INTRODUCTION
M yopia is one of the most commonly encountered refractive errors in humans and, as such, abundant literature exists exploring the mechanisms leading to its development and progression[1]. One of the areas of debate suggests an association between myopia onset and accommodative response[1]. Understanding the influence of accommodation on axial elongation may provide insight on myopia development.
Accommodation is a dynamic process characterized by temporal changes known as microfluctuations (MFs), even under steady viewing conditions. There is extensive debate on the actual role of MFs on the control and maintenance of the accommodative response[2-3]. MFs of accommodation were first investigated in 1960 through Fourier analysis, revealing low (<0.6 Hz) and high (1-2 Hz) frequency components[2,4].Low frequency MFs may arise from changes on the surface of the lens during accommodation[2]. They have also been associated to other factors governing depth of focus, such as,pupil miosis, thus supporting a possible role in the control of accommodative response[3]. Conversely, high frequency MFs seem to reflect noise from blood flow to the eye and orbit rather than actual optical fluctuations of the lens[5-7]. However,mostly for low frequencies, the amplitude of MFs increases with the level of accommodation up to approximately 5.00 D[3-4,8].Regarding the possible association of MFs and myopia, it has been suggested that increased aberrations and depth of focus in myopia may lead to a reduction in blur sensitivity[9-10], which in turn may result in increased accommodation variability and MFs, particularly at the low frequency range[4,8,11-12].Interestingly and probably related to the susceptibility to retinal blur, the amplitude of MFs was found to be larger for lateonset than early onset myopes[8]. However, research is required to determine whether myopia onset and progression is a cause or a consequence of retinal defocus and accommodation variability. Indeed, the study of MFs may contribute to this debate.
MFs are traditionally assessed by measuring the temporal variation in refractive error, as measured with an autorefractor.Recent research has documented the association between refractive error and the magnitude of MFs at the various temporal frequencies[8,11]. Some authors employ the autorefractor Power Refractor II (Plusoptix, Nurenberg, Germany) to record MFs[4]. This device, which is based on infrared eccentric photorefraction[12], has been extensively validated both for clinical practice and research[13-14].
In view of previous research, however, it remains unclear whether the changes in refraction obtained with the Power Refractor II arise exclusively from accommodation fluctuations.Therefore, the aim of the present study was to explore refractive changes in a sample of 10 healthy participants in low and high accommodation demand conditions. For this purpose, we define low accommodation demand as the situation in which participants fixate a distant target (>5 m)or when they fixate targets at any distance under cycloplegia.Similarly, to elicit a high accommodation demand participants were asked to fixate a target at a distance of 0.4 m (2.5 D)without the use of cycloplegia. The amplitude of the obtained MFs at each frequency was submitted to a Fourier analysis to investigate the actual role of accommodation in their origin.Given the documented noise inherent with the assessment of MFs, the difference between the amplitude of MFs at near and distance was calculated for each range of frequencies. It was hypothesized that, by determining the magnitude and influence of the non-accommodative factors, the use of this relative value would afford a better understanding of the actual contribution of accommodation to MFs.
SUBJECTS AND METHODS
Ethical Approval All participants provided written informed consent after the nature of the study was explained to them.The study was conducted in accordance with the tenets of the Declaration of Helsinki of 1975 (as revised in Tokyo in 2004),and received the approval of an Institutional Review Board(Universitat Politècnica de Catalunya).
A sample of 10 volunteers (5 females) aged 18 to 28y[mean±standard deviation (SD) of 23.4±3.4y) participated in the study. All participants had corrected visual acuity of 0.0 logMAR or better and refractive error ranging from -0.75 D to +1.00 D. Participants with ocular pathologies, having undergone refractive or ocular surgery, with accommodative or binocular vision alterations were excluded from the study, as well as those with hypersensitivity to any of the components of the cycloplegic eye drops employed in the study. All participants underwent a complete ocular and visual examination.
