Dear Editor,
I am Dr. Yukiko Shibata, from the Department of Ophthalmology,Faculty of Medicine and Graduate School of Medicine,Hokkaido University. I write to present the case of posterior scleritis simulating intraocular tumor.
A formal review and approval were waived by Institutional Review Board in Hokkaido University. The principles outlined in the Declaration of Helsinki were followed. Written informed consent on publishing the clinical and laboratory data was obtained from this patient.
A 59-year-old woman complained of blurred vision and eyelid swelling without obvious ocular pain in her right eye. When she visited a nearby clinic several months before, her bestcorrected visual acuity (BCVA) was 0.4 OD and 1.2 OS. Slitlamp examination revealed senile cataract OD. She underwent cataract surgery OD. Although her BCVA improved to 1.0 after the surgery, she noticed right upper lid swelling with mild irritation. Since magnetic resonance imaging (MRI)displayed markedly thickened scleral lesions, she was referred to Department of Ophthalmology, Hokkaido University Hospital for further examinations and management. Her BCVA was 1.0 OD within normal range of intraocular pressure at the initial presentation. Slit-lamp examination revealed subconjunctival elevated lesions with injection in the superonasal side OD (Figure 1A). Fundus examination (Figure 1B)showed choroidal folds in the supero-temporal side. B-mode ultrasonography displayed marked thickening of the posterior sclera which showed hyperreflective in the temporal and nasal sides, measuring 5-mm thickness in maximum (Figure 1C).OCT displayed an irregular retinal pigment epithelial layer in the temporal side of the macula (Figure 1D). MRI displayed marked posterior scleral thickening enhanced by gadolinium(Figure 1E). She did not have any other past medical history except for well-controlled hypertension. Family history was unremarkable. Blood test showed a positive for Hepatitis B virus antigen, and serum IgG and IgG4 levels were within the normal range; 1505 mg/dL and 108 mg/dL, respectively.The other serological findings such as C-reactive protein,rheumatoid factor, antinuclear antibody, anti-thyroglobulin antibody, and anti-thyroid peroxidase antibody were all negative.Based on clinical findings, nodular-type posterior scleritis or IgG4-related ophthalmic disease (IgG4-ROD) was initially considered; however, it was difficult to differentiate malignancies from benign lymphoproliferative disorders.Therefore, local resection of the episcleral lesions was scheduled to make a histopathological diagnosis. Whitish elevated lesions were exposed following conjunctival incision in the supero-nasal side, in which a part of the lesions was resected. Microscopic examination showed inflammatory cell infiltration located in the episclera (Figure 2A), made up of lymphocytes and plasma cells (Figure 2B1), with fibrous tissues (Figure 2B2). Immunohistochemical examination demonstrated that CD3-positive T-cells, CD20/5-positive B-cells, and CD138-positive plasma cells infiltrated into the tissue. Immunoglobulin light chain restriction by λ and κ deviation was not identified. Some plasma cells were positive for IgG, whereas the population of cells being positive for IgG4 was less than 40% (Figure 2C). There were no wellconfigured lymphoid follicles, granulomatous inflammation or necrosis. Based on the findings, clinical diagnosis in this case was nodular posterior scleritis, and pathological diagnosis was reactive lymphoid hyperplasia.
Systemic administration of prednisolone at the dose of 20 mg was given, which was tapered gradually, since scleral thickening was decreased. Slit lamp examination showed the elevated lesions with conjunctival injection are resolved (Figure 1F).B-mode image showed reduced scleral thickening (Figure 1H).

