INTRODUCTION
A cute eye pain (EP) is a very common ocular symptom in many ophthalmic diseases such as keratitis, ocular trauma and glaucoma et al. The cornea pain is one of the most common kinds of EP, and is always strong sharp, and causing visual disability. As a densely innervated structure, the cornea is one of the most sensitively tissues, which is supplied by the trigeminal nerve with the ophthalmic branch. Thus, infectious keratitis and corneal ulcer can cause serious EP. In China,the prevalence reported by other researcher was 0.148% in infectious keratitis[1] and the cornea pain always be the most frequently complain in keratitis patients. Cornea pain not only leads to the ocular discomfortable, but also might lead to the psychological disorders such as depressive and anxiety symptoms[2].
For corneal pain, the direct cause is that the damage of the cornea nerve triggers peripheral and central trigeminal sensory networks. Whether acute EP was processed at the level of the cortex remains unknown. In order to evaluate the alteration of intrinsic brain activity, functional magnetic resonance imaging (fMRI) had increasingly been applied in acute and chronic pain. Mathur et al[3] reported that the acute migraine pain individuals involved in cognitive-related neural activity changes. Increased function connectivity between insula and the anterior cingulate and the hippocampus were observed in the acute pressure pain[4]. Moreover, the anterior cingulated,primary sensory and motor cortex activity were involved in acute and persistent pain[5-6]. The chronic pain not only leads to brain function change but also lead to abnormilities of brain structure. Duke Han et al[7] demonstrated that increased functional connectivity occurred in the chronic pain between the left insula and posterior cingulate. The reduced grey matter volume was supposed to be associated with trigeminal neuralgia in the primary somatosensory and orbitofrontal cortices compared with health controls[8]. Yet it is little known on the resting state functional connectivity (rsFC) between homotopic regions in the whole brain in acute EP.
Hemispheric specialization is a notable feature of the human brain and is involved in memory[9], cognition[10] and emotion[11].Thus, hemispheric specialization provides a crucial evidence for neurophysiological mechanism. It has been reported previously that the fMRI imaging in EP patients has successful been applicated to assess the neurophysiological changes. It is an useful method by using fMRI analysis at resting-state to assessing activity of regional brain during rest with amplitude of low-frequency fluctuation (ALFF)[12-13], which is a reliable and reproducible method[14-15]. Our previously study has found abnormal ALFF in the precentral gyrus, and left precuneus, left caudate and parahippocampal gyri in patients with acute EP[16].Meanwhile, it's well known that visual sensation is strongly associated with interhemispheric synchrony[17]. The voxelmirrored homotopic connectivity (VMHC) method, an fMRI technology at resting state, was applied to evaluate the rsFC both in the functional hemisphere and its counterpart in the opposite part with the time series for a given voxel[18]. A previous VMHC study reported that decreased interhemispheric connectivity in the precuneus, the dorsolateral prefrontal and posterior cingulate cortex were studied in postherpetic neuralgia individuals[19]. However, so far, whether alterations of interhemispheric functional synchronization occur in EP subjects is still unknown.
Here, our aim of this study was to explore changes of interhemispheric rsFC in acute EP individuals by the VMHC analyze method. We speculated that acute EP might be correlated with the disrupted rsFC in the interhemispheric part.Furthermore, whether the mean VMHC values can be used as a useful clinical biomarker for acute EP.
SUBJECTS AND METHODS
Ethical Approval In this research, the protocol in methods complied with the Declaration of Helsinki and conformed to the principles of Medical Ethics of the Ophthalmology
Department, the First Affiliated Hospital of Nanchang University and the Ophthalmology Department of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University. Informed consent forms were provided to all volunteers.
Participants Totally 20 patients were recruited, and all of them were with acute EP (5 females and 15 males), from the Ophthalmology Department in the First Affiliated Hospital of Nanchang University and the Ophthalmology Department from the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University. The inclusion criteria of acute EP subjects in the study were: 1) acute EP patients due to corneal ulcers or keratitis; 2) bilateral eye involvement, with no ophthalmic diseases, such as myopia, cataracts, strabismus,optic degeneration, uveitis, retinal diseases, etc.
In our study, the exclusion criteria for EP were listed as follow:1) chronic symptoms with EP, more than three months; 2)ocular trauma related EP; 3) EP patients with a history of painkiller treatment before MRI scan; 4) serious complications related EP such as endophthalmitis, ocular perforating, orbital cellulitis, et al; 5) EP patients were complained with headache or pains from other body parts; 6) cardiovascular system diseases related EP, heart disease, hypertension, and so on; 7)psychiatric related problems and infarction diseases in cerebra(cerebral vascular malformations, infarction and hemorrhage cerebral disease).