The infrared photorefractor Power Refractor II (software version 3.5) was employed to determine temporal variations in objective refraction[13-14]. The monocular dynamic scan mode was selected, in which measurements are conducted every 0.04s. A minimum of 90s of continuous measurements were recorded for the right eye, while participants binocularly fixated a target with the aid of chin-and-forehead rest. Four different experimental conditions were tested three times each following a pre-established random order, although measurements always started with the non-cycloplegic conditions: without cycloplegic administration and target at a distance of 5 m (Far);without cycloplegia and target at 0.4 m (2.5 D; Near); with cycloplegia (Cyclopentolate) and target at 5 m (FarCyclo); and with cycloplegia and target at 0.4 m (NearCyclo). To ensure the attention of participants and continued fixation on the target over the required period an ad hoc eye chart was designed and displayed on a 9.7 inch, 4:3 display tablet (Energy i10 Quad SuperHD, Energy Sistem Soyntec S.A, Spain) at a resolution of 2048 per 1536 pixels. The target consisted in a tumbling E, which changed orientation at 1s intervals. Participants were instructed to use a mouse, which was linked to the tablet via Bluetooth, to signal when the tumbling E was oriented downwards. To avoid interfering with the visualization of the target the photorefractor was placed perpendicularly to the fixation axis and measurements were conducted with the aid of a hot mirror (reflective to infrared radiation and transparent to visible light) oriented at 45 degrees. This mirror was fixed at 5 cm from the corneal plane of the participant, and at 95 cm from the photorefractor, adding a total of 100 cm, which is the measurement distance recommended by the manufacturer.
For each experimental configuration, a measurement log or register was obtained. Occasionally, registers show interruptions, accounting for eyeblinks, and spikes, easily identifiable but unpredictable, which may arise from either the participant or the measurement apparatus. Therefore, any spike which showed a change in refraction over 10 D/s was deleted,whereupon, these blanks and those originating in interruptions were filled in by interpolation.
Figure 1A displays an example of a clean register without spikes or interruptions. A Fast Fourier Transform (FFT)algorithm was employed to convert the register from the original time domain, f(t), to a representation in the frequency domain, F(n), whereby the original 90s register resulted in a set of 1024 data points in the F(n), which corresponded to a range of frequencies from n=0 to n=12.5 Hz. To determine the magnitude of the refractive fluctuations at each frequency,A(n), in D2/Hz, the squared of the modulus of the Fourier transform, known as the power spectrum, was calculated from 0 to 12.5 Hz, in 0.1 Hz steps[8,11]. Figure 1B shows the corresponding power spectrum of the register used as an example.
Previous research has calculated the low (Aℓ) and high (Ah)components of the accommodation MFs as the summation of the A(n) values from n=0.1 to n=0.6 Hz and from n=1.0 to n=2.3 Hz, respectively[2,4]. In addition, a summation over all range of frequencies (n=0.1 to n=12.5 Hz) was performed to determine the total value of the amplitude of the MFs (Atot).Besides, for each range of temporal frequencies the values of the differences (Near-Far) and (NearCyclo-FarCyclo) were calculated, and compared with the Student's t-test for matchedpairs. A P-value of 0.05 or less was considered to denote statistical significance throughout the study.
RESULTS

Figure 1 Refraction changes over time in a near, non-cycloplegic experimental configuration A: Example of a clean register (without spikes or interruptions); B: Power spectrum of the same register showing the magnitude of the refractive MFs in 0.1 Hz frequency steps. The shaded rectangles correspond to the frequency regions used to determine the low and high frequency components of the MFs.
