INTRODUCTION
G laucoma was a leading cause of irreversible blindness worldwide[1-2], and could be categorized into two types:angle-closure glaucoma (ACG) and open angle glaucoma. In Asian, ACG is more prevalent than open angle glaucoma[3-5]. In terms of ACG, it could be divided into primary and secondary types. For the pathogenesis of primary ACG, besides the non-pupillary block mechanisms[6-11], the major mechanism was pupillary block[12-13]. As an initial option for primary ACG treatment, miotics could induce the contraction of the sphincter pupillae, which could then pull the peripheral iris away from the trabecular meshwork and therefore reopen the angle, and finally decrease intraocular pressure (IOP) and control the progression of glaucoma. For now, pilocarpine is still widely used in Asian due to the its inexpensiveness and effectiveness[14]. For the pathogenesis of secondary ACG, it would be different due to their different primary diseases. In terms of some secondary ACG, like secondary to zonular laxity and/or lens subluxation, the use of miotics may result in the further loosing of zonules and the forward movement of lens,causing the increase of pupillary block and the development of iris convex and angle closure[15-16]. The aim of this study was to evaluate the changes in anterior chamber structures in eyes with primary chronic angle-closure glaucoma (PCACG)and lens-induced secondary chronic angle-closure glaucoma(LSACG) after miosis using anterior segment optical coherence tomography (AS-OCT).
SUBJECTS AND METHODS
Ethical Approval The study was approved by the Ethics Committee of Tongji Hospital. All patients provided written informed consent ahead of participation. All study conduct adhered to the tenets of the Declaration of Helsinki.
Subjects Fourteen eyes from 14 patients with PCACG, twelve eyes from 12 patients with LSACG and fourteen eyes from 14 healthy subjects were recruited. Healthy subjects were included as negative control if 1) IOP of ≤21 mm Hg with no history of elevated IOP; 2) normal fundus, retinal nerve fiber thickness, visual field, and anterior chamber depth (ACD)with an open angle; 3) no family history of glaucoma. The chronic ACG was diagnosed based on 1) at least 180 degrees of angle closure obliterating pigmented part of trabecular meshwork, whether synechial or appositional, segmented or continuous; 2) requiring IOP-lowering medications, or IOP>21 mm Hg without IOP-lowering medications; 3) visual field loss compatible with glaucoma and/or glaucomatous optic disc changes[17]. None of them had a history of acute glaucoma attacks or signs of acute glaucoma attack. All the patients underwent slit-lamp, gonioscopy, AS-OCT (Visante, USA)and ultrasound biomicroscopy (UBM; iUltrasound, USA)examinations to evaluate the status of zonules and anterior chamber structures. Patients who exhibited zonular dialysis,iridodonesis, anterior chamber angle closure, and shallower ACD in the affected eye in comparison with the fellow eye were defined as cases of LSACG[18]. And after excluding all other secondary chronic angle-closure glaucoma (SCACG)(e.g. secondary to iridocorneal endothelial syndrome,neovascular glaucoma, uveitis), the rest of chronic ACG was defined as PCACG. Study subjects were excluded if they had a history of eye disease (excluding ACG) or surgery, systemic disease or poor OCT image quality[19]. For healthy subjects,one eye was randomly selected. For PCACG patients, if both eyes of the patient were involved in glaucomatous damage,the more severe eye would be chosen as “affected eye”; if only one eye of the patient was involved, it would be “affected eye”. For LSACG patients, the eye involved in glaucomatous damage was regarded as “affected eye”. And all the affected eyes were selected to receive pilocarpine, to observe the postmiosis changes in the anterior chamber structures. Patients with pilocarpine treatment were required to withdraw of pilocarpine for two weeks before participation.
Pre- and Post-miosis IOP and AS-OCT Examinations
IOPs were performed before miosis and every 5min after miosis (NIDEK RT-2100; Japan). If IOP was monitored to be constantly within 21 mm Hg after miosis, the subject would receive AS-OCT examinations 30min after miosis and the IOP value and OCT image at 30min after miosis would be regarded as the post-miosis IOP and OCT image. And if IOP was found >21 mm Hg after miosis, subject would receive AS-OCT examinations immediately instead of 30min postmiosis, and the IOP value and OCT image at this moment would be recorded as the post-miosis IOP and OCT image for further analysis. Moreover, the subject would also administrate oral methazolamide and be closely watched until the pupil and anterior chamber returned to pre-miosis level. AS-OCT were performed in the dark and the scan angle was horizontal across the center of the pupil. The operator adjusted the noise and optimized the polarization to ensure image quality.
