Lens status, n (%)Phakic 23 (37.1)Pseudophakic 39 (62.9)Pretreatment IOP (mm Hg) 35.2±8.3 (22-58)No. of AGD 3.2±0.4 (3-4)Follow-up period (mo) 13.6±1.4 (12-17)LogMAR BCVA 0.72±0.23 (0.4-1.2)
Table 2 IOP reductions and qualified success along the follow-up period of the included patients mean±SD
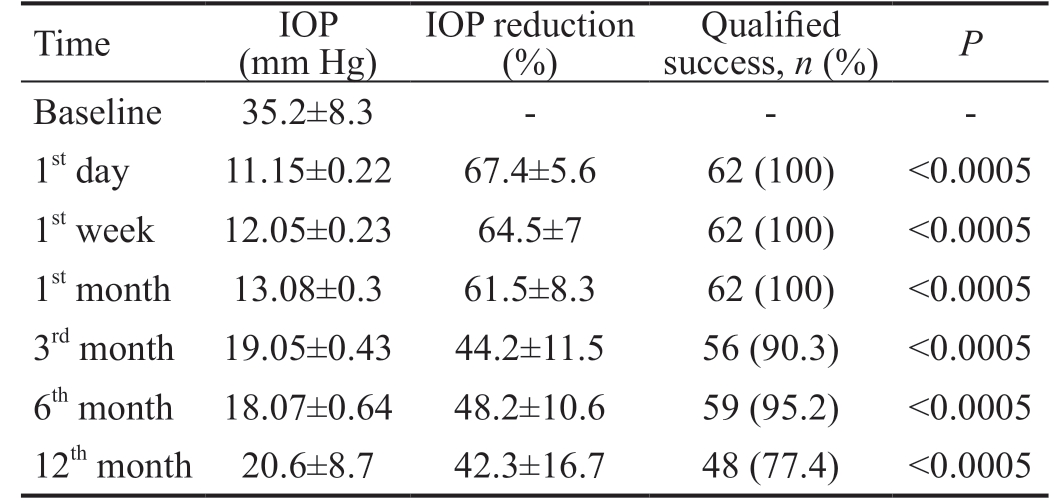
IOP: Intraocular pressure; SD: Standard deviation. P value denotes significance of the difference of the IOP at each follow-up point compared to the baseline value; test used Wilcoxon Signed-Rank test.Significance at P<0.05.
Time IOP(mm Hg)IOP reduction(%)Qualified success, n (%) P Baseline 35.2±8.3 - - -1st day 11.15±0.22 67.4±5.6 62 (100) <0.0005 1st week 12.05±0.23 64.5±7 62 (100) <0.0005 1st month 13.08±0.3 61.5±8.3 62 (100) <0.0005 3rd month 19.05±0.43 44.2±11.5 56 (90.3) <0.0005 6th month 18.07±0.64 48.2±10.6 59 (95.2) <0.0005 12th month 20.6±8.7 42.3±16.7 48 (77.4) <0.0005
reduction in the IOP at 1st day after treatment followed by rise over the next the 3mo. This was pursued by fairly stable IOP till the end of the follow-up.
The mean number of topical AGD decreased significantly from 3.2±0.4 before treatment to 2.1±1.02 at 12mo (P<0.0005).
Figure 3 demonstrates the outcome of UCP therapy for each diagnostic category of the included eyes. Of all the diagnoses,eyes with NVG and uveitic glaucoma showed the highest failure rate (60% and 45.5% respectively) with highly statistically difference compared with other diagnoses (P<0.005).
