INTRODUCTION
P rimary congenital glaucoma (PCG) is the most common childhood glaucoma accounting for 50%-70% of the cases[1-3]. It occurs due to the abnormal development of the trabecular meshwork (TM) and the anterior chamber angle. This anomaly is called trabeculodysgenesis or iridotrabeculodysgenesis according to the tissues involved[1,4].It is characterized by an elevation in intraocular pressure (IOP)in the early infantile period, which causes structural damage to the eyeball such as buphthalmos, corneal edema, Haab's striae and may result in optic nerve damage and subsequent permanent loss of vision[3,5]. The combined trabeculotomytrabeculectomy procedure with mitomycin-C (MMC) was recommended as a primary procedure in moderate to severe cases[6-7].
About 10%-40% of PCG cases are familial and may be associated with consanguinity[1,8]. Its incidence varies geographically; PCG is four times more common in Saudi Arabia than in Western societies with an incidence of 1:2500 and 1:10 000 live births respectively[1,3,8]. Four different loci have been linked to PCG (GLC3A, GLC3B, GLC3C and GLC3D)[8-12]. Mutations in the cytochrome P4501B1 gene(CYP1B1) located in the GLC3A locus on chromosome 2p21 are the most predominant (Online Mendelian Inheritance in Man, OMIM, ID No.231300). They were first reported in PCG patients in 1997[13].Sequence analyses have so far revealed approximately 243 different mutations in the CYP1B1 gene (Human Genome Mutation database; http://www.hgmd.cf.ac.uk/ac/index.php),of which about 159 are implicated in the pathogenesis of PCG which indicates excessive allelic heterogeneity[11,14-15]. The mutation spectra of CYP1B1 varies widely across different populations[15]; from almost 100% among Saudi Arabians[16]and Slovakian Gypsies[17], to 50% among Brazilians, and 20%in Japanese[13,18-19]. It was found that patients with CYP1B1 gene mutations required more surgeries to control the IOP than patients without these mutations[20-21].
Very little investigation has been done to study CYP1B1 and PCG in Egyptian patients. In this work, we perform a preliminary investigation to identify CYP1B1 mutations in PCG Egyptian patients and to evaluate the role of CYP1B1 mutations as a prognostic factor predicting the outcome of surgery in a cohort group of Egyptian PCG patients operated on with combined trabeculotomy-trabeculectomy with MMC.
SUBJECTS AND METHODS
Ethical Approval This prospective interventional study was approved by the Research Ethics Committee of Cairo University Hospitals and the declaration of Helsinki was respected. Subjects for the study were recruited randomly from the patients attending the Cairo University Pediatric Ophthalmology clinic who met the inclusion criteria for the study. All parents of patients signed an informed consent for the surgery and inclusion in the study.
Patients Inclusion criteria entailed being an Egyptian primary congenital glaucoma patient who was not operated on before in at least one eye and age of onset ranging from 0-2y.Secondary glaucoma patients and patients having additional developmental ocular and/or systemic anomalies were excluded from the study.
Surgical Procedure The patients were subjected to full ophthalmic assessment to confirm meeting the inclusion criteria, including detailed anterior segment examination with measurement of the horizontal corneal diameter, measurement of the IOP using Perkins applanation tonometer under general anesthesia, posterior segment evaluation (by ophthalmoscopy or ultrasonography), and axial length measurement. The clinical severity of the cases was assessed using a severity scale of mild, moderate and severe as proposed earlier[6].Children meeting the inclusion criteria underwent combined trabeculectomy-trabeculotomy with MMC done by the same surgeon in all patients. Postoperatively, the patients underwent full ophthalmic assessment (under sedation with oral chloral hydrate 25 mg/kg, once, 30min prior to examination) at one day, one week, and then monthly for one year including measurement of IOP, horizontal corneal diameter, and optic nerve head evaluation. Surgical result of the initial operation was graded into either success (maintaining IOP less than 21 mm Hg with or without anti-glaucoma medication at the end of the follow-up period), or failure (IOP more than 21 mm Hg with topical antiglaucoma medications at any point during the follow-up period).
CYP1B1 Gene Mutation Detection Peripheral blood samples were collected, at the time of the initial procedure, and genomic DNA was extracted using Generation capture column kit (Gentra, USA) following the manufacturer's protocol. The coding regions in CYP1B1 were amplified using 13 pairs of primers from a previous study[22].
Polymerase chain reaction (PCR) amplification of the coding regions of the CYP1B1 gene from genomic DNA and screening with single-strand conformation polymorphism(SSCP) were performed as previously described[22]. PCR products with SSCP variants were purified using Axyprep PCR cleanup kits (Axygene Biosciences, USA) adhering to the manufacturer's protocol. Analysis of the sequencing reactions was performed using Big Dye Terminator cycle sequencing kit (Applied Biosystem, Foster City, CA) on ABI Prism 3730 Genetic Analyzer automated sequencer (Applied Biosystems,Foster City, CA). For confirmation, sequencing was done in forward and reverse strands in case of suspected sequence alteration.
The sequence alignment was done against the wild-type CYP1B1 sequence (Genbank U56438.1) using the LALIGN software server (http://www.ch.embnet.org/software/LALIGN_form.html). CodonCode aligner software (CodonCode Corp.,Centerville MA, USA) was used for multiple alignment and comparison of different sequences. The amino acid sequence for the CYP1B1 was obtained from The National Center for Biotechnology Information (NCBI) Reference Sequence:NP_000095.2, and sequence alterations were studied using DNASIS® Max v3.0 software (Hitachi Solutions America,Ltd., San Francisco, USA). Evolutionary conserved regions were studied by comparing amino acid sequences across different species (obtained from NCBI). The impact of missense mutations was predicted by using the mutationtolerance-prediction software (SIFT) provided by Genome Institute of Singapore, available on the website (http://sift.bii.astar.edu.sg/). Patients with confirmed CYP1B1 sequence alteration were designated “mutation-present”, while those who could not be confirmed with sequence alteration were designated “mutation-absent”.
Statistical Analysis All statistical analyses were done using IBM SPSS v20.0 software (IBM Corporation, USA).Descriptive statistics were calculated, and the data were summarized as mean±standard deviation (SD) for continuous variables. Percentages and tables were used to describe categorical data.