Angle opening distance at 500 μm/750 μm from the scleral spur(AOD500/AOD750), trabecular-iris space area at 500 μm/750 μm from the scleral spur (TISA500/TISA750), ACD and pupil diameter (PD) were measured by the built-in software of the AS-OCT. The measurement methods were referred to the previous studies (Figure 1)[20-21].
Statistical Analysis All analyses were performed by SPSS 21.0. Comparison of demographic characteristics among three groups were performed by Kruskal-Wallis H test and Chi-square test. The general estimate equations were used to compare the AOD500/750 and TISA500/750 before and after miosis, and the paired sample t-test was used to compare ACD between two eyes of one subject, and to compare IOP, ACD and PD before and after miosis. All tests were two-tailed, and statistical significance was defined as a P value of <0.05.
RESULTS

Figure 1 Schematic of ACD, angle opening distance 500 µm/750 µm from the scleral spur (AOD500/AOD750) and trabecular-iris space area at 500 μm/750 µm from the scleral spur (TISA500/TISA750) measurements.
Subjects Characteristics The demographic data are shown in Table 1. There were no significant differences in age, sex, and central corneal thickness (CCT) among three groups. Axial length (AL) was significantly shorter, refractive error (RE) and IOP were significantly higher in PCACG group compared with LSACG and healthy groups.
ACD of Affected Eyes and Fellow Eyes in PCACG, LSACG and Healthy Groups Compared with fellow eyes, ACDs of the affected eyes were significantly shallower in both PCACG and LSACG groups, while no such significant difference was found in healthy group (Table 2). The difference between ACD value of fellow eyes and ACD value of affected eyes (ΔACD)were significant larger in PCACG and LSACG groups compared with healthy group. In terms of glaucomatous groups, no significant ΔACD difference between PCACG and LSACG groups was found (Table 3).
Comparison of AOD500/750, TISA500/750, ACD, PD and IOP of Affected Eyes Before and After Miosis in PCACG,LSACG and Healthy Groups Post-miosis ACD and IOP showed no significant changes, while post-miosis AOD500/750 and TISA500/750 increased significantly in PCACG group.Conversely, in terms of anterior chamber structure of LSACG and healthy groups, post-miosis AOD500/750, TISA500/750 and ACD decreased significantly. However, in terms of IOP,the change trends of LSACG and healthy groups were opposite to each other: LSACG group had a significant post-miosis increase in IOP, while healthy group had a significant postmiosis decrease in IOP (Table 4; Figure 2).
Table 1 Demographic characteristics comparison among PCACG, LSACG and healthy groups

aP value between PCACG and LSACG; bP value between PCACG and healthy group; cP value between LSACG and healthy group. dSignificance of difference: Kruskal-Wallis H test.
Parameters PCACG LSACG Healthy subject Pa Pb Pc Age (y) 43.1±8.2 38.3±6.1 38.9±8.6 0.128 0.152 0.975 Male (%) 42.86 41.67 50.00 0.951 0.705 0.671 CCT (μm) 542.5±24.2 533.3±24.4 537.1±30.4 0.262 0.878 0.330 AL (mm) 21.71±0.50 22.95±1.09 23.64±0.81 0.003c <0.001d 0.149 RE (D) 0.18±0.85 -0.97±1.71 -1.23±1.13 0.036c 0.003d 0.427 IOP (mm Hg) 18.57±1.68 16.16±1.54 16.31±1.65 0.002c 0.003d 0.767
Table 2 Comparison of ACD between affected eyes and fellow eyes in PCACG, LSACG and healthy groups

aSignificance of difference: paired t-test.
ACD (mm) Affected (selected) eyes Fellow (non-selected) eyes P PCACG 2.00±0.25 2.13±0.20 0.007a LSACG 2.39±0.20 2.59±0.23 <0.001a Healthy subjects 3.11±0.38 3.14±0.39 0.169
Table 3 Comparison of difference between ACD value of fellow eyes and ACD value of affected eyes (ΔACD)among PCACG, LSACG and healthy groups

ΔACD: The difference between ACD value of fellow eyes and ACD value of affected eyes. aP value between PCACG and LSACG; bP value between PCACG and healthy group; cP value between LSACG and healthy group.dSignificance of difference: Kruskal-Wallis H test.