For all range of frequencies, the largest amplitude values were obtained for the experimental condition Near (target at 0.4 m and without cycloplegia), thus suggesting that in these conditions the amplitude of MFs may contain information about the accommodative response. Indeed, statistically significant differences were found between the condition Near and Far (P=0.024 for Aℓ; P=0.035 for Atot), between Near and FarCyclo (P=0.005 for Aℓ; P<0.001 for Ah; P<0.001 for Atot) and between Near and NearCyclo (P=0.017 for Aℓ;P=0.005 for Ah; P=0.002 for Atot). In addition, the condition FarCyclo (target at 5 m and with cycloplegia) resulted in the smallest amplitude values, with statistically significant values between this condition and Far for both Ah (P=0.027) and Atot(P=0.011). The mean values for the differences near-far in the amplitude of the MFs at each range of frequencies, low (0.1 to 0.6 Hz; Aℓ), high (1.0 to 2.3 Hz; Ah) and total (0.1 to 12.5 Hz;Atot), with and without cycloplegia, and the results of the corresponding statistical analysis, are presented in Table 1. For all the tested ranges of frequencies, the difference between the amplitudes at near and far was largest when measurements were conducted without cycloplegia (P=0.029 for Aℓ; P=0.046 for Ah; P=0.024 for Atot), thus giving support to the possible role of accommodation in the origin of MFs. In addition,when comparing only low and high temporal frequencies, the smallest and largest near-far differences were found at high frequencies with cycloplegia and at low frequencies without cycloplegia, respectively.
DISCUSSION
The present research explored a new approach to exploremicrofluctuations related to accommodation. The findings revealed that the values of the amplitude of MFs for the experimental configurations in which accommodation was assumed to be absent (Far, FarCyclo and NearCyclo) were not negligible, irrespective of the range of frequencies under study,a finding that may support the existence of MFs not related to accommodation. Published literature places the possible origin of these other MFs at diverse rhythmic physiological variations such as arterial pulse[5-6] and heart rate[15] for the high frequency range, and breathing rhythm for low frequencies[7],amongst others. Interestingly, for low frequencies the value of the differences near-far with cycloplegia was only 32.9% of that obtained without cycloplegia, that is, other factors than accommodation, such as the convergence effort required for near vision, may account for the changes in refraction at near.Regarding high frequencies, the value of the differences nearfar with cycloplegia was the smallest of the relative values and only corresponded to 19.23% of that found without cycloplegia. This finding may suggest that, for low frequencies,the contribution of factors other than accommodation is superior when fixating a near target than the same contribution for a distant target, whereas for high frequencies the contribution of these non-accomodative factors is similar at near and distance. Thus, factors such as convergence and other ocular movements related to near vision would contribute to low rather than high frequency MFs.
Table 1 Differences near-far in the amplitude of the MFs without and with cycloplegia
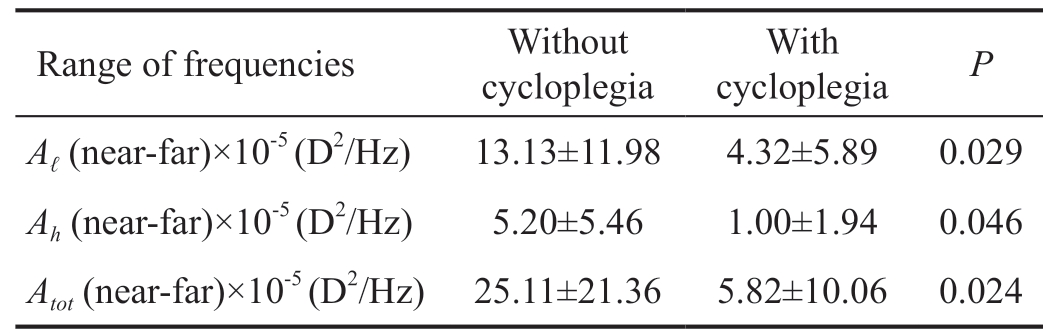
Range of frequencies Without cycloplegia With cycloplegia P Aℓ (near-far)×10-5 (D2/Hz) 13.13±11.98 4.32±5.89 0.029 Ah (near-far)×10-5 (D2/Hz) 5.20±5.46 1.00±1.94 0.046 Atot (near-far)×10-5 (D2/Hz) 25.11±21.36 5.82±10.06 0.024
It must be noted that some interference of the experimental configuration may not be completely discarded. Indeed, we employed a hot mirror, which is a specialized dielectric mirror that reflects infrared light, while allowing visible light to pass.Although this mirror should not restrict normal human vision,it must be acknowledged that the mirror had a relatively small frame, that is, even if participants were instructed to fixate a more distant target, a possible source of disturbance of the actual frame may not be entirely ruled out. As the mirror was used in all measurements, however, the present approach,based on the relative rather than absolute values of amplitude of MFs, should compensate for any possible interference related to the experimental set-up. In addition, only 10 subjects participated in the study, i.e. results describing absolute values of MFs need to be interpreted with caution. However, this should not invalidate de purpose of the investigation, which was to present a novel approach to reduce unwanted noise when exploring MFs.