Figure 1 CYP1B1 genomic, protein structure and homology A: CYP1B1 genomic structure with intron and exon boundaries locations(coding exons are shaded in blue) and positions of mutations and SNPs identified in the study. Novel mutations are shown in bold & italics.Nucleotide positions are in reference to CYP1B1 GenBank accession number U56438. B: CYP1B1 protein diagram showing locations of detected mutation marked on different regions of the protein. The evolutionary conserved regions are shaded in blue with annotations below.Locations of different missense mutations detected in the study are outlined above. Novel mutations are shown in bold. Short horizontal lines indicate substrate recognition sites (SRSs). C: Comparison of CYP1B1 protein sequence among species to detect evolutionary conservation at the areas of missense mutations.
Comparisons between categorical data were done using Pearson's Chi-square test or Fisher's exact test. Comparisons of non-paired continuous variables were done using independent samples t-test, while comparisons of paired continuous variables were carried out using paired sample t-test. Correlations between non-parametric variables were done by Spearman's rank correlation coefficient. Cumulative success of the initial procedure between both groups was examined using Kaplan-Meier survival analysis. A P-value<0.05 was considered statistically significant.
RESULTS
Patients Data The study included 42 eyes in 29 Egyptian children who underwent combined trabeculotomy-trabeculectomy with MMC as an initial procedure and were followed-up for 12mo postoperatively. Table 1 summarizes the demographic data collected from the 42 eyes (29 patients) included in the study. The mean age at presentation of the 29 patients was 8.57±10.63mo. No difference was found in clinical severity between the two subgroups in our study (Table 2). Table 3 shows the summary of the preoperative and postoperative IOP and corneal diameter of the 42 eyes included in the study.
Table 1 Demographic data
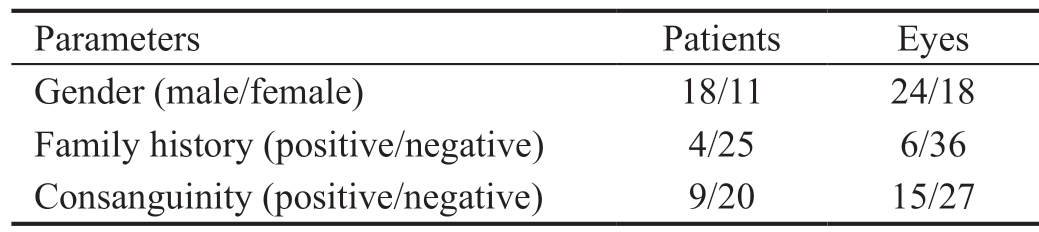
Parameters Patients Eyes Gender (male/female) 18/11 24/18 Family history (positive/negative) 4/25 6/36 Consanguinity (positive/negative) 9/20 15/27
Table 2 Preoperative clinical data of the eyes included in the study in both subgroups
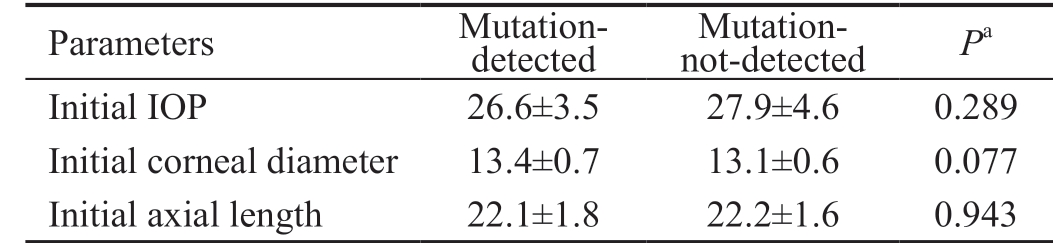
IOP: Intraocular pressure. aIndependent samples t-test.
Parameters Mutationdetected Mutation-not-detected Pa Initial IOP 26.6±3.5 27.9±4.6 0.289 Initial corneal diameter 13.4±0.7 13.1±0.6 0.077 Initial axial length 22.1±1.8 22.2±1.6 0.943
Mutation Detection All PCR products were subjected to SSCP to detect possible mutations through variation in band migration. Most band shifts appeared in the fragments corresponding to exon 2 where the Hinge region and both substrate binding sites are located (Figures 1A). DNA sequencing of the shifted fragments identified 15 different mutations, in 14 patients, that mostly lie in the second exon (Figures 1B, Table 4). Eight of these alterations were previously reported in different populations (HGMD; http://www.hgmd.cf.ac.uk/); three were previously reported SNP(3793 C>T, R48G, and D449D). Seven were novel mutations of which only 2 were predicted to be tolerated [4054 C>G(D83E) and 4535 A>C (M244L)], and the remaining 5 were suggested to be damaging mutation according to “Tolerance Simulation on SIFT”. The novel mutation 4545insC resulted in terminating the protein at amino acid 326 eliminating exon 3 from the resulting truncated protein. All patients with novel mutations had more than one mutation except for patient 14 who had only 4545insC (Ter@326) chain termination mutation. The mutation most frequently found in this study is the previously reported damaging mutation G61E which was found in 5 patients. Most of the mutated amino acids lie in areas that are evolutionary conversed as shown in Figure 1C.The genotype-phenotypes encountered in our study are summarized in Tables 5 and 6. Table 5 shows the 14 patients identified with different mutations. A wide variability in phenotype is noticed with all mutations. From Table 6, it is clear that mutations with severe phenotype and bad outcome were G61E, the truncating mutation Y81X and the frameshift 4339delG. One patient was homozygous while the other had compound heterozygous genotype (G61E+G211D). All these mutations were reported previously with severe phenotypes and bad prognosis[19].
Table 3 Preoperative and postoperative IOP and corneal diameter from eyes included in the study mean±SD

IOP: Intraocular pressure; N/A: Not available. aPaired samples t-test vs preoperative.