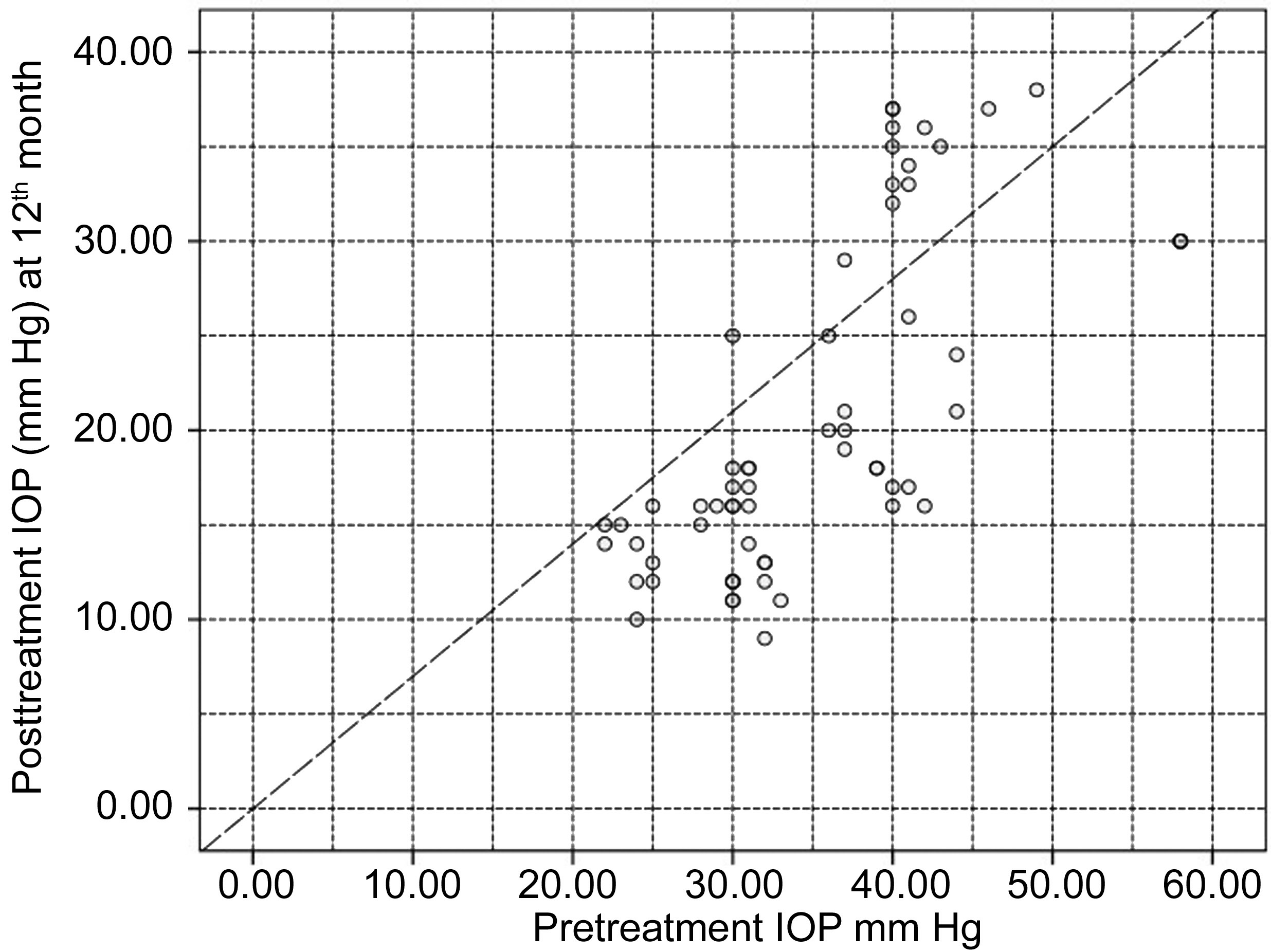
Figure 1 Scatter gram plot for IOP before treatment (X axis)and IOP at 12mo after treatment (Y axis) All the points below the diagonal dashed lines represent eyes with IOP reduction ≥30% from the baseline values.
