INTRODUCTION
C oats' disease is an exudative retinopathy characterized by light-bulb aneurysms, capillary non-perfusion,progression to exudative retinal detachment and, if untreated,neovascular glaucoma and phthisis bulbi[1]. It is typically a unilateral disease affecting boys and girls in a 3:1 ratio with an average age of onset between 8 and 16y[2]. Despite the average age, new-onset Coats' disease can occur in adults into their eighth decade[3]. Treatment strategies vary and include cryotherapy, laser photocoagulation, external drainage of subretinal fluid, scleral buckling, and pars plana vitrectomy(PPV)[4]. Adjunctive intravitreal injection of corticosteroids[5-6]and, more recently, anti-vascular endothelial growth factor(VEGF) have also been implemented[7-9]. Treatment is individualized based on presenting symptoms and progression of disease, yet there have been no randomized, clinical trials to demonstrate efficacy of one modality over another. Moreover,the frequency of complications including vision-limiting vitreoretinal fibrosis, tractional retinal detachment (TRD),neovascular glaucoma and enucleation are unknown, and their relation to individual treatment strategies is especially unclear.Ocular VEGF levels have been shown to be elevated in patients with Coats' disease[10-14]. This finding prompted investigational use of anti-VEGF agents as both primary and adjuvant therapy with variable success[10,14-16]. However, the role of VEGF in Coats' disease is unclear. Although Coats' disease is generally an exudative and not a proliferative retinopathy, occasionally vision may be limited by macular scarring from type 3 choroidal neovascularization (CNV)[17-19]. If present, this may respond well to anti-VEGF therapy[4]. Since the implementation of anti-VEGF agents for Coats' disease, even in the absence of CNV, certain concerns have surfaced. Ramasubramanian and Shields[20] reported a case series of eight patients with Coats'disease who underwent intravitreal injection of bevacizumab as well as cryotherapy and/or laser-photocoagulation. Eyes of half of these patients developed vitreoretinal fibrosis and 37.5% developed TRD in less than nine months after initial injection[20]. Gaillard et al[21] reported similar findings in their experience with nine Coats' disease children. In the Gaillard study, eyes of five patients went on to develop fibrotic vitreoretinopathy as early as five months after intravitreal anti-VEGF injections. Four out of these five patients also developed cataracts and one progressed to TRD[21]. In 2016, a larger cohort of patients (n=69) treated by this group was described but the relationship between vitreoretinal fibrosis and anti-VEGF agents was unclear in this cohort[22]. Finally, Bhat et al[23] reported findings of three patients that developed TRD after treatment with intravitreal bevacizumab, cryotherapy, and subretinal fluid drainage.
Tractional retinopathy after anti-VEGF treatment is not specific to Coats' disease and has been reported in patients with diabetic retinopathy[24], retinopathy of prematurity[25], and familial exudative vitreoretinopathy[26]. With ischemic diseases,such as diabetic retinopathy, a proposed angiofibrotic switch mediated by an imbalance in connective tissue growth factor(CTGF) and VEGF turns on a proliferative response that results in fibrosis and traction (popularly referred to as “crunch”)[27-28].However, is the internal milieu of growth factors and cytokines similar in exudative compared to vasoproliferative retinal disease? Furthermore, though complications from intravitreal injections are rare, i.e. subretinal hemorrhage[29-30], intraocular inflammation[31], endophthalmitis[32], and rhegmatogenous retinal detachment[33], these can precipitate permanent visual loss, especially in vulnerable eyes.
Despite the recent attention given to anti-VEGF agents,many of the patients reported in the above studies received both cryotherapy and anti-VEGF for more advanced Coats'disease (Stage≥3A). Cryotherapy is a long-standing and effective treatment, especially when large exudative retinal detachment prevents laser photocoagulation. Some fear that extensive cryotherapy leads to epiretinal membrane[34-35] and vitreoretinal fibrosis[36-37], while limited cryoapplication[38] or cryoapplication after air-fluid exchange[39] appears to be safe.With this in mind, many factors could contribute to vitreoretinal fibrosis with subsequent TRD in Coats' disease including, release of retinal pigment epithelial cells leading to proliferative vitreoretinopathy (PVR) after cryotherapy[36,40], an anti-VEGF “crunch” effect[25,41], or simply, the natural history of advanced-stage Coats' disease[22,42]. Accordingly, there are many unknown factors that impact outcomes in Coats' disease.The goal, therefore, is to understand the potential hazards of each treatment option and to use this information to anticipate adverse outcomes.We set out to elucidate whether an association exists between treatment modality, including laser photocoagulation,cryotherapy or intravitreal anti-VEGF injections, and occurrence of vitreoretinal fibrosis. Given the absence of prospective data, a pooled data analysis of all published,observational reports was undertaken.