Parameters Preoperative Postoperative 1d 1wk 1mo 3mo 6mo 12mo IOP (mm Hg) 27.30±4.11 12.19±3.40 14.02±3.86 15.97±5.34 16.90±6.12 17.85±6.32 18.5±7.42 Pa N/A <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 Corneal diameter (mm) 13.23±0.69 13.23±0.69 13.22±0.67 13.20±0.65 13.17±0.65 13.17±0.65 13.17±0.65 Pa N/A N/A 0.323 0.183 0.058 0.058 0.058
Table 4 Summary of CYP1B1 mutations/SNP observed in 29 Egyptian PCG patients
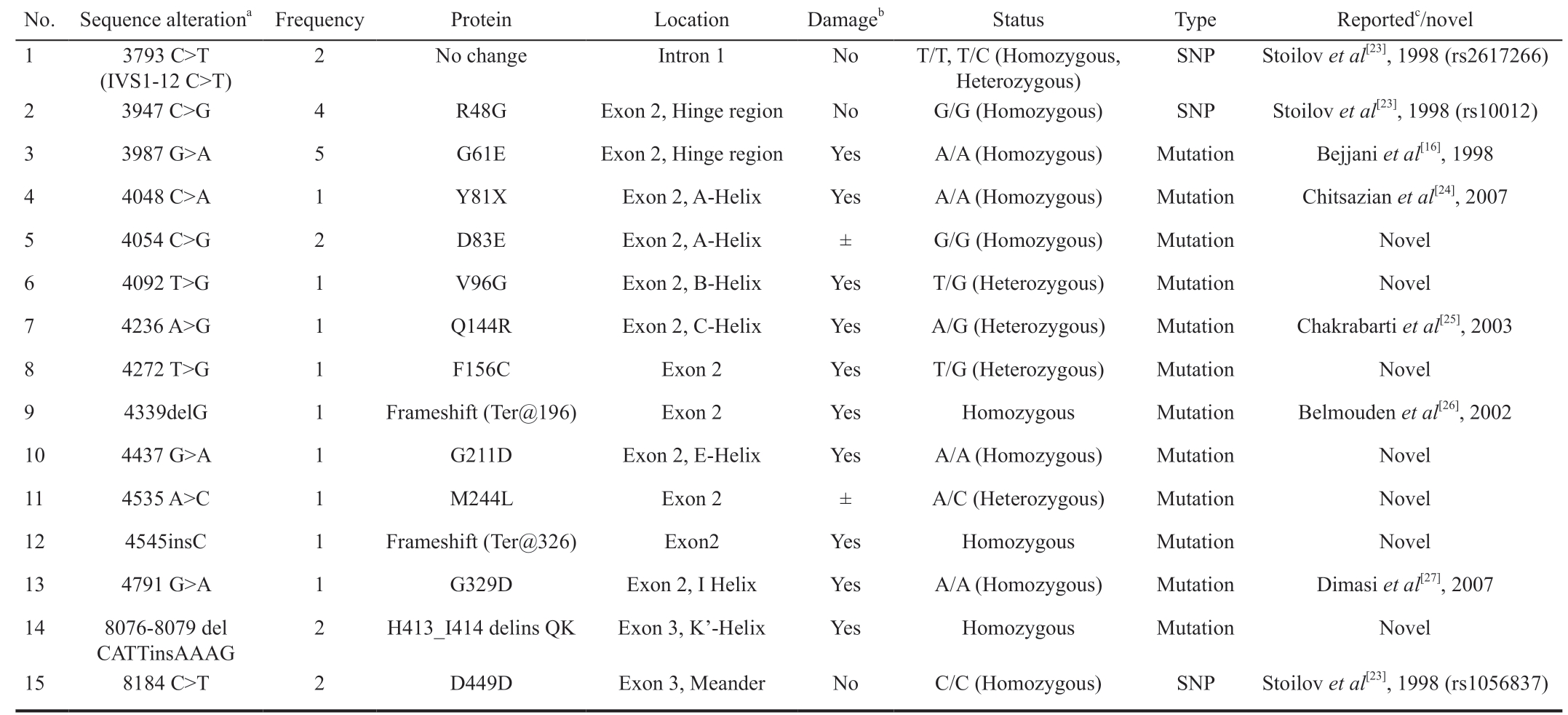
SNP: Single nucleotide polymorphism. aMutations are described in accordance with human CYP1B1 reference sequence at Genbank accession number U56438; bAccording to position on protein and tolerance simulation on SIFT result; cAccording to Human Genome Mutation Database(HGMD).
No. Sequence alterationa Frequency Protein Location Damageb Status Type Reportedc/novel 1 3793 C>T(IVS1-12 C>T)2 No change Intron 1 No T/T, T/C (Homozygous,Heterozygous)SNP Stoilov et al[23], 1998 (rs2617266)2 3947 C>G 4 R48G Exon 2, Hinge region No G/G (Homozygous) SNP Stoilov et al[23], 1998 (rs10012)3 3987 G>A 5 G61E Exon 2, Hinge region Yes A/A (Homozygous) Mutation Bejjani et al[16], 1998 4 4048 C>A 1 Y81X Exon 2, A-Helix Yes A/A (Homozygous) Mutation Chitsazian et al[24], 2007 5 4054 C>G 2 D83E Exon 2, A-Helix ± G/G (Homozygous) Mutation Novel 6 4092 T>G 1 V96G Exon 2, B-Helix Yes T/G (Heterozygous) Mutation Novel 7 4236 A>G 1 Q144R Exon 2, C-Helix Yes A/G (Heterozygous) Mutation Chakrabarti et al[25], 2003 8 4272 T>G 1 F156C Exon 2 Yes T/G (Heterozygous) Mutation Novel 9 4339delG 1 Frameshift (Ter@196) Exon 2 Yes Homozygous Mutation Belmouden et al[26], 2002 10 4437 G>A 1 G211D Exon 2, E-Helix Yes A/A (Homozygous) Mutation Novel 11 4535 A>C 1 M244L Exon 2 ± A/C (Heterozygous) Mutation Novel 12 4545insC 1 Frameshift (Ter@326) Exon2 Yes Homozygous Mutation Novel 13 4791 G>A 1 G329D Exon 2, I Helix Yes A/A (Homozygous) Mutation Dimasi et al[27], 2007 14 8076-8079 del CATTinsAAAG 2 H413_I414 delins QK Exon 3, K'-Helix Yes Homozygous Mutation Novel 15 8184 C>T 2 D449D Exon 3, Meander No C/C (Homozygous) SNP Stoilov et al[23], 1998 (rs1056837)
Thirty-one eyes (76%) in 22 patients showed success, while eleven eyes (24%) in 7 patients showed failure of the initial procedure and required re-surgery. Five out of 7 patient with failed initial procedure had confirmed mutations. While 9 out of 22 patients with successful initial procedure had mutations(Table 7). Positive consanguinity was found to be strongly correlated to failure of the initial procedure (P=0.016) with an odds ratio of 11.25 (Table 7). Age at presentation was found to have a significant directly proportional correlation with survival time, i.e. the older the child, the longer the initial procedure controls the IOP (rho=0.542, P<0.001). A weaker inverse relationship (rho=-0.332, P=0.031) was present between initial IOP and the survival time (Table 8). Kaplan-Meier survival analysis was done to compare the survival time between the mutation-present and the mutation-absent subgroups a difference was revealed in favor of mutationabsent subgroup (Figure 2). This difference was found to be highly significant (log-rank test, P=0.015).