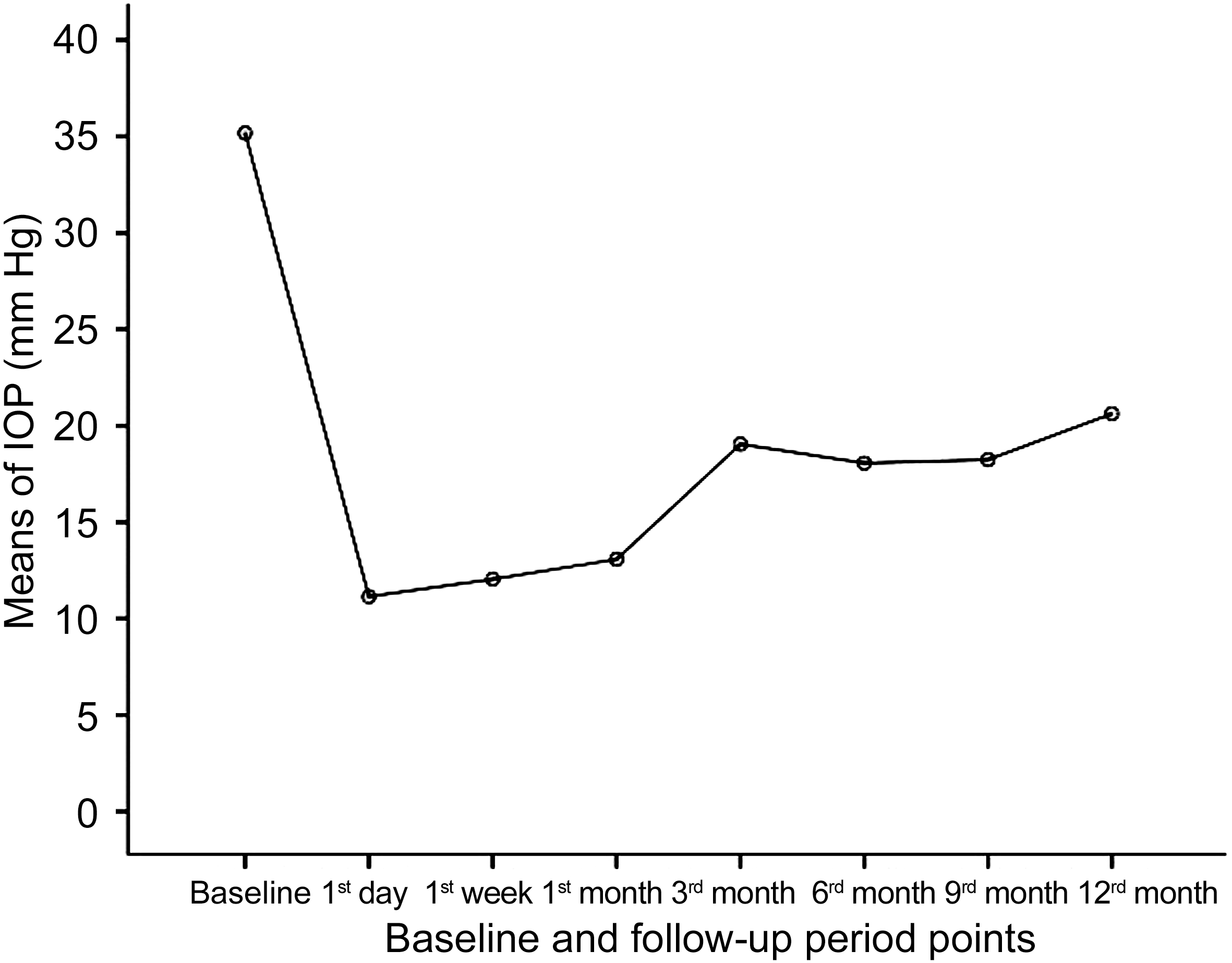
Figure 2 Means of IOP of the included eyes at baseline and at different points along the follow-up period.