METHODS
Eligibility for Considering Studies for This Review
Randomized clinical trials, retrospective case series and case reports with description of clinical course (clinical presentation, treatment decision, and follow up) were included to obtain a total number of patients with Coats' disease.Papers were excluded if there was no discussion of Coats'disease, if there were no clinical descriptions, or if the patient was not diagnosed with Coats' disease. At minimum, the patient description had to include presenting clinical features compatible with diagnosis of Coats' disease, treatment administered (including enucleation or observation), and posttreatment description of clinical outcome. The corresponding author of the report was contacted if information was missing.No personal identifiable information was reviewed in this study and the reporting herein is HIPAA compliant.
Search Method for Identifying Studies A PubMed (Medline,National Institutes of Health, USA) database search for the search term “Coats disease” was last completed November 18, 2017 and returned 489 results. There was no time period or language restriction. Institutional review board approval was not required as no identifiable patient information was reviewed. Non-English language articles were excluded unless a translated version was made available by the publisher.
Study Selection Author Adeniran JF performed the initial search then Adeniran JF and Duff SM performed the review for eligible eyes and quality of evidence assessment using the GRADE criteria[43]. If there was a disagreement for study quality or eye eligibility, the study was presented to pediatriconcology-trained ophthalmologist Ramasubramanian A for final decision.
Data Synthesis and Analysis Individual data were extracted from each paper and included patient age, Stage of Coats' by Shields Classification[44] if published after 2001, treatment administered, presence of any form of fibrosis (macular,peripheral, subretinal, epiretinal) and TRD upon presentation or following treatment, treatment administered (including if observation), surgical therapy (if applicable) and length of follow up. As different studies used different combinations of therapy, each treatment was analyzed individually as a binary variable (whether or not the eye received that treatment) in the primary analysis. If a study did not specifically identify whether laser and/or cryotherapy was performed, it was labeled as “ablative therapy” and excluded from analysis. The primary
Table 1 Demographics and interventions in eyes with Coats’ disease
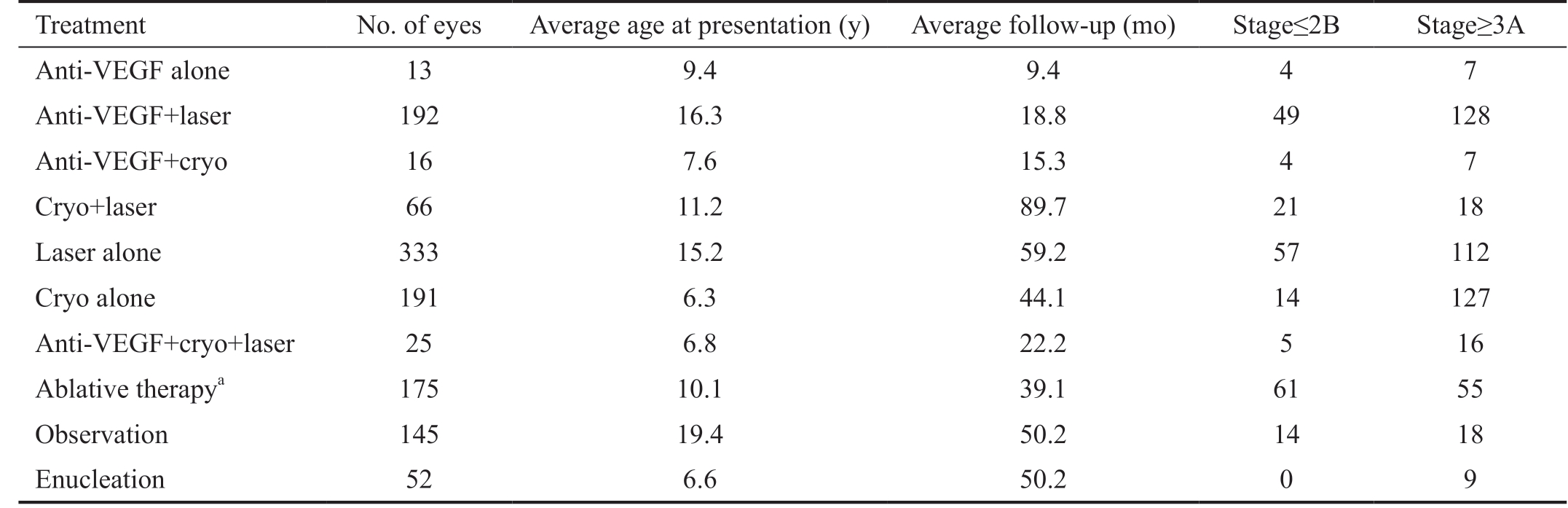
VEGF: Vascular endothelial growth factor. aAblative therapy includes those eyes receiving cryotherapy, laser, or both but details not given in original report.