Table 5 Genotype-Phenotype correlations of the patients included in the study
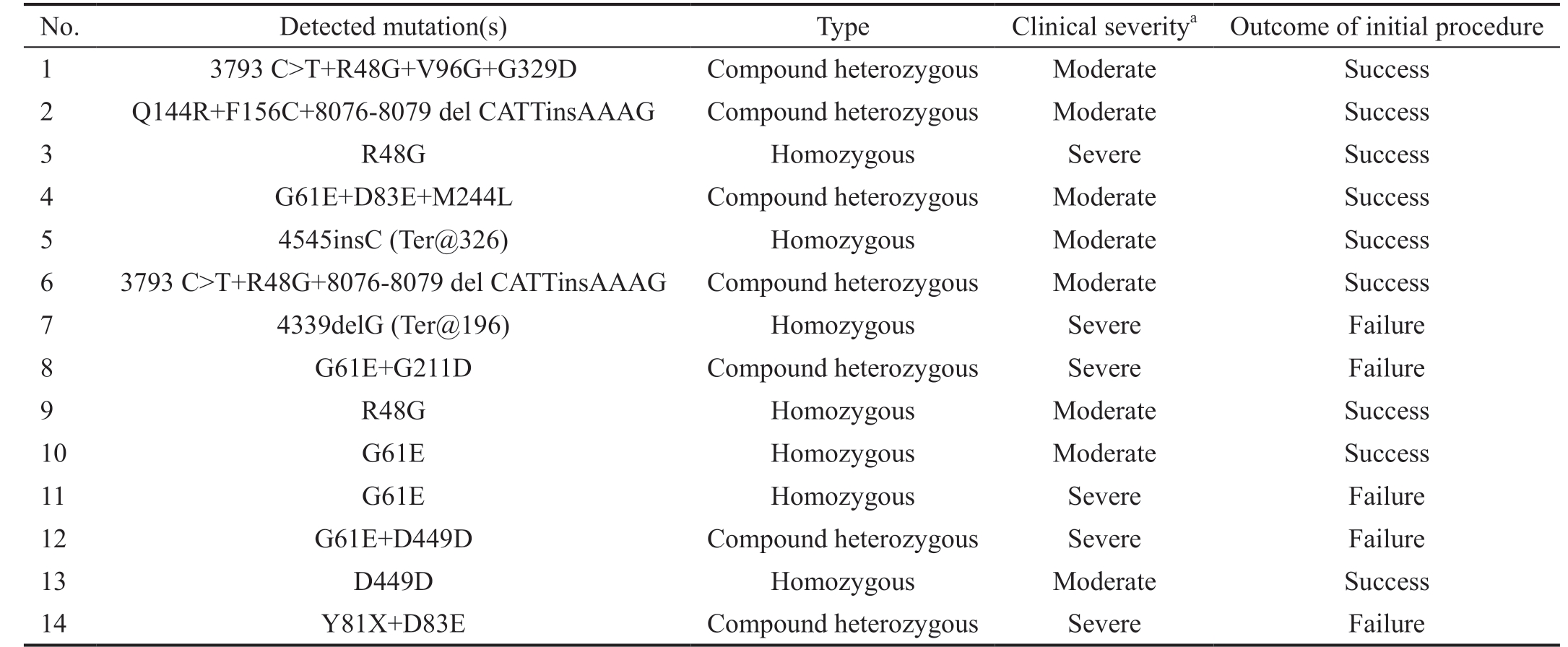
aBased on Al-Hazmi et al[6], 2005.
No. Detected mutation(s) Type Clinical severitya Outcome of initial procedure 1 3793 C>T+R48G+V96G+G329D Compound heterozygous Moderate Success 2 Q144R+F156C+8076-8079 del CATTinsAAAG Compound heterozygous Moderate Success 3 R48G Homozygous Severe Success 4 G61E+D83E+M244L Compound heterozygous Moderate Success 5 4545insC (Ter@326) Homozygous Moderate Success 6 3793 C>T+R48G+8076-8079 del CATTinsAAAG Compound heterozygous Moderate Success 7 4339delG (Ter@196) Homozygous Severe Failure 8 G61E+G211D Compound heterozygous Severe Failure 9 R48G Homozygous Moderate Success 10 G61E Homozygous Moderate Success 11 G61E Homozygous Severe Failure 12 G61E+D449D Compound heterozygous Severe Failure 13 D449D Homozygous Moderate Success 14 Y81X+D83E Compound heterozygous Severe Failure
Table 6 Severe phenotype associated with mutations detected in Egyptian PCG patients
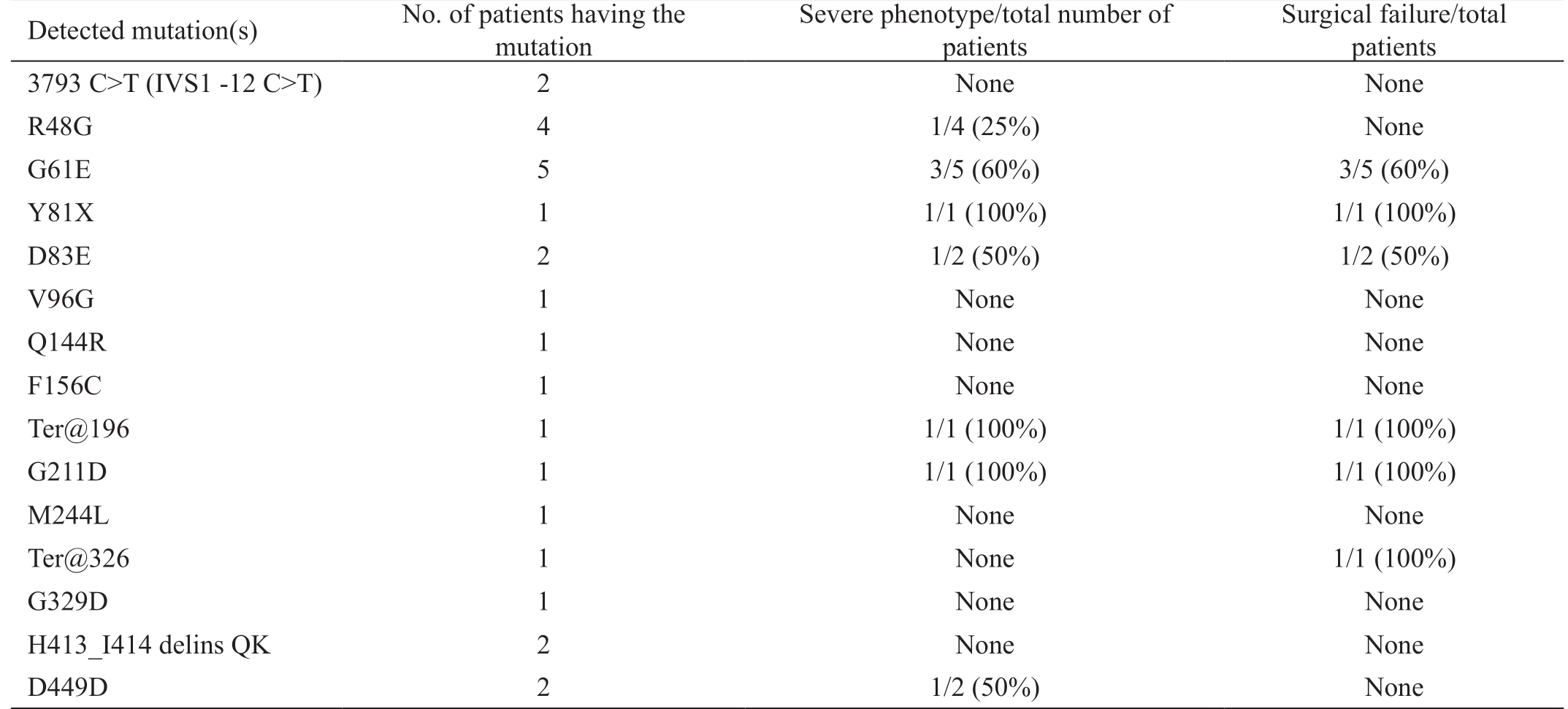
Detected mutation(s) No. of patients having the mutation Severe phenotype/total number of patients Surgical failure/total patients 3793 C>T (IVS1 -12 C>T) 2 None None R48G 4 1/4 (25%) None G61E 5 3/5 (60%) 3/5 (60%)Y81X 1 1/1 (100%) 1/1 (100%)D83E 2 1/2 (50%) 1/2 (50%)V96G 1 None None Q144R 1 None None F156C 1 None None Ter@196 1 1/1 (100%) 1/1 (100%)G211D 1 1/1 (100%) 1/1 (100%)M244L 1 None None Ter@326 1 None 1/1 (100%)G329D 1 None None H413_I414 delins QK 2 None None D449D 2 1/2 (50%) None
Table 7 Mutation status and severity in relation to outcome of the initial procedure

aPearson's Chi-square=6.77; bFisher's exact test.
Mutation status Present Absent P Failure 5 2 0.159a (odds ratio=3.611,95%CI, 0.56-22.89, P=0.173)Success 9 13 Total 14 15 Consanguinity Positive Negative Failure 5 2 0.016b (odds ratio=11.25,95%CI, 1.57-80.30,P=0.0158)Success 4 18 Total 9 20
DISCUSSION