Figure 1 Findings of slit-lamp examination and imaging before and after treatment A-E: Before treatment; F-I: After treatment. A: Slit lamp examination shows elevated lesions with conjunctival injection in the supero-nasal side OD; B: Fundus photo shows choroidal folds in the supero-temporal side; C: B-mode image shows marked posterior scleral thickening before treatment; D: OCT reveals an irregular retinal pigment epithelial layer in the temporal side of the macula; E: MRI detects marked posterior scleral thickening enhanced by gadolinium; F: The elevated lesions with conjunctival injection are resolved; G: Fundus displays resolved choroidal folds at the macular region; H: B-mode image shows reduced scleral thickening; I: OCT at the macular region reveals smooth retinal pigment epithelial layer.
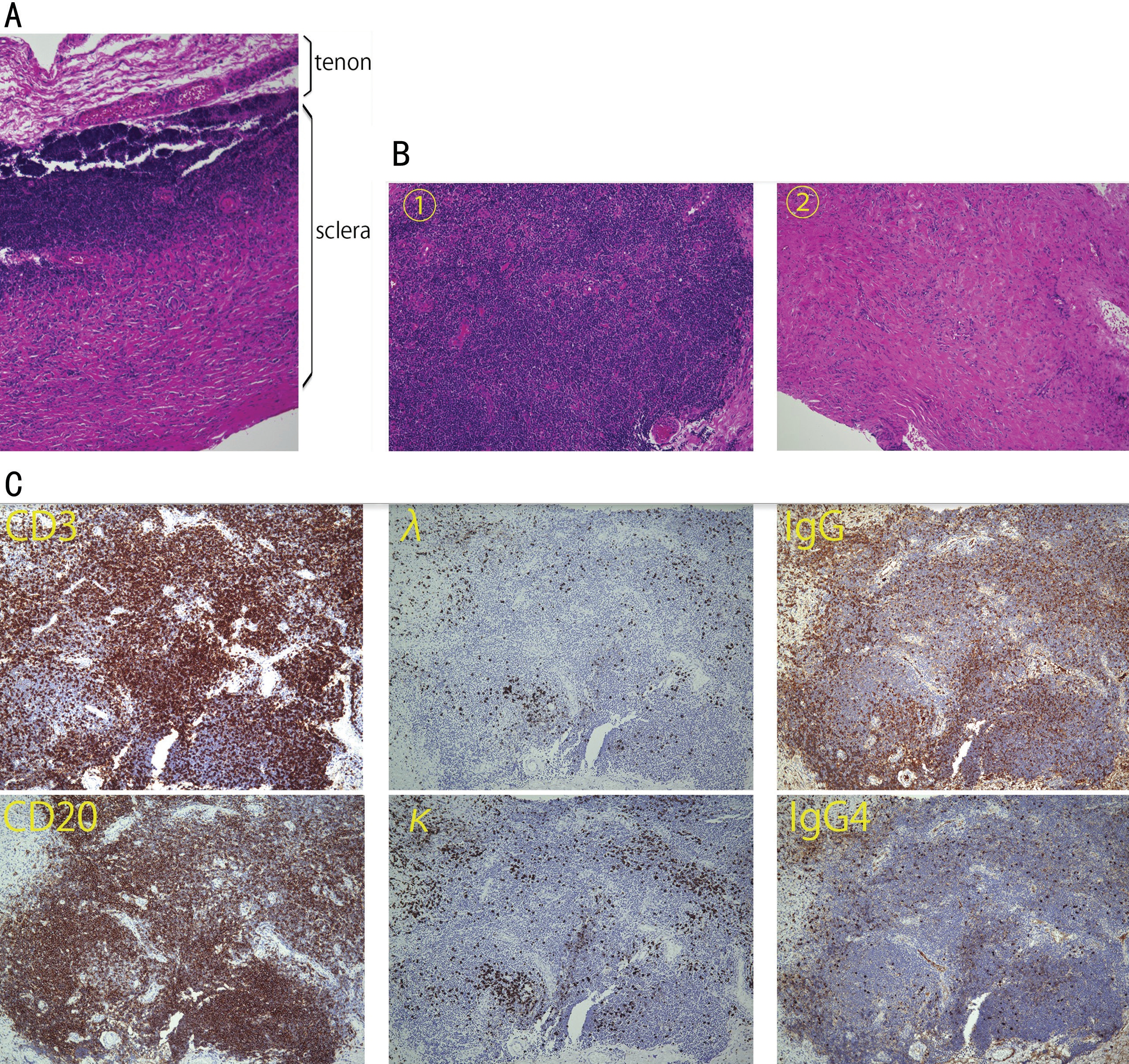
Figure 2 Findings of histopathological examination of the scleral lesion A: Inflammation takes place on the episclera (90 degree rotation from the original specimen of B, hematoxylin-eosin staining, magnification ×40); B: There are cellular component (B1) and fibrous tissue (B2)in the episclera (magnification ×100). In the former component, there are a variety of CD3-positive T-cells and CD20-positive B-cells infiltrating into the tissue. Immunoglobulin light chain restriction is not identified by λ and κ deviation; C: Some plasma cells are positive for IgG, while the cells positive for IgG4 are less than 40% (magnification ×100).