There were 20 healthy controls (HCs; 5 females and 15 males)enrolled in the study and they were closely matched in demographics information such as sex, age, and education level to the EP subjects. All health subjects followed the listed criteria: 1) with no eye problem and the best corrected visual acuity above 1; 2) no psychiatric related disorders (such as bipolar disorder or depression, and so on); 3) had no cardiac pacemaker or metal devices implanted in body and so on, all of which ensued the HCs be able to be examined with MRI scan.
Magnetic Resonance Imaging Data Acquisition All participants were examined by MRI on a 3.0T system which had eight-channel head coil (Siemens, Munich, Germany).Under spoiled gradient-recalled echo sequence, the T1-weighted whole-brian scans were performed with the parameters: (thickness=1.0 mm, repetition time at 1900ms,acquisition matrix with 256×256, echo time at 2.26ms, view field: 250×250 mm2, gap=0.5 mm, flip angle=9°). During 8-min resting-state scan with parameters as follow (echo time at 30ms, repetition time about 2000ms, gap=1.2 mm,thickness=4.0 mm, acquisition matrix with 64×64, flip angle=90°, 29 axial, view field =220×220 mm2) covered whole brain were corrected. During the fMRI scan, each participant was keeping awake during the whole examination.
Data Preprocessing in Functional Magnetic Resonance Imaging As shown in previous studys[20-21], the functional data was entered into the Data Processing Assistant, which was designed as Resting-State fMRI (rs-fMRI) Advanced Edition(DPARSFA; http://rfmri.org/DPARSFA)[20] and Statistical Parametric Mapping (SPM8; refer to http://www.fil.ion.ucl.ac.uk/spm) on the basis of MATLAB2013a (Mathworks,Natick, MA, USA).
Statistical Analysis in Voxel-mirrored Homotopic Connectivity In order to correct the normality, the VMHC maps in individuals were converted to z values by applying a Fisher z-transformation in the REST software (http://restingfmri.sourceforge.net). As shown in a previous study[18], a random effect of two sampled t-test was applied to analyze z-maps in individual to identify the changes in VMHC between two groups, in a voxel-wise manner with the global VMHC as covariate. The voxel level (P<0.05) was set as the statistical threshold for the multiple comparisons with Gaussian random field (GRF) theory (z>2.3, cluster >40 voxels, P<0.01,corrected FDR).
Statistical Analysis In this study, the cumulative clinical measurements were statistically analyzed with independent sample t test with the SPSS software (SPSS version 16.0, Inc.,Chicago, IL, USA). With the SPM8 toolki, the analysis of general linear model was performed for the statistical analysis,and the paired t-tests was applied to evaluate the changes in the z-maps of VMHC between the health controls and the EP groups.The receiver operating characteristic (ROC) curves was applied to evaluate the changes in the mean values of the VMHC from different brain regions between EP and healthy individuals.The relationship of behavioral performances and the mean values of VMHC in different brain regions was illustrated by Pearson correlation between the EP group and healthy subjects(P<0.05 with significant differences).
RESULTS
Demographics and Visual Measurements In the study, we don't found any significant differences for age with P=0.796 and for weight with P=0.845, between the HCs and acute EP individuals (P>0.05). The duration for EP was 13.17±6.21d showed in the mean±standard deviation (Table 1).
Voxel-mirrored Homotopic Connectivity Differences By compared to HCs, the VMHC decreased in acute EP individuals in lingual/calcarine, and medial frontal gyrus (MFG) and precentral/postcentral gyrus (PreCG/PosCG) (Figure 1, Table 2).In these two groups, we found the mean values of VMHC altered, and the values were displaced in a histogram (Figure 1C). In the EP individuals, our results showed no association between their clinical symptoms and the mean values of VMHC in different brain regions with P>0.05 showed no significant difference.
Receiver Operating Characteristic Curve Our study speculated that by comparing the differences between the EP and HCs individuals, the VMHC might be a very useful clinical markers. Furthermore, the mean VMHC values were compared and analyzed by the ROC curves method in different brain areas. The under ROC curve area (AUCs) stands for diagnostic rate. The AUCs for VMHC values were 0.933 with P<0.001, and the 95%CI about 0.731-0.984, for lingual/calcarine; PreCG/PosCG was 0.938 (P<0.001, 95%CI: 0.704-0.961); for MFG (0.990; P<0.001; 95%CI: 0.786-0.999),showed in Figure 2, EPs<HCs.