PCG is the most common childhood glaucoma. Despite being rare, its occurrence increases 10 times in some parts of the world because of consanguinity[28]. It has been repeatedly found that mutations in CYP1B1 are involved in the development of the disease. Mutations of this gene have not been properly analyzed in Egypt. In this study, we presented the association between mutations of the CYP1B1 gene and the failure of surgery in PCG. It was found that patients with confirmed CYP1B1 mutations were more likely to fail their initial surgical procedure earlier than patients without mutations as shown from the survival analysis of the surgeries done to the patients enrolled in the study.
Table 8 Correlations between different preoperative factors and the survival time of the initial procedure
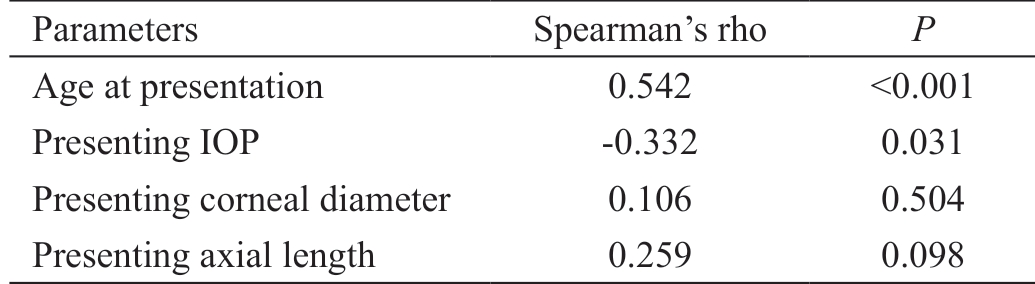
Parameters Spearman's rho P Age at presentation 0.542 <0.001 Presenting IOP -0.332 0.031 Presenting corneal diameter 0.106 0.504 Presenting axial length 0.259 0.098
The mechanism by which CYP1B1 mutations cause the disease is not fully understood[29]. However, it is hypothesized that these mutations result in either reduced activity or abundance of the CYP1B1 enzyme or both[30]. G61E mutation may be implicated with reduced activity, while frameshift mutations and Y81X mutation can result in a decreased abundance of the protein[30]. The reduction or absence of the cytochrome p450 enzyme 1B1 may affect the spatial and temporal regulation of the development of the anterior chamber angle or lead to arrest of its development through accumulation of toxic metabolites[31]. Hollander et al[32] in 2006 studied a small group of PCG patients and classified their angle anomalies histologically into three categories and tried to correlate it with the severity of the disease and the detected CYP1B1 mutations.His work highlighted the possibility that CYP1B1 mutations affect the severity of the disease through affecting the severity of the induced angle anomalies.