Treatment No. of eyes Average age at presentation (y) Average follow-up (mo) Stage≤2B Stage≥3A Anti-VEGF alone 13 9.4 9.4 4 7 Anti-VEGF+laser 192 16.3 18.8 49 128 Anti-VEGF+cryo 16 7.6 15.3 4 7 Cryo+laser 66 11.2 89.7 21 18 Laser alone 333 15.2 59.2 57 112 Cryo alone 191 6.3 44.1 14 127 Anti-VEGF+cryo+laser 25 6.8 22.2 5 16 Ablative therapya 175 10.1 39.1 61 55 Observation 145 19.4 50.2 14 18 Enucleation 52 6.6 50.2 0 9
outcome measure was the odds ratio for development of fibrosis or TRD after exposure to 1 of 3 treatment modalities:laser photocoagulation, cryotherapy, or intravitreal anti-VEGF agent. The primary analysis was performed on the historical data from all included studies at any time point (1921-2017)while secondary analysis was performed on data extracted from the anti-VEGF era (2007-2017). Multivariate analysis was performed comparing combination treatment head-tohead, laser and intravitreal anti-VEGF versus cryotherapy and intravitreal anti-VEGF.
Statistical analysis was performed using Minitab 17 (Minitab,Inc, State College, PA, USA). For the analysis of continuous data Student's t-test was used for normally distributed variables and Kruskal-Wallis for non-parametric variables.For the analysis of categorical variables, Chi-square or Fishers' exact test were used and when applicable, odds ratio values with 95% confidence intervals (CIs) were calculated.Binary logistic regression with fibrosis at presentation and treatment type as independent variables was performed in an attempt to determine predictors of TRD. Multivariate analysis was utilized to examine the effect of combination therapy.In all analyses, a two-sided P value <0.05 was considered statistically significant. All presented means are accompanied by their respective standard deviations.
RESULTS
Four-hundred and eighty-nine articles resulted from the initial search. Three-hundred and fourteen were excluded for reasons including, non-English language (n=87), lack of adequate clinical descriptions (n=135) or were unrelated to or did not significantly discuss Coats' disease (n=92). Following these exclusions, 175 papers detailed treatment and clinical course of 1183 eyes. The first included study was from 1921[45] and the first report of intravitreal anti-VEGF use was in 2007[14].All studies were deemed to be of low to very low quality due to their retrospective nature. Of the 1183 eyes whose clinical courses were reviewed (Table 1), data for age (mean 13.7y)and length of follow up (mean 45.5mo) was missing for 42.3% of patients (n=502 missing for both age and follow up).Coats' disease staging, based on the Shields Classification[44],was available in papers published after 2001 (excludes 352 eyes). For the papers after 2001 that had adequate clinical descriptions with wide-field imaging, staging was imposed by the authors if none was explicitly stated. This accounted for staging of 84 eyes. Altogether, ≤Stage 2B accounted for 229 eyes and 530 were ≥Stage 3A.