DISCUSSION
As we known, the VMHC is a novel and reliable rs-fMRI method to illustrate the alterations of functional interhemispheric connectivity. Our study is the first to explore the functional interhemispheric connectivity involved in acute EP individuals.In this research, comparing with HCs, the significant decreased VMHC values were observed in the bilateral parts of lingual/calcarine, bilateral PreCG/PosCG and bilateral MFG in the acute EP individuals (Table 2). Compared with HCs, the following regions demonstrated very significantly increased DC values to various extents: 1-lingual/calcarine (L; BA30,t=-4.529), 2-lingual/calcarine (R; BA30, t=-4.529), 3-(PreCG/PosCG) (L; t=-3.692 for BA), 4-(PreCG/PosCG) (R; BA4, t=-3.692), 5-MFG (L; BA8 with t=-5.123) and 6-MFG (R; BA8,t=-5.123) in acute EP individuals (Figure 3).
Lingual gyrus and calcarine sulcus are both parts of the occipital lobe. The primary visual cortex (V1) concentratedin the calcarine sulcus as reported[22], and the lingual gyrus located between the posterior part of the collateral sulcus and the calcarine sulcus, which plays a very important role in vision and dreaming[23]. Various neuroimaging studies demonstrated that the occipital lobe was related to many chronic pain processes including headache and low back pain[24-25]. Moreover, a VBM study reported that chronic low back pain individuals were supposed to be closely associated with the decreased volume in occipital lobe by gray matter[26].Meanwhile, Cutrer et al[27] revealed that the migraine patients displaced cerebral blood flow reduction in the occipital cortex.In this study, acute EP individuals displaced that VMHC values decreased in lingual/calcarine, which indicated impaired function of interhemispheric functional connectivity in lingual/calcarine. Therefore, it supposed to be that acute EP in individuals might lead to interhemispheric dysfunction in pain processes brain region.
Table 1 Demographics and clinical measurements for EP and HC group
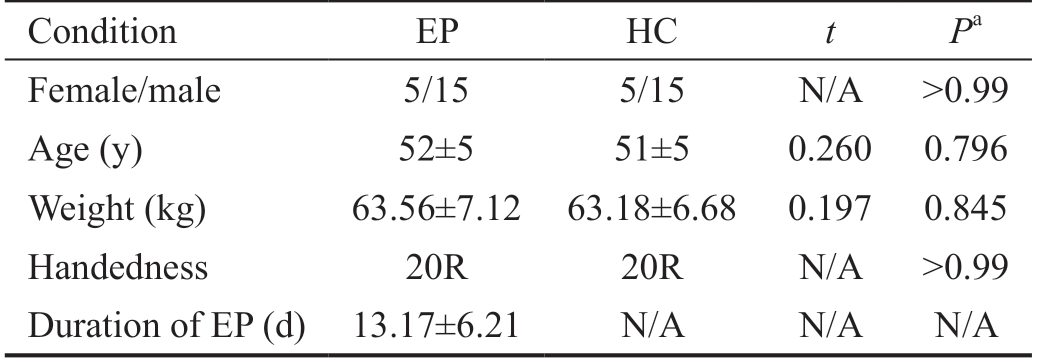
EP: Eye pain; HC: Healthy control; N/A: Not applicable. aIndependent t-tests comparing two groups.
Condition EP HC t Pa Female/male 5/15 5/15 N/A >0.99 Age (y) 52±5 51±5 0.260 0.796 Weight (kg) 63.56±7.12 63.18±6.68 0.197 0.845 Handedness 20R 20R N/A >0.99 Duration of EP (d) 13.17±6.21 N/A N/A N/A
Table 2 Brain areas demonstrated significantly different VMHC values between EP and HC group
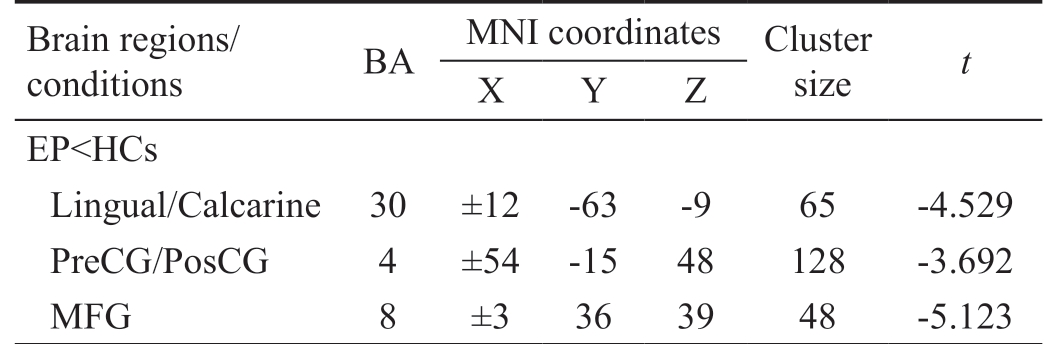
VMHC: Voxel-mirrored homotopic connectivity; EP: Eye pain; HC:Healthy control; BA: Brodmann area; MNI: Montreal Neurological Institute; MFG: Medial frontal gyrus; PreCG/PosCG: Precentral/postcentral gyrus.