Parameters PCACG LSACG Healthy subject Pa Pb Pc ΔACD (mm) 0.14±0.16 0.20±0.11 0.03±0.08 0.206 0.045d 0.001d
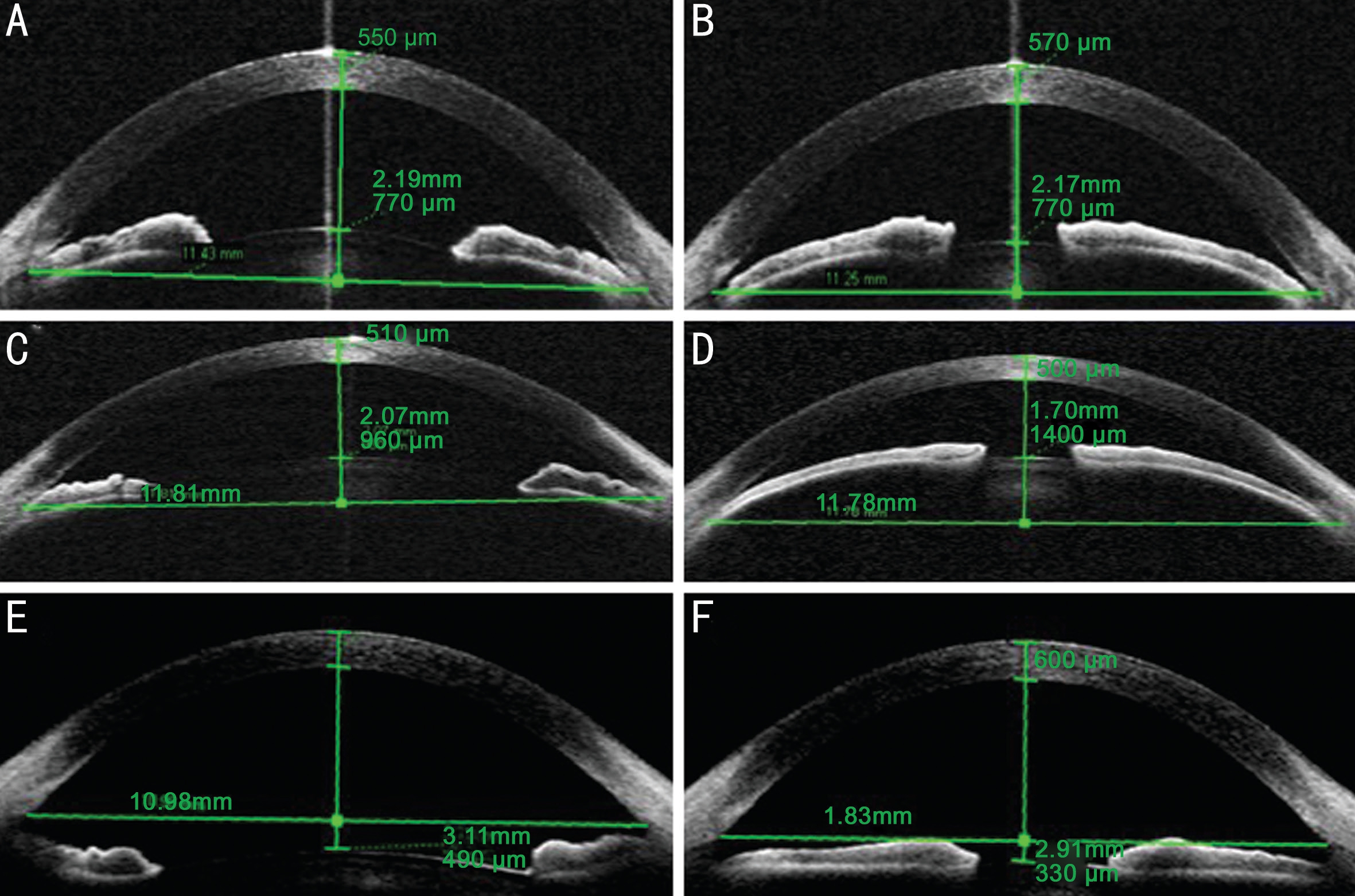
Figure 2 Changes in the anterior chamber after miosis The ACD of the eye with PCACG stayed relatively unchanged and the anterior chamber angle became wider after miosis (A, B). In contrast, the ACD decreased significantly by 0.37 mm in the eye with LSACG after miosis,and the anterior chamber angle also became narrower (C, D). For healthy eyes, after miosis, the ACD decreased significantly and the anterior chamber angle became less wide (E, F).