Of the included eyes, 221 received anti-VEGF agents intravitreally. Thirteen were treated with anti-VEGF agents alone, 192 received intravitreal anti-VEGF plus laser, 16 received intravitreal anti-VEGF plus cryotherapy, and 25 received all three treatment modalities. Additionally, 40 eyes received intravitreal steroids alone while 25 received intravitreal steroids in combination with intravitreal anti-VEGF. Since the study was not structured to assess intravitreal steroids independently, these eyes were grouped according to whether also receiving anti-VEGF agent, ablative therapy or observation. Of those reported, most eyes received bevacizumab (n=201), while the rest included ranibizumab(n=47) or pegatinib (n=2). The vast majority of eyes were treated with ablative therapy including cryotherapy and/or laser photocoagulation without anti-VEGF agents (n=765).While we could not distinguish whether laser, cryotherapy or both were used in 175 eyes (ablative therapy), 66 eyes received both, 333 eyes received laser photocoagulation alone, and 191 eyes received cryotherapy alone. Observation occurred in 145 eyes while 36 were initially enucleated. An additional 17 eyes underwent enucleation after laser photocoagulation alone (n=2),cryotherapy alone (n=6), both laser and cryotherapy (n=1),injection plus cryotherapy (n=1) and a period of observation(n=7). Surgery was undertaken in 184 eyes (Table 2) and included vitrectomy alone±external drainage of subretinal fluid

Figure 1 Color fundus photo of representative patient is shown A: Typical clinical manifestations of Coats' disease are demonstrated here with central exudate and peripheral telangiectasia; B: Fluorescein angiography showing the telangiectatic vessels and the peripheral nonperfusion; C: Following laser photocoagulation, majority of the telangiectatic vessels responded with the exception of a temporal vessel; D:Cryotherapy to the temporal quadrant resulted in regression of the telangiectasia but the occurrence of vitreoretinal fibrosis (arrows) with no retinal detachment.
Table 2 Summary of final clinical observations and surgical interventions eyes

VEGF: Vascular endothelial growth factor; TRD: Tractional retinal detachment; PPV: Pars plana vitrectomy. aExternal drainage performed without PPV or SB; bDoes not include 175 eyes that did not clearly state laser versus cryotherapy as individual or joint therapy.
Treatment (total) Fibrosis Presence of TRD post-treatment Surgical intervention Pre-treatment Post-treatment PPV SB PPV+SB External drainage Anti-VEGF alone (13) 0 1 0 1 0 0 2a Anti-VEGF+laser (192) 11 18 2 4 2 4 10 Anti-VEGF+cryo (16) 2 6 4 2 0 1 1 Laser alone (333) 30 50 11 37 1 1 11 Cryo alone (191) 7 16 8 21 4 18 13 Cryo+Laser (66)b 3 6 3 9 1 2 5 Anti-VEGF+cryo+laser (25) 2 7 5 1 0 0 6 Ablative therapy (175) 0 44 12 6 0 0 4 Observation (145) 3 6 1 7 0 0 9
(SRF; n=89), scleral buckle (SB)±external drainage of SRF(n=8), vitrectomy and SB±external drainage of SRF (n=26),or external drainage of SRF alone (n=61). Where fibrosis was discussed in more descriptive terms, we classified based on macular or peripheral and epiretinal or subretinal locations.Fibrosis (Table 2) was not an uncommon finding on initial presentation (5.4%; 61/1133). An epiretinal membrane was noted in 23 of these cases (2.0%) and 2 eyes presented with macular holes (0.17%). Additionally, there were 5 (0.44%)cases of TRD on presentation. At final follow up, any form of fibrosis (epiretinal, subretinal; peripheral or macular) occurred in 158 eyes (15.5%), macular holes occurred in an additional 2 eyes (0.35%), and an additional 44 eyes (3.9%) had TRD.
One eye that did not initially present with TRD, developed TRD after a period of observation. Eyes with TRD posttreatment and the corresponding treatment given is as shown in Table 2. Of those receiving intravitreal anti-VEGF and laser, 2.5% (1/192) developed TRD, while 25% (4/16) had cryotherapy and anti-VEGF, 4.5% (3/66) had both laser and cryotherapy, 2.7% (9/333) had laser alone, 4.7% (9/192) had cryotherapy alone, and 20% (5/25) had all 3 treatments. Of the 175 eyes having had “ablative therapy”, 12 developed TRD(6.9%). An example of progression of vitreoretinal fibrosis in an unpublished case is presented in the Figure 1.
Table 3 OR (95%CI) for treatment association with vitreoretinal fibrosis and TRD
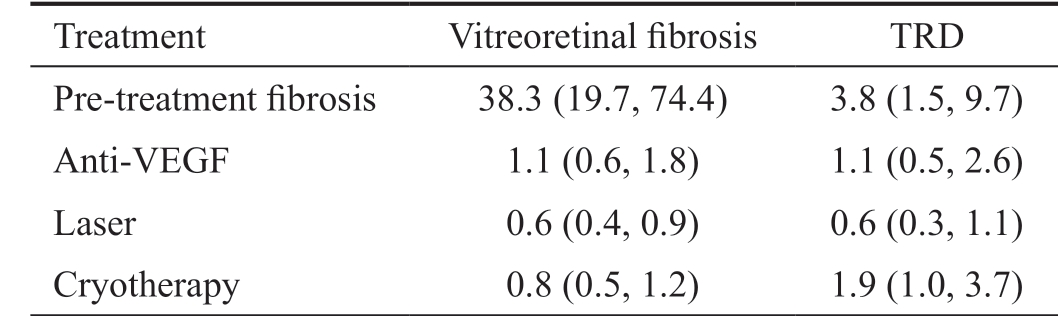
TRD: Tractional retinal detachment; CI: Confidence interval.