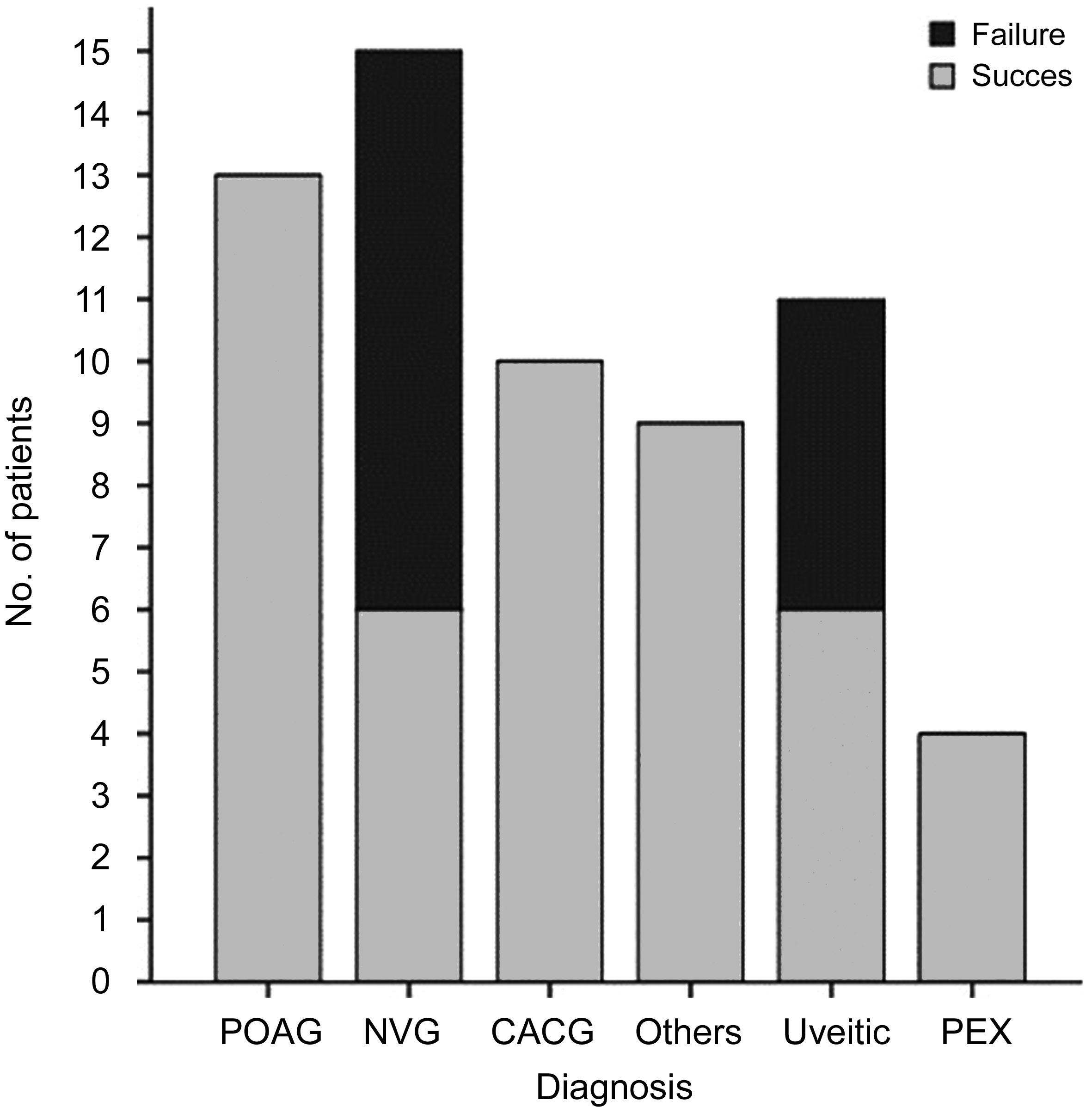
Figure 3 Success rates among different categories of glaucoma included in the study POAG: Primary open angle glaucoma; CACG:Chronic angle-closure glaucoma; NVG: Neovascular glaucoma; PEX:Pseudoexfoliative glaucoma.
Visual Acuity There was statistically insignificant change in the logMAR mean BCVA from baseline of 0.72±0.23 to 0.73±0.26 at 12mo' post-treatment (P=0.6).