Choroidal folds and retinal pigment epithelial layer irregularity were resolved (Figure 1G and 1I, respectively). So far, no recurrence of scleral inflammation has been observed for 9mo from the onset.
It is likely that patients with posterior scleritis suffer from ocular pain. McCluskey et al[1] found that 55 of 99 patients who were diagnosed with posterior scleritis had ocular pain.In contrast, patients with posterior scleritis did not always suffer from ocular pain[2], and further the symptoms would not be associated with inflammation. Deaner et al[3] reported that a 58-year-old woman who had unrelenting ocular pain was diagnosed with choroidal metastasis from lung cancer. Shields et al[4] reported that 7% of patients with metastasis to the ocular regions had an ocular pain. Although B-mode ultrasonography showed hyperreflectivity which might be diagnosed scleritis,it was difficult to exclude IgG4-ROD or choroidal melanoma according to other clinical findings including MRI. Therefore,it may be clinically difficult to differentiate posterior scleritis from intraocular malignant tumors based on clinical symptoms including ocular pain[2,5-6]. Our case presented with markedly thickened sclera and lack of obvious ocular pain.
There is a concern about the need of biopsy in this case. If the inflammation was considered most likely in differential diagnoses, systemic corticosteroid might have been given instead of biopsy, leading to regression of the thickening.However, there are at least 3 reasons why the biopsy was conducted. First of all, Goto and Ueda[7] reported an interesting case of IgG4-ROD complicated by local scleral thickening without ocular pain, which initially simulated intraocular tumors. The diagnosis was eventually made based on clinicopathological findings[7]. The clinical findings are similar with our case. IgG4-ROD is an ophthalmic manifestation in systemic IgG4-related disease, which can represent different clinical course from that in posterior scleritis. Second is that our patient was HBV carrier. If systemic steroid therapy started, managements of HBV reactivation are needed together with primary physician. Therefore, justification of use of steroids should be required by histological findings.Third, there are previous reports showing scleral melanoma and malignant lymphoma[5-6,8]. Demirci et al[5] reported that nonpigmented appearance of the lesion, normal intrinsic choroidal vascular pattern, and transmission of lights on transillumination were important clinical clues suggestive of posterior scleritis. Finger et al[8] reported that it was difficult to diagnose posterior scleritis when the patient did not have ocular pain in spite of modern diagnostic aids of fluorescein angiography and ultrasonography. Sielert et al[9] reported that posterior nodular scleritis may present as a painless subretinal mass with features concerning for malignancy. They also reported that the uniform high internal reflectivity of posterior scleritis found on B-scan helped distinguish it from a choroidal amelanotic melanoma, which has low internal reflectiveity.Taken together, it was mandatory to make a histological diagnosis in this case.