Brain regions/conditions BA MNI coordinates Cluster size t X Y Z EP<HCs Lingual/Calcarine 30 ±12 -63 -9 65 -4.529 PreCG/PosCG 4 ±54 -15 48 128 -3.692 MFG 8 ±3 36 39 48 -5.123
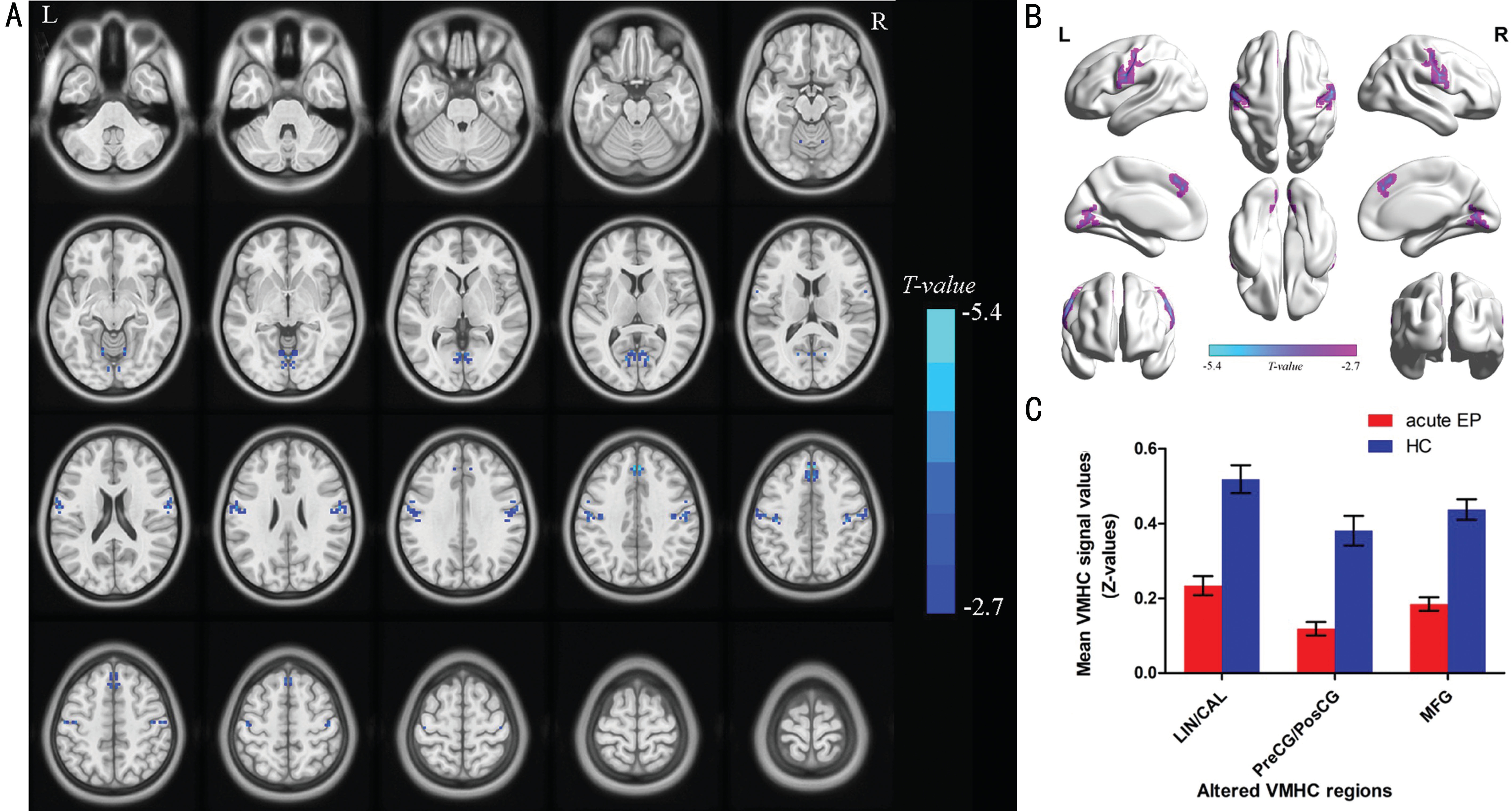
Figure 1 Interhemispheric connectivity in the EPs versus HCs A: The lower VMHC values was indicated by blue areas showed P<0.01 for multiple comparisons analyze within GRF theory, P<0.01, z>2.3, cluster above 40 voxels with corrected FDR; B: The mean VMHC values were altered between the EPs and HC individuals; C: The differences observed in the interhemispheric connectivity were significant in the lingual/calcarine, PreCG/PosCG, MFG.