In conclusion, the present findings evidenced that the relative values of amplitude of MFs, that is, the difference in amplitude near-far for each range of frequencies, with and without cycloplegia, are useful in isolating a large percentage of the MFs not directly related to accommodation, that is, nonaccommodative noise. Therefore, we would advise researchers to employ relative rather than absolute MFs amplitude values as a tool to furthering the understanding of MFs of accommodation and their possible link with myopia onset and progression.
ACKNOWLEDGEMENTS
Conflicts of Interest: Lupón N, None; Gispets J, None;Cardona G, None; Tàpia A, None; Abril H, None.
1 Mutti DO. Hereditary and environmental contributions to emmetropization and myopia. Optom Vis Sci 2010;87(4):255-259.
2 Leahy C, Leroux C, Dainty C, Diaz-Santana L. Temporal dynamics and statistical characteristics of the microfluctuations of accommodation:dependence on the mean accommodative effort. Opt Express 2010;18(3):2668-2681.
3 Yao P, Lin H, Huang J, Chu R, Jiang BC. Objective depth-offocus is different from subjective depth-of-focus and correlated with accommodative microfluctuations. Vision Res 2010;50(13):1266-1273.
4 Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res 2006;46(16):2581-2592.
5 Winn B, Pugh JR, Gilmartin B, Owens H. Arterial pulse modulates steady-state ocular accommodation. Curr Eye Res 1990;9(10):971-975.
6 Owens H, Winn B, Gilmartin B, Pugh JR. Effect of a topical betaadrenergic receptor antagonist on the dynamics of steady-state accommodation. Ophthalmic Physiol Opt 1991;11(2):99-104.
7 Collins M, Davis B, Wood J. Microfluctuations of steady-state accommodation and the cardiopulmonary system. Vision Res 1995;35(17):2491-2502.
8 Day M, Strang NC, Seidel D, Gray LS, Mallen EA. Refractive group differences in accommodation microfluctuations with changing accommodation stimulus. Ophthalmic Physiol Opt 2006;26(1):88-96.
9 Collins MJ, Buehren T, Iskander DR. Retinal image quality, reading and myopia. Vision Res 2006;46(1-2):196-215.
10 Rosenfield M, Abraham-Cohen JA. Blur sensitivity in myopes. Optom Vis Sci 1999;76(5):303-307.
11 Seidel D, Gray LS, Heron G. Retinotopic accommodation responses in myopia. Invest Ophthalmol Vis Sci 2003;44(3):1035-1041.
12 Langaas T, Riddell PM, Svarverud E, Ystenaes AE, Langeggen I,Bruenech JR. Variability of the accommodation response in early onset myopia. Optom Vis Sci 2008;85(1):37-48.
13 Choi M, Weiss S, Schaeffel F, Seidemann A, Howland HC, Wilhelm B,Wilhelm H. Laboratory, clinical, and kindergarten test of a new eccentric infrared photorefractor (PowerRefractor). Optom Vis Sci 2000;77(10):537-548.
14 Jainta S, Jaschinski W, Hoormann J. Measurement of refractive error and accommodation with the photorefractor PowerRef II. Ophthalmic Physiol Opt 2004;24(6):520-527.
15 van der Heijde GL, Beers AP, Dubbelman M. Microfluctuations of steady-state accommodation measured with ultrasonography. Ophthalmic Physiol Opt 1996;16(3):216-221.