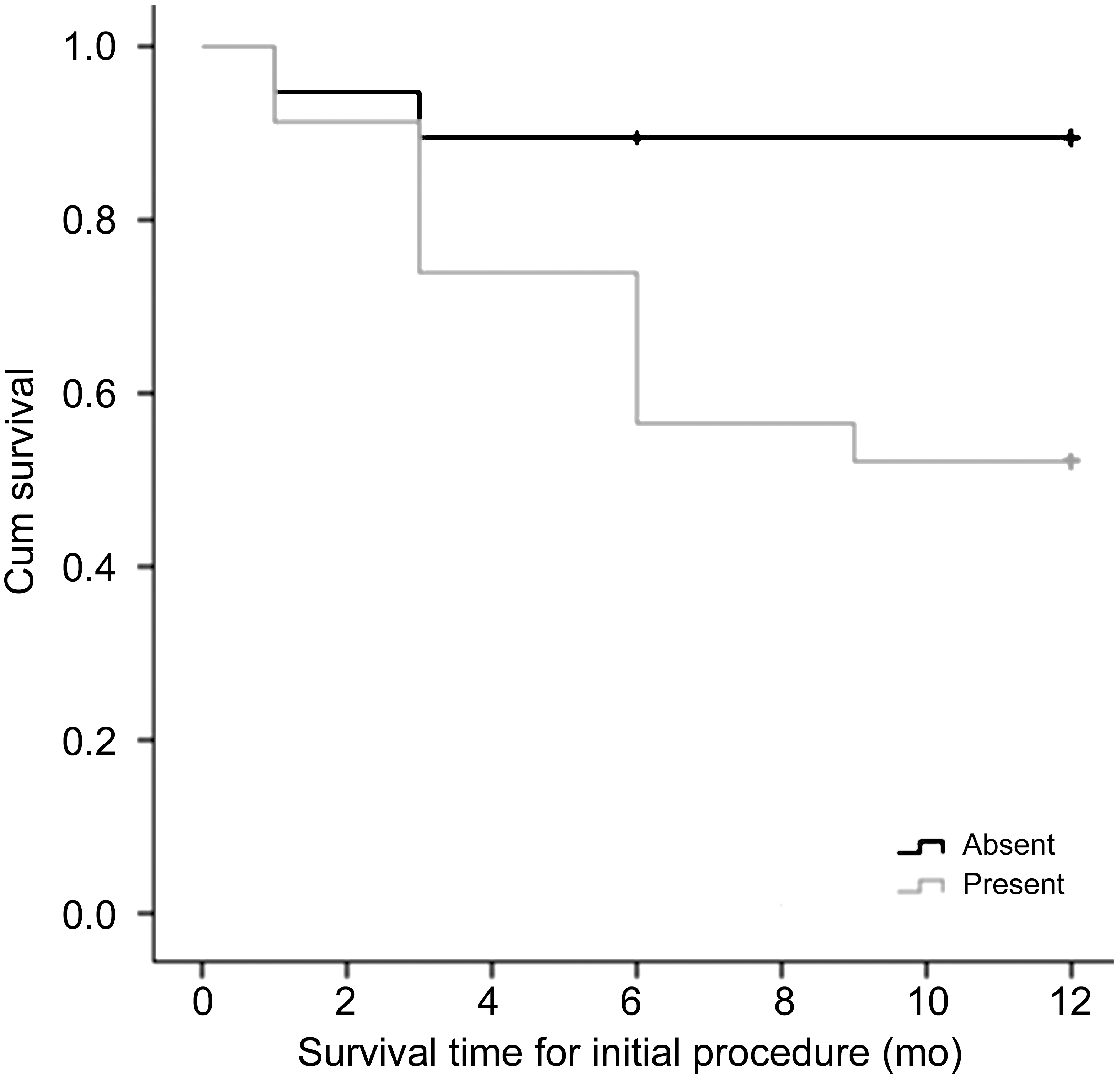
Figure 2 Kaplan-Meier survival analysis: comparing survival time (in months) of the combined procedure in mutation-absent eyes (19 eyes in 15 patients) and mutation-present eyes (23 eyes in 14 patients) subgroups.
Few studies addressed the association of CYP1B1 mutations with poor surgical prognosis in PCG[3,20-21,33-34]. Della Paolera et al[20] in 2010 reported that Brazilian patients with CYP1B1 mutations required significantly more surgical procedures to control the IOP than those without mutations. The same finding was reported in a group of Arab and Jewish patients[21].However, in both of these studies, the surgical procedures performed for the patients were not identified. Chakrabarti et al[33] in 2010 analyzed a CYP1B1 promoter polymorphism and studied the survival of IOP control in patients with or without mutations and found a significant difference between the two groups. The mutation group had poorer control of the IOP over the postoperative period of follow-up. These results are in agreement with the current study in which the initial procedure had more tendencies to fail early in the group with confirmed mutations.
Abu-Amero et al[3] in 2011 studied a group of 74 eyes of Saudi patients with PCG. They reported a non-significant correlation between success or failure of the surgery with the mutation status. These results are in contrast with the results of the current study. This may be attributed to the fact that their patients were operated without randomization using two different surgical procedures; namely combined trabeculectomy-trabeculotomy and nonpenetrating deep sclerectomy with adjuvant MMC. The later procedure is usually inadequate in presence of an anomalous angle[35].
The success rate of the first procedure in Middle Eastern PCG patients (66%) is lower than Western PCG patients(80%-90%)[36]. Geyer et al[21] in 2011 noticed that Jewish PCG patients of European descent had a milder form of the disease and required lesser number of procedure to control the IOP than Arabs and Druze PCG patients. They related this to the lower incidence of CYP1B1 mutations detected in the group of Jewish patients than the other two groups in the study.The success rate in our study was 69% which is comparable to the results reported by Mullaney et al[7] in 1999 and Al-Hazmi et al[6] in 2005 for combined trabeculotomy-trabeculectomy with MMC in moderate to severe cases (70%-80%). Both studies were carried on Saudi patients who are close in ethnicity to our group of patients.
Overview of the factors related to the success and failure of the surgical procedure in our group revealed that positive consanguinity was significantly associated with more failure of the initial procedure. Furthermore, presence of CYP1B1 mutations, early age at presentation and high initial IOP were all associated with the lower survival and early failure of the initial procedure.
In our cohort, the younger the patient at the initial diagnosis,the higher his presenting IOP and the earlier he might fail the surgery. The early onset and onset at birth has been always related to the poor outcome of the surgery[37-39]. However, in 2005, Levy et al[36] underwent a study of prognostic factors for PCG operated in the first 3mo of life in Arab Bedouins. They found that their failure group had significantly higher initial IOP than the successful group, otherwise no other factors;such as mean age at presentation or corneal diameter, was associated with the final outcome. They proposed that initial IOP could be the prognostic factor for the final outcome in PCG. High presenting IOP is considered a severe phenotype that has been associated with presence of CYP1B1 mutations in PCG patients[29,40].