Treatment Vitreoretinal fibrosis TRD Pre-treatment fibrosis 38.3 (19.7, 74.4) 3.8 (1.5, 9.7)Anti-VEGF 1.1 (0.6, 1.8) 1.1 (0.5, 2.6)Laser 0.6 (0.4, 0.9) 0.6 (0.3, 1.1)Cryotherapy 0.8 (0.5, 1.2) 1.9 (1.0, 3.7)
As shown in Table 3, patients presenting with any form of fibrosis were at significantly higher risk worsening of vitreoretinal fibrosis post-treatment (OR: 38.3, 95%CI: 19.7-74.4) and TRD (OR: 3.8, 95%CI: 1.5-9.7). While laser was protective from vitreoretinal fibrosis (OR: 0.6, 95%CI: 0.4-0.9),neither cryotherapy (OR 0.8, 95%CI 0.5-1.2) nor intravitreal anti-VEGF (OR: 1.1, 95%CI: 0.6-1.8) were significantly associated with vitreoretinal fibrosis.
While laser tends to protect from TRD (OR: 0.6, 95%CI: 0.3-1.1), there was a greater association with cryotherapy (OR:1.9, 95%CI: 1.0-3.7), but no clear association of intravitreal anti-VEGF agents with TRD (OR: 1.1, 95%CI: 0.5-2.6).Exploring the suggestive association with cryotherapy and TRD further, a secondary analysis was performed on data only since the first reported injection of intravitreal anti-VEGF for Coats' disease[14]. In this subset, cryotherapy showed a significantly higher risk for TRD (OR: 4.9, 95%CI: 2.3-10.6).Laser continued to be protective in the anti-VEGF era from vitreoretinal fibrosis (OR: 0.2, 95%CI: 0.1-0.3) and TRD (OR:0.25, 95%CI: 0.1-0.6). The clinical staging of Coats' disease was first published in 2001, and in the pooled analysis, eyes with exudative retinal detachment (≥3A) were not found to be at higher risk for TRD (OR: 1.2, 95%CI: 0.5-2.7).
Given the increased risk for TRD in cases published since 2007, we looked at combination therapy in the anti-VEGF era. Laser combined with intravitreal anti-VEGF therapy was protective against both post-treatment vitreoretinal fibrosis(OR: 0.1, 95%CI: 0.03-0.35) and TRD (OR: 0.05, 95%CI:0.01-0.23) when compared to cryotherapy combined with intravitreal anti-VEGF (P<0.001).
DISCUSSION
Coats' disease occurs “without signs of significant vitreous traction”[18,46]; hence, treatment itself likely has an impact on the development or worsening of vitreoretinal traction. If certain triggers for vitreoretinal fibrosis and traction can be identified,TRD may be avoided, ultimately improving outcomes. Our initial purpose for this study was to determine the relationship between anti-VEGF agents and the development of TRD due to earlier reports of such events[20,22-23]. However, our pooled data analysis failed to show increased risk of vitreoretinal fibrosis and TRD with use of intravitreal anti-VEGF agents alone. We did, however, find a significant association between the development of TRD in patients that had been treated with cryotherapy in the anti-VEGF era. Additionally, we noted that treatment with laser photocoagulation alone and in combination with intravitreal anti-VEGF therapy decreases the risk of vitreoretinal fibrosis and TRD.
Intravitreal anti-VEGF therapy in exudative retinal diseases such as Coats' disease has been reported to decrease subretinal exudates, macular edema, size and number of telangiectatic vessels, improve retinal detachments, and improve visual outcomes. Although there are reports[20-21,23] that show a possible association of intravitreal bevacizumab or ranibizumab with TRD in Coats' disease, other authors have attributed the adverse outcomes to the use of aggressive cryotherapy which may elicit proliferative vitreoretinopathy[47]. Daruich et al[22]failed to find a significant difference between those eyes with TRD after cryotherapy compared to laser (P=0.07) in their cohort from years 1989 to 2013. However, the pooled data analysis reported herein shows a possible association between increased TRD in eyes treated with both cryotherapy and anti-VEGF agents. This question, however, would be best studied prospectively.