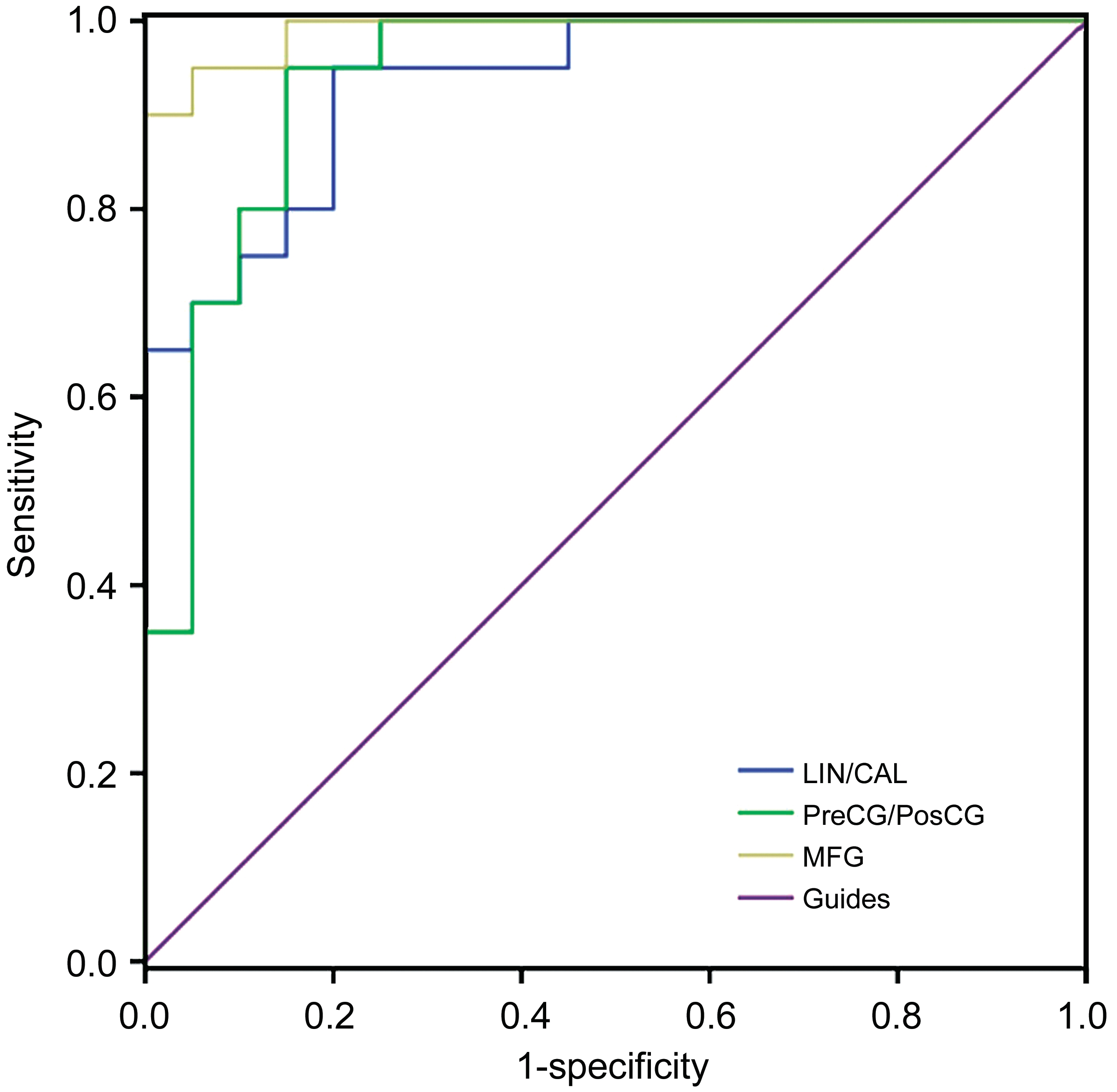
Figure 2 The analysis of ROC curve in the mean values of VMHC for different brain regions.