From our results, patients with CYP1B1 mutations were more likely to have lower survival time for the first procedure than the other patients. The patients with mutations had distorted anatomy at the area of the corneoscleral limbus, which made the identification of Schlemm's canal more difficult and the surgeon noticed increased resistance to the trabeculotome insertion into the canal in this group than the non-mutation group of patients. This observation may signal an under- or abnormal development of Schlemm's canal in these patients.These findings suggest that presence of CYP1B1 could be considered as a prognostic factor for the outcome of the surgery, along with clinical prognostic factors such as poor corneal clarity, high presenting IOP and early onset of the disease. This is especially important in populations with high incidence of CYP1B1 mutations as in the Middle East and India. Belmouden et al[26] in 2002 and Abu-Amero et al[3] in 2011 proposed screening for the founder mutation, G61E,in PCG patients from Middle Eastern descent due to its high prevalence. However, all sequence alterations of CYP1B1 should be considered.
A limitation of this study may be the small sample size, but the controlled nature of this prospective study compensated for this weakness. All patients were chosen with the same criteria and from the Egyptian population only. In addition, they were operated upon with the same procedure, done by the same surgeon in all patients. This prospective cohort controlled study design is the best to evaluate risk factors and their impact on the natural history of the disease or intervention[41]. The lack of normal control for CYP1B1 mutations and SNPs in the Egyptian population is another limitation, but we compensated for this by comparative sequence alignment with other CYP1B1 orthologous proteins and computer-assisted mutation tolerance simulation. None the less, the screening for SNPs in normal Egyptian population should be considered in further studies. In addition, the presence of compound mutations makes it harder to identify the phenotypic outcome associated with a specific mutation without further investigations.In summary, this study suggests that CYP1B1 mutations participate in the development of primary congenital glaucoma in Egyptian patients. Seven novel CYP1B1 mutations were detected in our group that could be specific to the Egyptian population. Five of these were suggested to be damaging mutations according to “Tolerance Simulation on SIFT”.The novel mutation 4545insC resulted in terminating the protein at amino acid 326 eliminating exon 3 from the protein.Further studies are required to determine the effects of these mutations on the function of the protein and consequently the possible role in the development of PCG. On the other hand,G61E mutation was the most frequently encountered in our group with potential adverse effects on the clinical severity and surgical prognosis of PCG. Patients harboring CYP1B1 mutations suffer from early failure and poorer prognosis than patients with no mutations of CYP1B1.
Therefore, CYP1B1 mutations might be considered as a prognostic factor for the surgery in PCG together with clinical prognostic factors. From the outcomes of the current research,we recommend that at least patients with severe phenotype should undergo genetic testing which will give a good prognostic idea regarding the outcomes of their subsequent surgery.
ACKNOWLEDGEMENTS
Clinical part of the work was performed at the Ophthalmology Department, Faculty of Medicine, Cairo University, Egypt. The rest of the work at the Cancer Biology Department, National Cancer Institute, Cairo University, Egypt.
Conflicts of Interest: Khafagy M, None; El-Guendy N,None; Tantawy M, None; Eldaly M, None; Elhilali H, None;Abdel Wahab A, None.
1 Walton DS. Infantile, Childhood, and Juvenile Glaucomas. In: The Glaucoma Book. New York, NY: Springer New York; 2010:567-579.
2 Campos-Mollo E, López-Garrido MP, Blanco-Marchite C, Garcia-Feijoo J, Peralta J, Belmonte-Martínez J, Ayuso C, Escribano J. CYP1B1 mutations in Spanish patients with primary congenital glaucoma:phenotypic and functional variability. Mol Vis 2009;15:417-431.
3 Abu-Amero KK, Osman EA, Mousa A, Wheeler J, Whigham B,Allingham RR, Hauser MA, Al-Obeidan SA. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma:genotype-phenotype correlation. Mol Vis 2011;17:2911-2919.
4 Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma(trabeculodysgenesis). Trans Am Ophthalmol Soc 1982;80:321-325.
5 Reddy AB, Panicker SG, Mandal AK, Hasnain SE, Balasubramanian D. Identification of R368H as a predominant CYP1B1 allele causing primary congenital glaucoma in indian patients. Invest Ophthalmol Vis Sci 2003;44(10):4200-4203.
6 Al-Hazmi A, Awad A, Zwaan J, Al-Mesfer SA, Al-Jadaan I, Al-Mohammed A. Correlation between surgical success rate and severity of congenital glaucoma. Br J Ophthalmol 2005;89(4):449-453.
7 Mullaney PB, Selleck C, Al-Awad A, Al-Mesfer S, Zwaan J. Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Arch Ophthalmol 1999;117(4):457-460.
8 Sarfarazi M, Stoilov I. Molecular genetics of primary congenital glaucoma. Eye (Lond) 2000;14( Pt 3B):422-428.
9 Akarsu AN, Turacli ME, Aktan SG, Barsoum-Homsy M, Chevrette L,Sayli BS, Sarfarazi M. A second locus (GLC3B) for primary congenital glaucoma (Buphthalmos) maps to the 1p36 region. Hum Mol Genet 1996;5(8):1199-1203.
10 Sarfarazi M. Recent advances in molecular genetics of glaucomas.Hum Mol Genet 1997;6(10):1667-1677.
11 Sarfarazi M, Stoilov I, Schenkman JB. Genetics and biochemistry of primary congenital glaucoma. Ophthalmol Clin North Am 2003;16(4):543-554,vi.
12 Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA,Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K,Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y,Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 2009;84(5):664-671.
13 Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet 1997;6(4):641-647.
14 Chakrabarti S, Kaur K, Kaur I, Mandal AK, Parikh RS, Thomas R, Majumder PP. Globally, CYP1B1 mutations in primary congenital glaucoma are strongly structured by geographic and haplotype backgrounds. Invest Ophthalmol Vis Sci 2006;47(1):43-47.
15 Weisschuh N, Wolf C, Wissinger B, Gramer E. A clinical and molecular genetic study of German patients with primary congenital glaucoma. Am J Ophthalmol 2009;147(4):744-753.