DISCUSSION
As a miotic, besides its effect on the pupil, the administration of pilocarpine could also induce the contraction of the ciliary muscle, then the relaxation of zonule and changes in the
Table 4 Comparison of AOD500/750, TISA500/750, ACD, PD and IOP of affected eyes before and after miosis in PCACG, LSACG and healthy groups
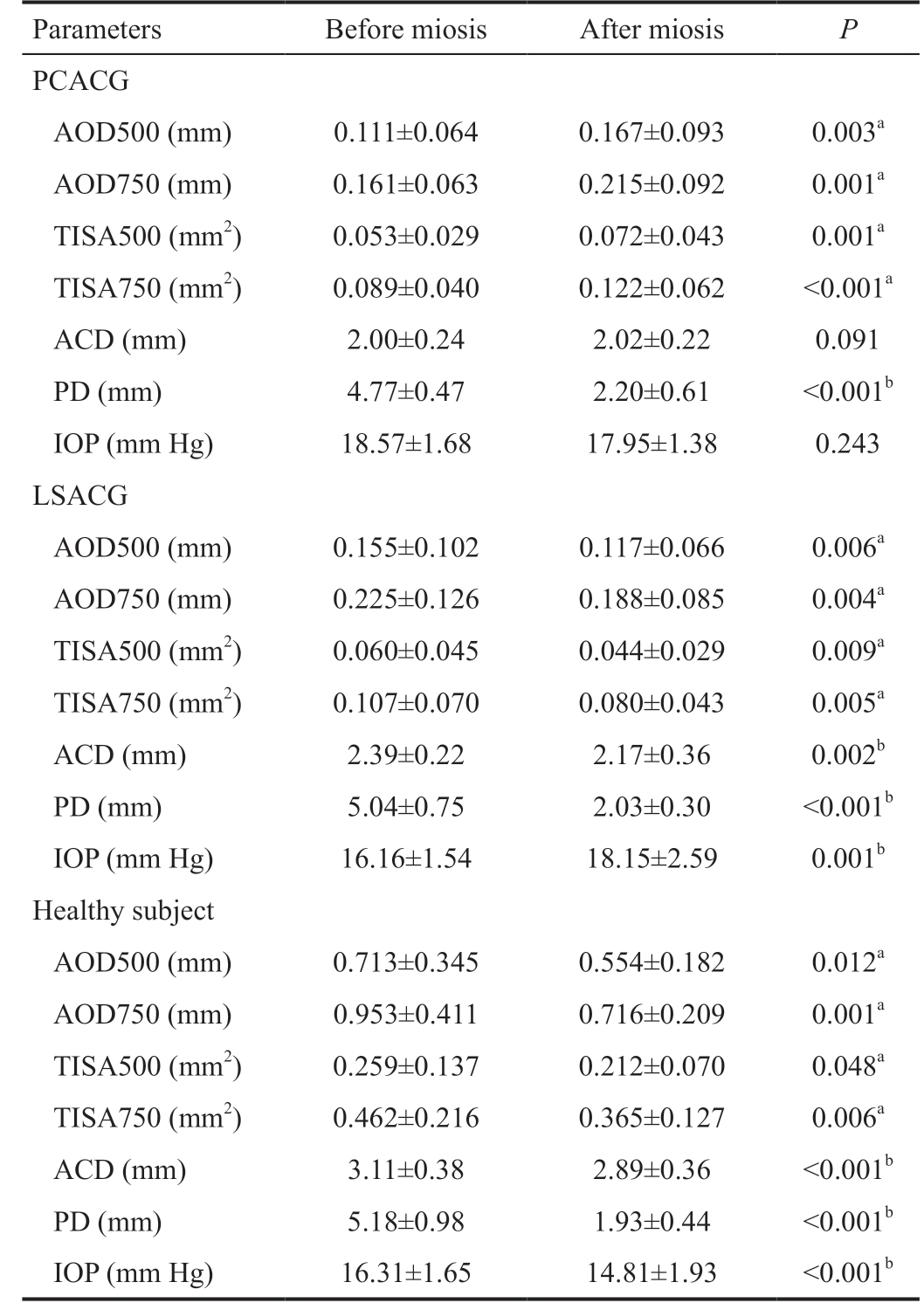
Parameters Before miosis After miosis P PCACG AOD500 (mm) 0.111±0.064 0.167±0.093 0.003a AOD750 (mm) 0.161±0.063 0.215±0.092 0.001a TISA500 (mm2) 0.053±0.029 0.072±0.043 0.001a TISA750 (mm2) 0.089±0.040 0.122±0.062 <0.001a ACD (mm) 2.00±0.24 2.02±0.22 0.091 PD (mm) 4.77±0.47 2.20±0.61 <0.001b IOP (mm Hg) 18.57±1.68 17.95±1.38 0.243 LSACG AOD500 (mm) 0.155±0.102 0.117±0.066 0.006a AOD750 (mm) 0.225±0.126 0.188±0.085 0.004a TISA500 (mm2) 0.060±0.045 0.044±0.029 0.009a TISA750 (mm2) 0.107±0.070 0.080±0.043 0.005a ACD (mm) 2.39±0.22 2.17±0.36 0.002b PD (mm) 5.04±0.75 2.03±0.30 <0.001b IOP (mm Hg) 16.16±1.54 18.15±2.59 0.001b Healthy subject AOD500 (mm) 0.713±0.345 0.554±0.182 0.012a AOD750 (mm) 0.953±0.411 0.716±0.209 0.001a TISA500 (mm2) 0.259±0.137 0.212±0.070 0.048a TISA750 (mm2) 0.462±0.216 0.365±0.127 0.006a ACD (mm) 3.11±0.38 2.89±0.36 <0.001b PD (mm) 5.18±0.98 1.93±0.44 <0.001b IOP (mm Hg) 16.31±1.65 14.81±1.93 <0.001b
aSignificance of difference: General estimate equations; bSignificance of difference: Paired t-test. AOD500/AOD750: Angle opening distance at 500 μm/750 μm from the scleral spur; TISA500/TISA750:Trabecular-iris space area at 500 μm/750 μm from the scleral spur.lens shape, resulting in the increase in the lens thickness and anterior surface curve[22]. Given that ACD was measured as the length of central perpendicular line between posterior surface of the cornea and anterior