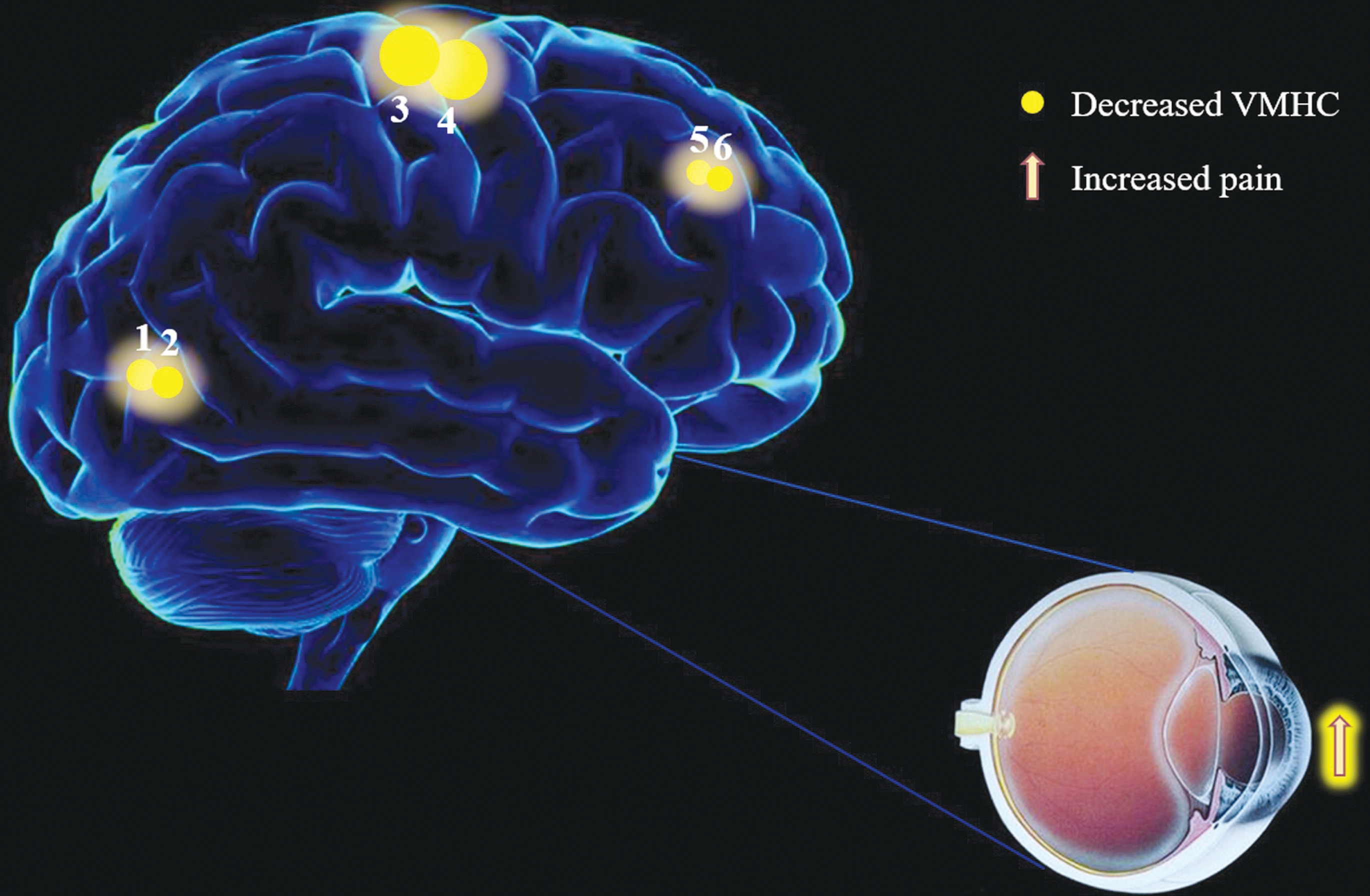
Figure 3 The VMHC results of brain activity in the acute EP individuals.
Primary somatosensory cortex (S1) was found to be located in postcentral gyrus, which is correlated with sensation[28].