While many advocate use of cryotherapy in presence of exudative RD, there are several reports to suggest that laser is just as effective to treat Coats' disease when the retina is detached[48]. When treatment is targeted to the abnormal vasculature, either green or yellow laser is effectively taken up by hemoglobin in the telangiectatic vessels, precluding the need to target pigment in the retinal pigment epithelium[49-50].Five eyes did present with TRD; one was diagnosed based on enucleation specimen in a 3 year old[51], one in a Coats' plus disease in a 2-year-old[52], one in a 31-year-old that presented with low vision for an “extended period” of time and had anterior chamber cell and flare[42], and two reported in a single case series[53] where patients (17 and 18 years old) presented with advanced disease. Similar to all of these cases is the presence of long-standing, advanced disease. Interestingly,higher stage of Coats' disease (≥3A) was not significantly associated with TRD in the current analysis.
There are many limitations to this analysis, including lack of standardization of treatment regimens, a wide variety of reporting styles, and missing information such as Coats'disease staging, age, follow up time, and other demographic data. As such, odds ratios were used to estimate associations rather than risk ratios to predict outcomes since this could not be calculated from the available data. Notably, many studies stated that cryotherapy or laser was performed without further details regarding treatment provided and thus were excluded from analysis. Importantly, this accounted for 12 eyes with TRD. We attempted to address the issue of a bias towards use of cryotherapy and worse outcomes in more advance eyes; however, there was no association of Stage ≥3A eyes with TRD. Overall, there is a high risk of reporting bias in any retrospective study and, especially relevant to this study,authors may be reluctant to report or publish cases with significant fibrosis and vitreous traction. Lastly, the questions posed herein would be ideally studied in a prospective,controlled setting.
Conclusion When faced with Coats' disease, there are several treatment approaches. While no definitive conclusion may be drawn from a pooled data analysis, we have presented evidence that suggests cryotherapy places patients at a higher risk for post-treatment TRD in this anti-VEGF era. It remains to be seen whether it is the combination of cryotherapy and anti-VEGF that is the culprit. Also evident is that patients presenting with any form of fibrosis are at higher risk to develop progressive vitreoretinal fibrosis and TRD later along with poorer visual acuity[22]. In these cases, one may use cryotherapy and anti-VEGF agents judiciously. Although many questions arise from this analysis, we conclude that treatment of Coats' disease is multi-modal, including various medical and surgical strategies, but that ultimately, the most efficacious regimen remains to be seen. Laser photocoagulation, intravitreal steroids and anti-VEGF agents may represent the safest strategy initially to limit potentially devastating complications from vitreoretinal fibrosis.
ACKNOWLEDGEMENTS
We especially thank those investigators that took the time to clarify or provide additional information from their published reports.
Authors’ contributions: Dr. Ramasubramanian has had full access to all the data in the study and takes responsibility for the integrity of the data.
Foundation: Unrestricted institutional grant from Research to Prevent Blindness.
Conflicts of Interest: Adeniran JF, None; Duff SM, None;Mimouni M, None; Lambert N, None; Ramasubramanian A, None.
1 Shields JA, Shields CL. Review: coats disease: the 2001 LuEsther T.Mertz lecture. Retina 2002;22(1):80-91.
2 Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of Coats'disease: a review of the literature. Ophthalmologica 2012;227(4):175-182.3 Rishi E, Rishi P, Appukuttan B, Uparkar M, Sharma T, Gopal L. Coats'disease of adult-onset in 48 eyes. Indian J Ophthalmol 2016;64(7):518-523.4 Sigler EJ, Randolph JC, Calzada JI, Wilson MW, Haik BG. Current management of Coats disease. Survey of Ophthalmology 2014;59(1):30-46.5 Saatci AO, Doruk HC, Yaman A. Intravitreal dexamethasone implant(ozurdex) in Coats' disease. Case Rep Ophthalmol 2013;4(3):122-128.
6 Ghazi NG, Al Shamsi H, Larsson J, Abboud E. Intravitreal triamcinolone in Coats' disease. Ophthalmology 2012;119(3):648-649.