16 Bejjani BA, Lewis RA, Tomey KF, Anderson KL, Dueker DK, Jabak M, Astle WF, Otterud B, Leppert M, Lupski JR. Mutations in CYP1B1,the gene for cytochrome P4501B1, are the predominant cause of primary congenital glaucoma in Saudi Arabia. Am J Hum Genet 1998;62(2):325-333.17 Plásilová M, Stoilov I, Sarfarazi M, Kádasi L, Feráková E, Ferák V.Identification of a single ancestral CYP1B1 mutation in Slovak Gypsies(Roms) affected with primary congenital glaucoma. J Med Genet 1999;36(4):290-294.
18 Ohtake Y, Tanino T, Suzuki Y, Miyata H, Taomoto M, Azuma N,Tanihara H, Araie M, Mashima Y. Phenotype of cytochrome P4501B1 gene (CYP1B1) mutations in Japanese patients with primary congenital glaucoma. Br J Ophthalmol 2003;87(3):302-304.
19 Li N, Zhou Y, Du L, Wei ML, Chen XM. Overview of Cytochrome P450 1B1 gene mutations in patients with primary congenital glaucoma.Exp Eye Res 2011;93(5):572-579.
20 Della Paolera M, de Vasconcellos JP, Umbelino CC, Kasahara N,Rocha MN, Richeti F, Costa VP, Tavares A, de Melo MB. CYP1B1 gene analysis in primary congenital glaucoma Brazilian patients: novel mutations and association with poor prognosis. J Glaucoma 2010;19(3): 176-182.
21 Geyer O, Wolf A, Levinger E, Harari-Shacham A, Walton DS, Shochat C, Korem S, Bercovich D. Genotype/phenotype correlation in primary congenital glaucoma patients from different ethnic groups of the Israeli population. Am J Ophthalmol 2011;151(2):263-271.e1.
22 Stoilov IR, Costa VP, Vasconcellos JP, Melo MB, Betinjane AJ, Carani JC, Oltrogge EV, Sarfarazi M. Molecular genetics of primary congenital glaucoma in Brazil. Invest Ophthalmol Vis Sci 2002;43(6):1820-1827.
23 Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, Turacli ME, Or M, Lewis RA, Ozdemir N, Brice G, Aktan SG, Chevrette L,Coca-Prados M, Sarfarazi M. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet 1998;62(3):573-584.
24 Chitsazian F, Tusi BK, Elahi E, Saroei HA, Sanati MH, Yazdani S, Pakravan M, Nilforooshan N, Eslami Y, Mehrjerdi MA, Zareei R, Jabbarvand M, Abdolahi A, Lasheyee AR, Etemadi A, Bayat B,Sadeghi M, Banoei MM, Ghafarzadeh B, Rohani MR, Rismanchian A,Thorstenson Y, Sarfarazi M. CYP1B1 mutation profile of Iranian primary congenital glaucoma patients and associated haplotypes. J Mol Diagn 2007;9(3):382-393.
25 Chakrabarti S, Komatireddy S, Mandal AK, Balasubramanian D. Gene symbol: CYP1B1. Disease: glaucoma, primary congenital. Hum Genet 2003;113(6):556.
26 Belmouden A, Melki R, Hamdani M, Zaghloul K, Amraoui A, Nadifi S, Akhayat O, Garchon HJ. A novel frameshift founder mutation in the cytochrome P450 1B1 (CYP1B1) gene is associated with primary congenital glaucoma in Morocco. Clin Genet 2002;62(4):334-339.
27 Dimasi DP, Hewitt AW, Straga T, Pater J, MacKinnon JR, Elder JE, Casey T, Mackey DA, Craig JE. Prevalence of CYP1B1 mutations in Australian patients with primary congenital glaucoma. Clin Genet 2007;72(3):255-260.28 Chouiter L, Nadifi S. Analysis of CYP1B1 gene mutations in patients with primary congenital glaucoma. J Pediatr Genet 2017;6(4):205-214.
29 Kaur K, Mandal AK, Chakrabarti S. Primary congenital glaucoma and the involvement of CYP1B1. Middle East Afr J Ophthalmol 2011;18(1):7-16.30 Chavarria-Soley G, Sticht H, Aklillu E, Ingelman-Sundberg M,Pasutto F, Reis A, Rautenstrauss B. Mutations in CYP1B1 cause primary congenital glaucoma by reduction of either activity or abundance of the enzyme. Hum Mutat 2008;29(9):1147-1153.
31 Stoilov I, Rezaie T, Jansson I, Schenkman JB, Sarfarazi M. Expression of cytochrome P4501b1 (Cyp1b1) during early murine development. Mol Vis 2004;10:629-636.
32 Hollander DA, Sarfarazi M, Stoilov I, Wood IS, Fredrick DR, Alvarado JA. Genotype and phenotype correlations in congenital glaucoma. Trans Am Ophthalmol Soc 2006;104:183-195.
33 Chakrabarti S, Ghanekar Y, Kaur K, Kaur I, Mandal AK, Rao KN,Parikh RS, Thomas R, Majumder PP. A polymorphism in the CYP1B1 promoter is functionally associated with primary congenital glaucoma.Hum Mol Genet 2010;19(20):4083-4090.
34 Chen XL, Chen YH, Wang L, Jiang DK, Wang WZ, Xia MY, Yu L, Sun XH. CYP1B1 genotype influences the phenotype in primary congenital glaucoma and surgical treatment. Br J Ophthalmol 2014;98(2):246-251.
35 Roche O, Beby F, Parsa A, Orssaud C, Dufier JL, Parsa CF.Nonpenetrating external trabeculectomy for congenital glaucoma: a retrospective study. Ophthalmology 2007;114(11):1994-1999.
36 Levy J, Carmi R, Rosen S, Lifshitz T. Primary congenital glaucoma presenting within the first three months of life in a Bedouin population:prognostic factors. J Glaucoma 2005;14(2):139-144.
37 deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma). Surv Ophthalmol 1983;28(1):1-19.
38 Beck AD. Diagnosis and management of pediatric glaucoma.
Ophthalmol Clin North Am 2001;14(3):501-512.
39 Biglan AW. Glaucoma in children: are we making progress? J AAPOS 2006;10(1):7-21.
40 Panicker SG, Mandal AK, Reddy AB, Gothwal VK, Hasnain SE.Correlations of genotype with phenotype in Indian patients with primary congenital glaucoma. Invest Ophthalmol Vis Sci 2004;45(4):1149-1156.
41 Hartung DM, Touchette D. Overview of clinical research design. Am J Health Syst Pharm 2009;66(4):398-408.