Meanwhile, the S1 play a very critical role in the processes sensation of pain[29]. A recent research reported that functional and structural plasticity of S1 occurred in chronic pain individuals[30]. An fMRI study revealed that corneal pain subjects showed activations in S1 and primary motor cortex[31]. Moreover, a phantom limb pain patient showed increased sensitivity of S1 to pain-related[32]. In our study, our results exhibited that significant decreased VMHC values was observed in postcentral gyrus, which indicated that EP individuals might associate with hemispheric specialization dysfunction in S1.
Precentral gyrus, known as primary motor cortex (M1), which played a very essential role in motor control. Many previous studies demonstrated that the dysfunction of M1 occurred in complex regional pain syndrome[33]. A neuroimaging study revealed that acute muscle orofacial and cutaneous pain were highly associated with signal change within the contralateral M1 in form of intensity decreases[34]. Meanwhile, acute muscle pain led to the altered activity in S1/M1[6]. In our research,the results showed that acute EP individuals demonstrated significant decreased VMHC values in bilateral precentral gyrus. That is to say, the EP individuals might associate with impaired hemispheric specialization in M1.
MFG is involved in higher cognitive function. The MFG played a critical role in executive and cognition function[35].Our results exhibited that significant decreased VMHC values were observed in the bilateral MFG in individuals with acute eye pan. We speculated that acute pain in eye might cause the dysfunction in cognition.
ROC curve provides a standardized and statistical method to distinguish disorder condition from healthy individuals. When an AUC value is 0.7-0.9, the accuracy is perceived as perfect.A value between 0.5 and 0.7 is considered moderate, and less than 0.5 means the discrimination result is low. The analysis of ROC curve in our research showed that the AUCs of each brain regions were over 0.9, which might represent these specific VMHC differences have a proper diagnostic accuracy in identifying EP. In brief, these results showed that the method of the VMHC might be a sensitive measurement of rs-fMRI,indicating that the lingual gyrus/calcarine, PreCG/PosCG and MFG might be appropriate clinical markers for EP patients in future. In sum, our research suggested that individuals with acute EP demonstrated impaired hemispheric specialization in the M1/S1 regions, the limbic system and emotion regions,which could possibly provide primarily evidences to explain the neurological mechanisms involved in sensorimotor and emotion dysfunction in EP individuals.
Some limitations should be mentioned in our research. First of all, the relatively small sample size should be taken into account. Second, our study did not find the relationship between their clinical features and the mean values of VMHC in many brain areas. Third, in this study, the VMHC methods were applied to investigate hemispheric specialization changes in acute EP individuals. Future study, multi-modal methods of neuro imaging should be used to illustrate the alterations of brain activities.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81660158; No.81400372); Natural Science Key Project of Jiangxi Province (No.20161ACB21017); Health Development Planning Commission Science Foundation of Jiangxi Province (No.20175116).
Conflicts of Interest: Dong ZZ, None; Zhu FY, None; Shi WQ, None; Shu YQ, None; Chen LL, None; Yuan Q, None;Lin Q, None; Zhu PW, None; Liu KC, None; Min YL, None;Ye L, None; Shao Y, None.
1 Cao J, Yang YN, Yang WJ, Wu RX, Xiao X, Yuan J, Xing YQ, Tan XD.Prevalence of infectious keratitis in Central China. BMC Ophthalmol 2014;14:43.
2 Szakáts I, Sebestyén M, Németh J, Birkás E, Purebl G. The role of health anxiety and depressive symptoms in dry eye disease. Curr Eye Res 2016;41(8):1044-1049.
3 Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin 2015;7:347-358.
4 Ichesco E, Puiu T, Hampson JP, Kairys AE, Clauw DJ, Harte SE, Peltier SJ, Harris RE, Schmidt-Wilcke T. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain 2016;20(7):1079-1089.
5 Buffington AL, Hanlon CA, McKeown MJ. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain 2005;113(1-2):172-184.
6 Burns E, Chipchase LS, Schabrun SM. Primary sensory and motor cortex function in response to acute muscle pain: a systematic review and meta-analysis. Eur J Pain 2016;20(8):1203-1213.
7 Duke Han S, Buchman AS, Arfanakis K, Fleischman DA, Bennett DA.Functional connectivity networks associated with chronic musculoskeletal pain in old age. Int J Geriatr Psychiatry 2013;28(8):858-867.
8 Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia.Neuroimage 2013;74:352-358.
9 Manoach DS, White NS, Lindgren KA, Heckers S, Coleman MJ, Dubal S, Holzman PS. Hemispheric specialization of the lateral prefrontal cortex for strategic processing during spatial and shape working memory.Neuroimage 2004;21(3):894-903.
10 Rosa C, Lassonde M, Pinard C, Keenan JP, Belin P. Investigations of hemispheric specialization of self-voice recognition. Brain Cogn 2008;68(2):204-214.