Safety
Intraoperative All the patients tolerated well to the procedure.None of the cases encountered any complications during the treatment (Table 3).
Postoperative None of the eyes developed hypotony (IOP≤5 mm Hg), choroidal detachments or phthisis. IOP spikes were not recorded for any of the eyes. All the cases developed moderate anterior chamber reaction that was treated by topical steroids for 2wk. Three eyes (4.8%) developed transient macular edema resolved after 1st month under topical nonsteriodal anti-inflammatory drugs with no impact on the final BCVA. Two cases (3.2%) presented with mydriasis that lasted till the end of the follow-up. Punctate keratitis was recorded in 6 eyes (9.8%). Of the 14 eyes with insufficient response to UCP, 4 patients required glaucoma surgery; one patient required diode laser cyclophotocoagulation (CPC), and 3 patients had Ahmed valve surgery. These secondary treatments were carried out after 12mo from the UCP treatment. Patients with earlier intervention before 12mo follow-up were not included in the study (Table 3).
DISCUSSION
UCP is considered a revolutionary step in cyclodestructive maneuvers involved in glaucoma treatment. The procedure incorporates the selective application of HIFU over the ciliary processes in a non-invasive, single-step, computer-supported method[6]. Various reports studied the efficacy and the safety of UCP in controlling IOP especially in patients with refractory glaucoma[7-9]. The current study was designed and conducted to assess the safety and efficacy of UCP for the first time among Kuwaiti patients with either primary or secondary glaucoma who were naïve to glaucoma surgeries. In total, 62 eyes of 62 patients were enrolled in the study retrospectively. All the patients had completed the 12mo' follow-up period.
The enrolled eyes presented with moderate to severe stages of the disease. Accordingly, we chose the target IOP reduction to be equal to or higher than 30% from the baseline values, meeting the requirements of some clinical studies as“Advanced Glaucoma Intervention Study”. These studies recommended a relatively stringent IOP target in eyes with advanced glaucoma in attempt to impede further progression in their visual field defects. Target IOP reduction of 20% was commonly used in previous studies evaluating the role of UCP in glaucoma patients. Thus, it might be rather complicated while comparing our results to those obtained previously.
In our study, the mean percentage of IOP reduction at 12mo was 42.3% which was higher than that reported previously in earlier studies, with IOP reduction ranging from 30% to 38%[7-9,12]. In the current study, none of patients had a history of preceding glaucoma surgery, whereas the former studies included refractory glaucoma patients who had -at least- previously one failed filtration surgery. Thus, the mean pretreatment IOP in our study was higher than that reported in the earlier reports with resultant higher reduction rates. In this aspect, our study would be more correspondent to the 2 studies published by Aptel et al[10] and Deb-Joardar and Reddy[13] including patients naïve to filtration surgery. They noted 30% and 32.3% reduction in IOP at 12mo respectively. Nevertheless, their study comprised only open angle glaucoma (OAG) patients with mean pretreatment IOP of 28.2 and 23.5 mm Hg which were also far less than our baseline values (35.2±8.3 mm Hg).
Qualified success was achieved in 77.4% in our study.Scrutinizing the results of the different glaucoma categories included in our study revealed lower success rates in NVG and uveitic eyes (40% and 55.5% respectively). It was suggested that patients with secondary glaucoma with pre-existing lower trabecular meshwork outflow of aqueous humor would have suboptimal response to a decrease in aqueous production and thus induce poorer effect on IOP control[12]. In contrary,Giannaccare and associates[14] found a higher percentage of IOP reduction in patients with NVG than POAG. They suggested that, this could be related to the higher preoperative IOP in NVG patients than OAG participants and to the different exposure time of the ultrasound utilized as well as the small sample number of the included NVG.