surface of the lens[21], the increase in lens thickness and anterior surface curve could cause the observed decrease in ACD. And the increase in the lens thickness and anterior surface curve could also pull the iris anteriorly to the cornea, making the anterior chamber angle less wide. In addition, although the anterior chamber of healthy subject became shallower after miosis, post-miosis IOP of healthy subject showed a significant decrease. The reason for that could be the effect of miotic on the conventional aqueous humor outflow pathway. Even if the normal anterior chamber angle became less wide after miosis, however, it still remained open, ensuring the normal aqueous humor drainage.Additionally, the contraction of ciliary muscle induced by pilocarpine could also stretch the trabecular meshwork and Schlemm's canal via scleral spur and connecting fibrils between ciliary body and Schlemm's canal, and then increase the aqueous humor outflow facility and decrease in IOP[23-25]. For PCACG group, the post-miosis changes in anterior chamber structure was opposite to healthy subjects, showing a wider anterior chamber angle. For LSACG group, although its change trend of anterior chamber structure was similar to healthy subjects (shallower anterior chamber and narrower anterior chamber angle after miosis), its change trend of IOP was completely contrary to healthy subject (post-miosis IOP of LSACG showed a significant increase while that of healthy subject showed a significant decrease). Thus, the post-miosis changes in PCACG and LSACG groups were different from healthy subject, indicating that the post-miosis changes in these two glaucomatous groups were not physiological but pathological, and those pathological changes could be relevant to the disease of ACG itself.