7 Chaudhary KM, Mititelu M, Lieberman RM. An evidence-based review of vascular endothelial growth factor inhibition in pediatric retinal diseases: part 2. Coats' disease, best disease, and uveitis with childhood neovascularization. J Pediatr Ophthalmol Strabismus 2013;50(1):11-19.8 Kaul S, Uparkar M, Mody K, Walinjkar J, Kothari M, Natarajan S.Intravitreal anti-vascular endothelial growth factor agents as an adjunct in the management of Coats' disease in children. Indian J Ophthalmol 2010;58(1):76-78.
9 Cakir M, Cekiç O, Yilmaz OF. Combined intravitreal bevacizumab and triamcinolone injection in a child with Coats disease. J AAPOS 2008;12(3):309-311.
10 He YG, Wang H, Zhao B, Lee J, Bahl D, McCluskey J. Elevated vascular endothelial growth factor level in Coats' disease and possible therapeutic role of bevacizumab. Graefes Arch Clin Exp Ophthalmol 2010;248(10):1519-1521.
11 Kase S, Rao NA, Yoshikawa H, Fukuhara J, Noda K, Kanda A, Ishida S. Expression of vascular endothelial growth factor in eyes with Coats'disease. Invest Ophthalmol Vis Sci 2013;54(1):57-62.
12 Zhang H, Liu ZL. Increased nitric oxide and vascular endothelial growth factor levels in the aqueous humor of patients with Coats' disease.
J Ocul Pharmacol Ther 2012;28(4):397-401.
13 Zhao Q, Peng XY, Chen FH, Zhang YP, Wang L, You QS, Jonas JB.Vascular endothelial growth factor in Coats' disease. Acta Ophthalmol 2014;92(3):e225-e228.
14 Sun Y, Jain A, Moshfeghi DM. Elevated vascular endothelial growth factor levels in Coats disease: rapid response to pegaptanib sodium.
Graefes Arch Clin Exp Ophthalmol 2007;245(9):1387-1388.
15 Venkatesh P, Mandal S, Garg S. Management of Coats disease with bevacizumab in 2 patients. Can J Ophthalmol 2008;43(2):245-246.
16 Teh SS, Ahem A, Bastion ML. Intravitreal ranibizumab in the management of stage 2B Coats disease in a young adult Malay man. BMJ Case Rep 2013;2013:bcr2013009697.
17 Sigler EJ, Calzada JI. Retinal angiomatous proliferation with chorioretinal anastomosis in childhood Coats disease: a reappraisal of macular fibrosis using multimodal imaging. Retina 2015;35(3):537-546.18 Jumper JM, Pomerleau D, McDonald HR, Johnson RN, Fu AD,Cunningham ET Jr. Macular fibrosis in Coats disease. Retina 2010;30(4 Suppl):S9-S14.
19 Vezzola D, Mapelli C, Canton V, Viola F, Ratiglia R. Macular fibrosis in Coats disease. Retina 2011;31(10):2137-2138.
20 Ramasubramanian A, Shields CL. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96(3):356-359.
21 Gaillard MC, Mataftsi A, Balmer A, Houghton S, Munier FL.ranibizumab in the management of advanced Coats disease Stages 3B and 4: long-term outcomes. Retina 2014;34(11):2275-2281.
22 Daruich A, Matet A, Tran HV, Gaillard MC, Munier FL. Extramacular fibrosis in Coats' disease. Retina 2016;36(10):2022-2028.
23 Bhat V, D'Souza P, Shah PK, Narendran V. Risk of tractional retinal detachment following intravitreal bevacizumab along with subretinal fluid drainage and cryotherapy for stage 3B Coats' disease. Middle East Afr J Ophthalmol 2016;23(2):208-211.
24 Arevalo JF, Maia M, Flynn HW Jr, Saravia M, Avery RL, Wu L, Eid Farah M, Pieramici DJ, Berrocal MH, Sanchez JG. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol 2008;92(2):213-216.
25 Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2008;246(7):1061-1063.
26 Quiram PA, Drenser KA, Lai MM, Capone A Jr, Trese MT. Treatment of vascularly active familial exudative vitreoretinopathy with pegaptanib sodium (Macugen). Retina 2008;28(3 Suppl):S8-S12.
27 Kuiper EJ, van Nieuwenhoven FA, de Smet MD, van Meurs JC,Tanck MW, Oliver N, Klaassen I, van Noorden CJ, Goldschmeding R,Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 2008;3(7):e2675.
28 van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, van Noorden CJF, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol 2012;96(4):587-590.