We employed the 2nd generation probe in the current study with its noticeable advantages over its antecedent. The size of the transducer had increased from 2.5 mm in the 1st generation probe to 4 mm. The increased size allows wider treatment zone, thus abolishing the influence of the possible anatomical variations of the ciliary body. Higher success rate (67%) was reported by Rouland and Aptel[15] utilizing the 2nd generation probe with a mean IOP reduction of 44% in these patients.Giannaccare et al[16] showed that the second-generation probes resulted in a highly statically significant IOP reduction(P<0.05). Denis[17] conducted a metanalysis study aiming mainly at comparing the efficacy of the two generations of the probe and found that at 6-month post therapy, the 2nd generation probe achieved higher mean IOP reduction (35%)compared to 29% by its comparator.
Table 3 Intraoperative and postoperative complications

Complication n (%)Postoperative Anterior chamber reaction 62 (100)Punctate keratitis 6 (9.8)Macular edema 3 (4.8)Mydriasis 2 (3.2)
Regarding the exposure time, favorable IOP control was achieved with longer exposure time in a study conducted by Denis et al[12] comparing 6s vs 4s treatment. The 8s exposure protocol standard with the 2nd generation probe was proved to be superior to the 1st generation treatments with shorter exposure times (4 and 6s)[7,18-19]. Furthermore; Deb-Joardar and Reddy[13] compared more prolonged protocols of 8 and 10s.Though insignificant difference in IOP reduction was noticed(45% with 8s vs 41% with 10s); patients with 10s protocol experienced higher incidence of scleral marks and anterior chamber reaction.
In our study, we did not record any major complications either during the procedure or along the follow-up period.Particularly, severe hypotony or phthisis, which are commonly reported following most of the known cyclodestructive procedures[20-22]. No records for IOP spikes or significant IOP rise either in the early or all over the follow up period.Clinical examinations reported moderate signs of intraocular inflammation without hypopyon or synechiae as signs of severe inflammation. Punctate keratitis encountered in the study was supposed to be due to mechanical effect caused by the suction cone during its placing. Visual acuity remained statistically unchanged at the last follow-up. These signs of well tolerability of the UCP was compatible with previous studies[8-10].
The retrospective nature of the study with the relatively small sample size represent the limitations of the study. However,widening our inclusion categories to include angle-closure as well as secondary glaucoma naïve to surgery with long term follow-up constitute points of strength to our current study.Further prospective randomized comparative studies are required to prove the superiority of this modality over standard cyclodestructive procedures as diode CPC.
The present study shows that UCP using 2nd generation highintensity focused ultrasound using 8s protocol proved to be effective in reducing IOP in non-refractory glaucoma patients. However, lower efficacy seems to occur with cases of neovascular and uveitic glaucoma.
ACKNOWLEDGEMENTS
Authors’ contributions: Torky MA and Al Zafiri YA were responsible for the design, surgical treatment and data collection. Torky MA and Hagras SM participated in the interpretation of the data and wrote the first draft of the manuscript. Hagras SM worked on the statistical analysis.Torky MA and Mokbel TH shared in intellectual content of the draft. Torky MA, Khattab AM and Bassiouny RM shared in revising the manuscript. All the authors perused and approved the final manuscript version that was submitted for publication.
Conflicts of Interest: Torky MA, None; Al Zafiri YA, None;Hagras SM, None; Khattab AM, None; Bassiouny RM,
None; Mokbel TH, None.
1 Fankhauser F, Kwasniewska S, Van der Zypen E. Cyclodestructive procedures. I. Clinical and morphological aspects: a review. Ophthalmologica 2004;218(2):77-95.
2 Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol 2007;91(12):1631-1635.
3 Coleman DJ, Lizzi FL, Driller J, Rosado AL, Burgess SE, Torpey JH, Smith ME, Silverman RH, Yablonski ME, Chang S. Therapeutic ultrasound in the treatment of glaucoma. II. Clinical applications.Ophthalmology 1985;92(3):347-353.
4 Sterk CC, vd Valk PH, van Hees CL, van Delft JL, van Best JA,Oosterhuis JA. The effect of therapeutic ultrasound on the average of multiple intraocular pressures throughout the day in therapy-resistant glaucoma. Graefe's Arch Clin Exp Ophthalmol 1989;227(1):36-38.