After the administration of pilocarpine, PCACG and LSACG groups showed a distinct change trend in anterior chamber structures. For PCACG patients, post-miosis anterior chamber angle widened significantly. Miosis could decrease or eliminate the pupillary block, pull the peripheral iris away from the trabecular meshwork, and finally open the anterior chamber angle[14,26]. In addition, although the anterior chamber angle widened after miosis, IOP only showed a non-significant decrease. In this study, our subjects were chronic glaucomatous patients. The long chronic disease course might have partly or totally damaged the aqueous humor outflow pathway and disabled the drainage ability of the affected eyes. Thus, even though miosis could open or widen the anterior chamber, the aqueous humor would still not fluently drainage through the damaged outflow pathway, resulting in the non-significant change in IOP after miosis. For LSACG group, pilocarpine showed a contrary effect with narrowing in the anterior chamber angle, shallowing in ACD and elevation in IOP.The mechanism might be the zonule. The zonular apparatus is the main support system of the lens. Weakened zonules allow the lens to be mobile and move anteriorly. This could result in the forward lens movement, a shallower anterior chamber, increased pupillary block and iris convexity[27-29],which increase the risk for an angle-closure event[29-30]. All the LSACG patients in this study showed certain evidences of zonule laxity, thus, the administration of pilocarpine would contract the ciliary muscle and further loose the zonule, leading to further displacement of lens, the exacerbation of pupillary block and forward movement of lens-iris diagram and finally the narrowing of anterior chamber angle and increase in IOP. Although both PCACG and LSACG showed signs of chronic ACG, their responses to miotics were different. Thus,we should pay more attention to the chronic ACG patients with suspect of secondary to zonular laxity and avoided the improper administration of pilocarpine on them, which would close the anterior chamber angle and elevate IOP further.
Our results suggested that the ACD difference between affected and fellow eyes in LSACG group was similar to that in PCACG group. For LSACG patients, the displacement of lens and its influence on the anterior chamber were not obvious,and there might also be no other significant signs (e.g. serious eye pain or headache, irisopsia) of elevated IOP. Combined with its chronic disease course, it could be very likely to misdiagnose LSACG as PCACG. Therefore, a detailed history taking, a slit-lamp examination, a necessary AS-OCT or UBM examination to evaluate the status of zonule were important for the distinction between PCACG and LSACG and also meaningful for the correct treatment selection.
In conclusion, we should pay attention to the distinction between PCACG and LSACG patients and the proper administration of pilocarpine in the treatment of patients with chronic ACG.
ACKNOWLEDGEMENTS
Foundation: Supported by the National Natural Science Foundation of China (No.81471744).
Conflicts of Interest: Li M, None; Yan XQ, None; Li GY,
None; Zhang H, None.
1 Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017;5(12):e1221-e1234.
2 Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014;121(11):2081-2090.
3 He MG, Foster PJ, Ge J, Huang WY, Zheng YF, Friedman DS, Lee PS,Khaw PT. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci 2006;47(7):2782-2788.
4 Vijaya L, George R, Arvind H, Baskaran M, Paul PG, Ramesh SV, Raju P, Kumaramanickavel G, McCarty C. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol 2006;124(3):403-409.
5 Foster PJ, Baasanhu J, Alsbirk PH, Munkhbayar D, Uranchimeg D, Johnson GJ. Glaucoma in Mongolia. A population-based survey in Hövsgöl province,northern Mongolia. Arch Ophthalmol 1996;114(10):1235-1241.
6 Diniz Filho A, Cronemberger S, Mérula RV, Calixto N. Comparative biometric study between plateau iris configuration and primary open angle glaucoma with narrow angle. Arq Bras Oftalmol 2009;72(3):302-307.
7 Lee RY, Kasuga T, Cui Q, Porco TC, Huang GF, He MG, Lin SC.Association between baseline iris thickness and prophylactic laser peripheral iridotomy outcomes in primary angle-closure suspects.Ophthalmology 2014;121(6):1194-1202.
8 Mizoguchi T, Ozaki M, Wakiyama H, Ogino N. Peripheral iris thickness and association with iridotrabecular contact after laser peripheral iridotomy in patients with primary angle-closure and primary angleclosure glaucoma. Clin Ophthalmol 2014;8:517-522.