29 Karagiannis DA, Mitropoulos P, Ladas ID. Large subretinal haemorrhage following change from intravitreal bevacizumab to ranibizumab. Ophthalmologica 2009;223(4):279-282.
30 Modarres M, Naseripour M, Falavarjani KG, Nikeghbali A, Hashemi M, Parvaresh MM. Intravitreal injection of 2.5 mg versus 1.25 mg bevacizumab (Avastin) for treatment of CNV associated with AMD.Retina 2009;29(3):319-324.
31 Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol 2011;56(2):95-113.
32 Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR.Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351(27):2805-2816.
33 Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27(7):787-794.
34 Jaccoma EH, Conway BP, Campochiaro PA. Cryotherapy causes extensive breakdown of the blood-retinal barrier. A comparison with argon laser photocoagulation. Arch Ophthalmol 1985;103(11):1728-1730.
35 Uemura A, Ideta H, Nagasaki H, Morita H, Ito K. Macular pucker after retinal detachment surgery. Ophthalmic Surg 1992;23(2):116-119.
36 Glaser BM, Vidaurri-Leal J, Michels RG, Campochiaro PA.Cryotherapy during surgery for giant retinal tears and intravitreal dispersion of viable retinal pigment epithelial cells. Ophthalmology 1993;100(4):466-470.
37 Dunker S, Faulborn J, Haller EM, Reich ME. The effect of retinal cryoapplication on the vitreous. Retina 1997;17(4):338-343.
38 Saran BR, Brucker AJ. Macular epiretinal membrane formation and treated retinal breaks. Am J Ophthalmol 1995;120(4):480-485.
39 Suesskind D, Altpeter E, Schrader M, Bartz-Schmidt KU, Aisenbrey S. Pars plana vitrectomy for treatment of advanced Coats' disease:presentation of a modified surgical technique and long-term follow-up.Graefes Arch Clin Exp Ophthalmol 2014;252(6):873-879.
40 Campochiaro PA, Kaden IH, Vidaurri-Leal J, Glaser BM. Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells.Arch Ophthalmol 1985;103(3):434-436.
41 Moradian S, Ahmadieh H, Malihi M, Soheilian M, Dehghan MH, Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2008;246(12):1699-1705.
42 Machemer R, Williams JM Sr. Pathogenesis and therapy of traction detachment in various retinal vascular diseases. Am J Ophthalmol 1988;105(2):170-181.
43 Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org.
44 Shields JA, Shields CL, Honavar SG, Demirci H, Cater J.Classification and management of Coats disease: the 2000 Proctor Lecture. Am J Ophthalmol 2001;131(5):572-583.
45 Davis AE. Coats' disease of the retina: report of two cases. Trans Am Ophthalmol Soc 1921;19:222-229.
46 Lai CH, Kuo HK, Wu PC, Kuo ML, Chen YJ. Manifestation of Coats'disease by age in Taiwan. Clin Exp Ophthalmol 2007;35(4):361-365.
47 Grosso A, Pellegrini M, Cereda MG, Panico C, Staurenghi G, Sigler EJ. Pearls and pitfalls in diagnosis and management of coats disease.Retina 2015;35(4):614-623.
48 Nucci P, Bandello F, Serafino M, Wilson ME. Selective photocoagulation in Coats' disease: ten-year follow-up. Eur J Ophthalmol 2002;12(6):501-505.49 Mrejen S, Metge F, Denion E, Dureau P, Edelson C, Caputo G.Management of retinal detachment in Coats disease. Study of 15 cases.Retina 2008;28(3 Suppl):S26-S32.
50 Shapiro MJ, Chow CC, Karth PA, Kiernan DF, Blair MP. Effects of green diode laser in the treatment of pediatric Coats disease. Am J Ophthalmol 2011;151(4):725-731.e2.
51 Rugwizangoga B, Mwabili T, Scanlan T, Meyer P, Kitinya J. Coats'disease in Tanzania: first case report and literature review. Afr Health Sci 2014;14(3):763-768.
52 Yannuzzi NA, Tzu JH, Ko AC, Hess DJ, Cristian I, Berrocal AM. Ocular findings and treatment of a young boy with Coats' plus.Ophthalmic Surg Lasers Imaging Retina 2014;45(5):462-465.
53 Muftuoglu G, Gulkilik G. Pars plana vitrectomy in advanced Coats'disease. Case Rep Ophthalmol 2011;2(1):15-22.