5 Aptel F, Charrel T, Lafon C, Romano F, Chapelon JY, Blumen-Ohana E, Nordmann JP, Denis P. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci 2011;52(12):8747-8753.
6 Mastropasqua R, Fasanella V, Mastropasqua A, Ciancaglini M, Agnifili L. High-intensity focused ultrasound circular cyclocoagulation in glaucoma: a step forward for cyclodestruction? J Ophthalmol 2017;2017:7136275.
7 Melamed S, Goldenfeld M, Cotlear D. High-intensity focused ultrasound device in refractory Glaucoma patients. Results at 1 year.
Invest Ophthalmol Vis Sci 2014;55(13):6171.
8 Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case series. Curr Med Res Opin 2014;30(8):1599-1605.
9 Melamed S, Goldenfeld M, Cotlear D, Skaat A, Moroz I. High-intensity focused ultrasound treatment in refractory glaucoma patients: results at 1y of prospective clinical study. Eur J Ophthalmol 2015;25(6):483-489.
10 Aptel F, Denis P, Rouland JF, Renard JP, Bron A. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol 2016;94(5):e268-e277.
11 Giannaccare G, Sebastiani S, Campos EC. Ultrasound cyclo plasty in eyes with glaucoma. J Vis Exp 2018(131).
12 Denis P, Aptel F, Rouland JF, Nordmann JP, Lachkar Y, Renard JP,Sellem E, Baudouin C, Bron A. Cyclocoagulation of the ciliary bodies by high-intensity focused ultrasound: a 12-month multicenter study. Invest Ophthalmol Vis Sci 2015;56(2):1089-1096.
13 Deb-Joardar N, Reddy KP. Application of high intensity focused ultrasound for treatment of open-angle glaucoma in Indian patients.Indian J Ophthalmol 2018;66(4):517-523.
14 Giannaccare G, Vagge A, Gizzi C, Bagnis A, Sebastiani S, Del Noce C,Fresina M, Traverso CE, Campos EC. High-intensity focused ultrasound
treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol 2017;255(3):599-605.
15 Rouland JF, Aptel F. Primary open angle glaucoma treated by high intensity focused ultrasound (HIFU): results at 18mo of a prospective pilot study on patients treated with the 2nd generation probe. Acta Ophthalmol 2016;94.
16 Giannaccare G, Vagge A, Sebastiani S, Urbini LE, Corazza P,Pellegrini M, Carmassi L, Bergamini F, Traverso CE, Campos EC.Ultrasound cyclo-plasty in patients with glaucoma: 1-year results from a multicentre prospective study. Ophthalmic Res 2018:1-6.
17 Denis P. Clinical research of ultrasound ciliary plasty and implications for clinical practice. European Ophthalmic Review 2016;10(2):108.
18 Aptel F, Rouland JF. Ultrasound ciliary plasty to treat glaucoma:efficacy and safety results on 152 patients. Acta Ophthalmol 2017;95.
19 Aptel F, Rouland JF, Stalmans I, Denis P. Ultrasound treatment in patients with Primary Open-Angle Glaucoma with a second generation probe: Results of a Multicenter Clinical Trial. Acta Ophthalmol 2016;94.
20 Ramli N, Htoon HM, Ho CL, Aung T, Perera S. Risk factors for hypotony after transscleral diode cyclophotocoagulation. J Glaucoma 2012;21(3):169-173.
21 Pucci V, Tappainer F, Borin S, Bellucci R. Long-term follow-up after transscleral diode laser photocoagulation in refractory glaucoma.Ophthalmologica 2003;217(4):279-283.
22 Graber M, Rothschild PR, Khoueir Z, Bluwol E, Benhatchi N, Lachkar Y. High intensity focused ultrasound cyclodestruction versus cyclodiode treatment of refractory glaucoma: a retrospective comparative study. J Fr Ophtalmol 2018;41(7):611-618.