In our case, microscopic examination revealed lymphoplasmacytic infiltration as well as fibrosis in the episclera that might be similar with the previous reported case[9]. On the contrary, according to the diagnostic criteria of IgG4-ROD[10],our present case did not satisfy the histological criteria.Moreover, this case showed no elevation of serum IgG4 levels.Therefore, the current case could not be diagnosed with IgG4-ROD. Riono et al[11] proposed histological classification of posterior scleritis, which is divided into four morphologic groups: zonal necrotizing granulomatous inflammation(Group 1), non-zonal diffuse granulomatous inflammation(Group 2), necrotizing inflammation with microabscesses(Group 3), and sarcoid granulomatous inflammation (Group 4).Our case might be partly consistent with Group 2; however, our case is unique because of episcleral involvement, but not total scleral wall involvement, made up of chronic inflammatory cells, without lymphoid follicle, granulomatous inflammation nor microfoci of necrosis, the latter of which could be found in Group 2 posterior scleritis[11].
A part of patients with posterior scleritis may be associated with systemic diseases including rheumatoid arthritis, systemic vasculitis, and granulomatosis with polyangiitis. Since she did not have medical history of collagen disease or any specific blood findings before, the cause of inflammation was considered idiopathic in this case. It has been demonstrated that scleritis associated with systemic autoimmune diseases and idiopathic scleritis had different pathological features[11].Scleritis with systemic autoimmune diseases was involved with an immune complex-mediated process, whereas idiopathic scleritis is less likely to have this process. Cases with zonal necrotizing granulomatous scleritis were primary associated with systemic autoimmune diseases, while those with nonzonal diffuse scleral inflammation showed no evidence of associated systemic diseases[11]. This observation is consistent with our case because our patient had a non-zonal diffuse nonspecific inflammation without any systemic disorders.
In conclusions, when ophthalmologists noticed markedly thickened sclera, intraocular tumors such as malignant lymphoma and melanoma as well as IgG4-ROD should be included as differential diagnoses. This nodular posterior scleritis showing markedly thickened sclera histologically revealed non-zonal diffuse inflammation.
ACKNOWLEDGEMENTS
Conflicts of Interest: Shibata Y, None; Kase S, None;Namba K, None; Ishida S, None.
1 McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis. Ophthalmology 1999;106(12):2380-2386.
2 Calthorpe CM, Watson PG, McCartney AC. Posterior scleritis: a clinical and histological survey. Eye (Lond) 1988;2(Pt 3):267-277.
3 Deaner JD, Pointdujour-Lim R, Say EA, Shields CL. Unrelenting ocular pain as a masquerading symptom of occult choroidal metastasis. Ocul Oncol Pathol 2017;3(1):56-59.
4 Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology 1997;104(8):1265-1276.5 Demirci H, Shields CL, Honavar SG, Shields JA, Bardenstein DS.Long-term follow-up of giant nodular posterior scleritis simulating choroidal melanoma. Arch Ophthalmol 2000;118(9):1290-1292.
6 Hoang-Xuan T, Bodaghi B, Toublanc M, Delmer A, Schwartz L, D'Hermies F. Scleritis and mucosal-associated lymphoid tissue lymphoma: a new masquerade syndrome. Ophthalmology 1996;103(4):631-635.
7 Goto H, Ueda S. Immunoglobulin G4-related ophthalmic disease involving the sclera misdiagnosed as intraocular tumor: report of one case.Ocul Oncol Pathol 2016;2(4):285-288.
8 Finger T, Perry D, Packer S, Erdey A, Weisman D, Sibony A. Posterior scleritis as an intraocular tumour. Br J Ophthalmol 1990;74(2):121-122.9 Sielert LA, Harris AR, Pyun JM, Campbell BJ, Swan RT. Posterior scleritis. Expert Review of Ophthalmology 2016;11(6):475-484.
10 Goto H, Takahira M, Azumi A. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn J Ophthalmol 2015;59(1):1-7.
11 Riono WP, Hidayat AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology 1999;106(7):1328-1333.