9 Moghimi S, Vahedian Z, Fakhraie G, Ghaffari R, Eslami Y, Jabarvand M, Zarei R, Mohammadi M, Lin S. Ocular biometry in the subtypes of angle closure: an anterior segment optical coherence tomography study.Am J Ophthalmol 2013;155(4):664-673,673.e1.
10 Peng PH, Nguyen H, Lin HS, Nguyen N, Lin S. Long-term outcomes of laser iridotomy in Vietnamese patients with primary angle closure. Br J Ophthalmol 2011;95(9):1207-1211.
11 Ng WT, Morgan W. Mechanisms and treatment of primary angle closure: a review. Clin Exp Ophthalmol 2012;40(4):e218-e228.
12 Heys JJ, Barocas VH, Taravella MJ. Modeling passive mechanical interaction between aqueous humor and iris. J Biomech Eng 2001;123(6):540-547.
13 Razeghinejad MR, Myers JS. Contemporary approach to the diagnosis and management of primary angle-closure disease. Surv Ophthalmol 2018;63(6):754-768.
14 Sun XH, Dai Y, Chen YH, Yu DY, Cringle SJ, Chen JY, Kong XM,Wang XL, Jiang CH. Primary angle closure glaucoma: what we know and what we don't know. Prog Retin Eye Res 2017;57:26-45.
15 Ritch R, Shields MB. The secondary glaucomas. Mosby: St Louis,MO, 1982.
16 Madill SA, Bain KE, Patton N, Bennett H, Singh J. Emergency use of pilocarpine and pupil block glaucoma in ectopia lentis. Eye (Lond)2005;19(1):105-107.
17 Tham CC, Kwong YY, Leung DY, Lam SW, Li FC, Chiu TY,Chan JC, Lam DS, Lai JS. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology 2009;116(4):725-731,731.e1-3.18 Luo LX, Li M, Zhong YM, Cheng B, Liu X. Evaluation of secondary glaucoma associated with subluxated lens misdiagnosed as acute primary angle-closure glaucoma. J Glaucoma 2013;22(4):307-310.
19 Lee RY, Kasuga T, Cui Q, Huang GF, He MG, Lin SC. Association between baseline angle width and induced angle opening following prophylactic laser peripheral iridotomy. Invest Ophthalmol Vis Sci 2013;54(5):3763-3770.
20 Zheng C, Guzman CP, Cheung CY, He YK, Friedman DS, Ong SH,Narayanaswamy AK, Chew PT, Perera SA, Aung T. Analysis of anterior segment dynamics using anterior segment optical coherence tomography before and after laser peripheral iridotomy. JAMA Ophthalmol 2013;131(1):44-49.
21 Li M, Song YW, Zhao Y, Yan XQ, Zhang H. Influence of exercise on the structure of the anterior chamber of the eye. Acta Ophthalmol 2018;96(2):e247-e253.
22 Koeppl C, Findl O, Menapace R, Kriechbaum K, Wirtitsch M, Buehl W, Sacu S, Drexler W. Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg 2005;31(7):1290-1297.
23 Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 2009;88(4):769-775.
24 Gong HY, Trinkaus-Randall V, Freddo TF. Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr Eye Res 1989;8(10):1071-1082.
25 Rosenquist RC Jr, Melamed S, Epstein DL. Anterior and posterior axial lens displacement and human aqueous outflow facility. Invest Ophthalmol Vis Sci 1988;29(7):1159-1164.
26 Papaconstantinou D, Georgalas I, Kourtis N, Krassas A, Diagourtas A,Koutsandrea C, Georgopoulos G. Lens-induced glaucoma in the elderly.
Clin Interv Aging 2009;4:331-336.
27 Tarongoy P, Ho CL, Walton DS. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol 2009;54(2):211-225.
28 Stangler F, Prietsch RF, Fortes Filho JB. Bilateral acute angle closure glaucoma in a young patient receiving oral topiramate: case report. Arq Bras Oftalmol 2007;70(1):133-136.
29 Asaoka R, Kato M, Suami M, Usami Y, Hotta Y, Sato M. Chronic angle closure glaucoma secondary to frail zonular fibres and spherophakia. Acta Ophthalmol Scand 2003;81(5):533-535.
30 Silver DM, Quigley HA. Aqueous flow through the iris-lens channel:estimates of differential pressure between the anterior and posterior chambers. J Glaucoma 2004;13(2):100-107.