11 Najt P, Bayer U, Hausmann M. Models of hemispheric specialization in facial emotion perception: a reevaluation. Emotion 2013;13(1):159-167.
12 Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, Wang YX. Longterm total sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: a resting-state fMRI study.
Neuropsychiatr Dis Treat 2015;11:761-772.
13 Zhang YX, Zhu CY, Chen H, Duan XJ, Lu FM, Li ML, Liu F, Ma XJ,Wang YF, Zeng L, Zhang W, Chen HF. Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder.J Affect Disord 2015;174:329-335.
14 Dong MH, Li J, Shi XF, Gao SD, Fu SJ, Liu ZQ, Liang FR, Gong QY, Shi GM, Tian J. Altered baseline brain activity in experts measured by amplitude of low frequency fluctuations (ALFF): a resting state fMRI study using expertise model of acupuncturists. Front Hum Neurosci 2015;9:99.
15 Guo WB, Liu F, Xue ZM, Gao KM, Liu ZN, Xiao CQ, Chen HF,Zhao JP. Decreased interhemispheric coordination in treatment-resistant depression: a resting-state fMRI study. PLoS One 2013;8(8):e71368.
16 Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L,Ma MY, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: a resting-state fMRI study. Neuropsychiatr Dis Treat 2018;14:251-257.
17 Foubert L, Bennequin D, Thomas MA, Droulez J, Milleret C.Interhemispheric synchrony in visual cortex and abnormal postnatal visual experience. Front Biosci (Landmark Ed) 2010;15:681-707.
18 Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, Milham MP.Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010;30(45):15034-15043.
19 Jiang J, Gu LL, Bao D, Hong SD, He W, Tan Y, Zeng XJ, Gong HH,Zhang DY, Zhou FQ. Altered homotopic connectivity in postherpetic neuralgia: a resting state fMRI study. J Pain Res 2016;9:877-886.
20 Yan CG, Zang YF. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010;4:13.
21 Goto M, Abe O, Aoki S, et al. Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects.Neuroradiology 2013;55(7):869-875.
22 Nasaruddin NH, Yusoff AN, Kaur S. Brain activation in response to randomized visual stimulation as obtained from conjunction and differential analysis: an fMRI study. Journal of Physics: Conference Series 546(546):012003.
23 Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci 2000;267(1455):1909-1913.
24 Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 1999;56(5):548-554.
25 Shi Y, Liu ZP, Zhang SS, Li Q, Guo SG, Yang JM, Wu W. Brain network response to acupuncture stimuli in experimental acute low back pain: an fMRI study. Evid Based Complement Alternat Med 2015;2015:210120.
26 Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S.Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex 2014;24(4):1037-1044.
27 Cutrer FM, Sorensen AG, Weisskoff RM, Ostergaard L, Sanchez del Rio M, Lee EJ, Rosen BR, Moskowitz MA. Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol 1998;43(1):25-31.28 Kaukoranta E, Hämäläinen M, Sarvas J, Hari R. Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Neuromagnetic recordings and statistical considerations. Exp Brain Res 1986;63(1):60-66.
29 Ploner M, Schmitz F, Freund HJ, Schnitzler A. Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol 2000;83(3):1770-1776.
30 Kim W, Kim SK, Nabekura J. Functional and structural plasticity in the primary somatosensory cortex associated with chronic pain. J Neurochem 2017;141(4):499-506.
31 Moulton EA, Becerra L, Rosenthal P, Borsook D. An approach to localizing corneal pain representation in human primary somatosensory cortex. PLoS One 2012;7(9):e44643.
32 Zhao J, Guo XL, Xia XL, Peng WW, Wang WC, Li SL, Zhang Y, Hu L. Functional reorganization of the primary somatosensory cortex of a phantom limb pain patient. Pain Physician 2016;19(5):E781-E786.
33 Di Pietro F, McAuley JH, Parkitny L, Lotze M, Wand BM, Moseley GL, Stanton TR. Primary motor cortex function in complex regional pain syndrome: a systematic review and meta-analysis. J Pain 2013;14(11):1270-1288.
34 Nash PG, Macefield VG, Klineberg IJ, Gustin SM, Murray GM,Henderson LA. Changes in human primary motor cortex activity during acute cutaneous and muscle orofacial pain. J Orofac Pain 2010;24(4):379-390.
35 Talati A, Hirsch J. Functional specialization within the MFG for perceptual go/no-go decisions based on “what,” “when,” and “where”related information: an fMRI study. J Cogn Neurosci 2005;17(7):981-993.